Evaluation of Cholinesterase Inhibitory Potential of Different Genotypes of Ziziphus nummularia, Their HPLC-UV, and Molecular Docking Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.2. DPPH Radical Scavenging Capacity
2.3. ABTS Radical Scavenging Capabilities
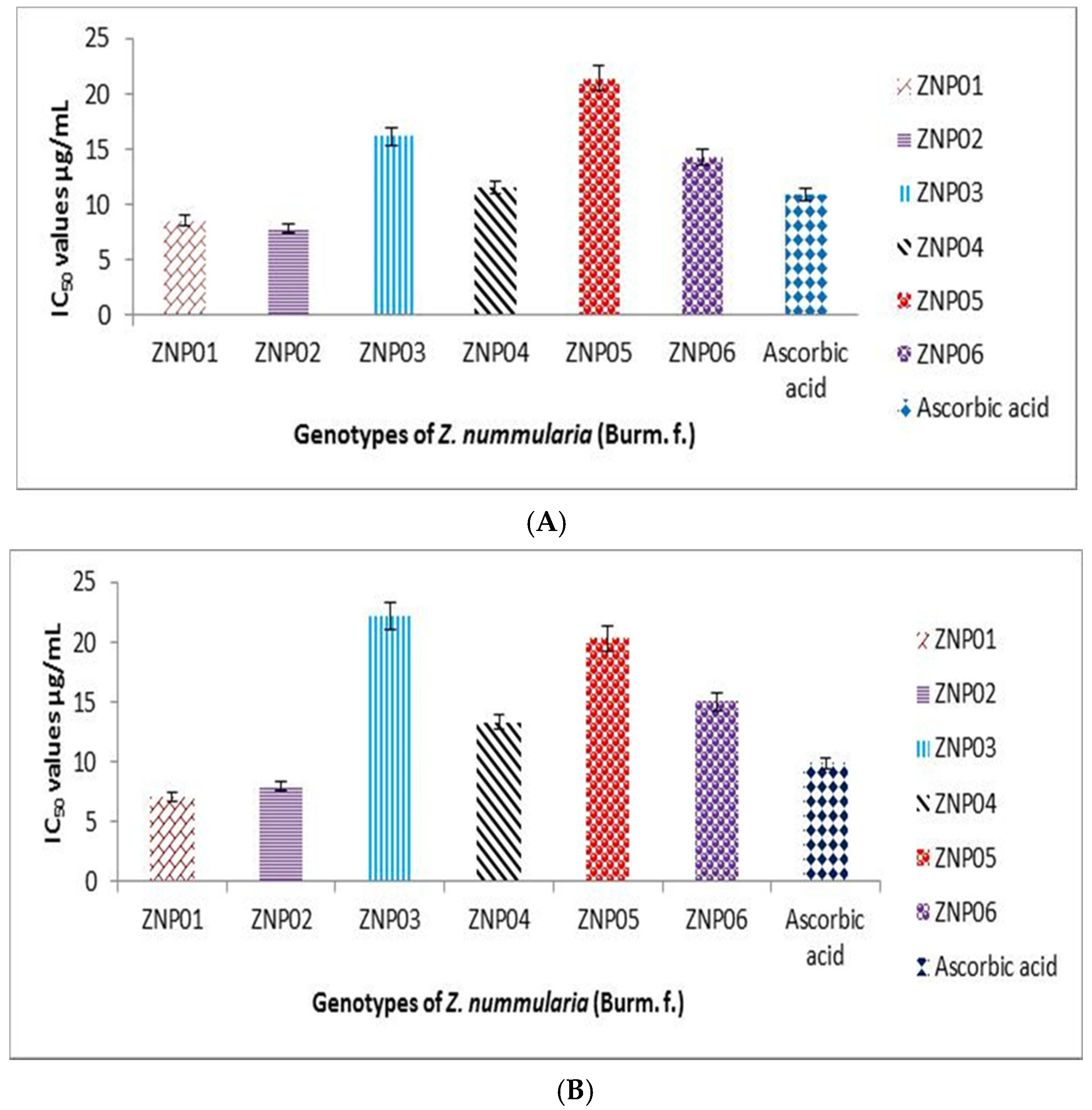
2.4. Acetyl Cholinesterase Inhibition Potential
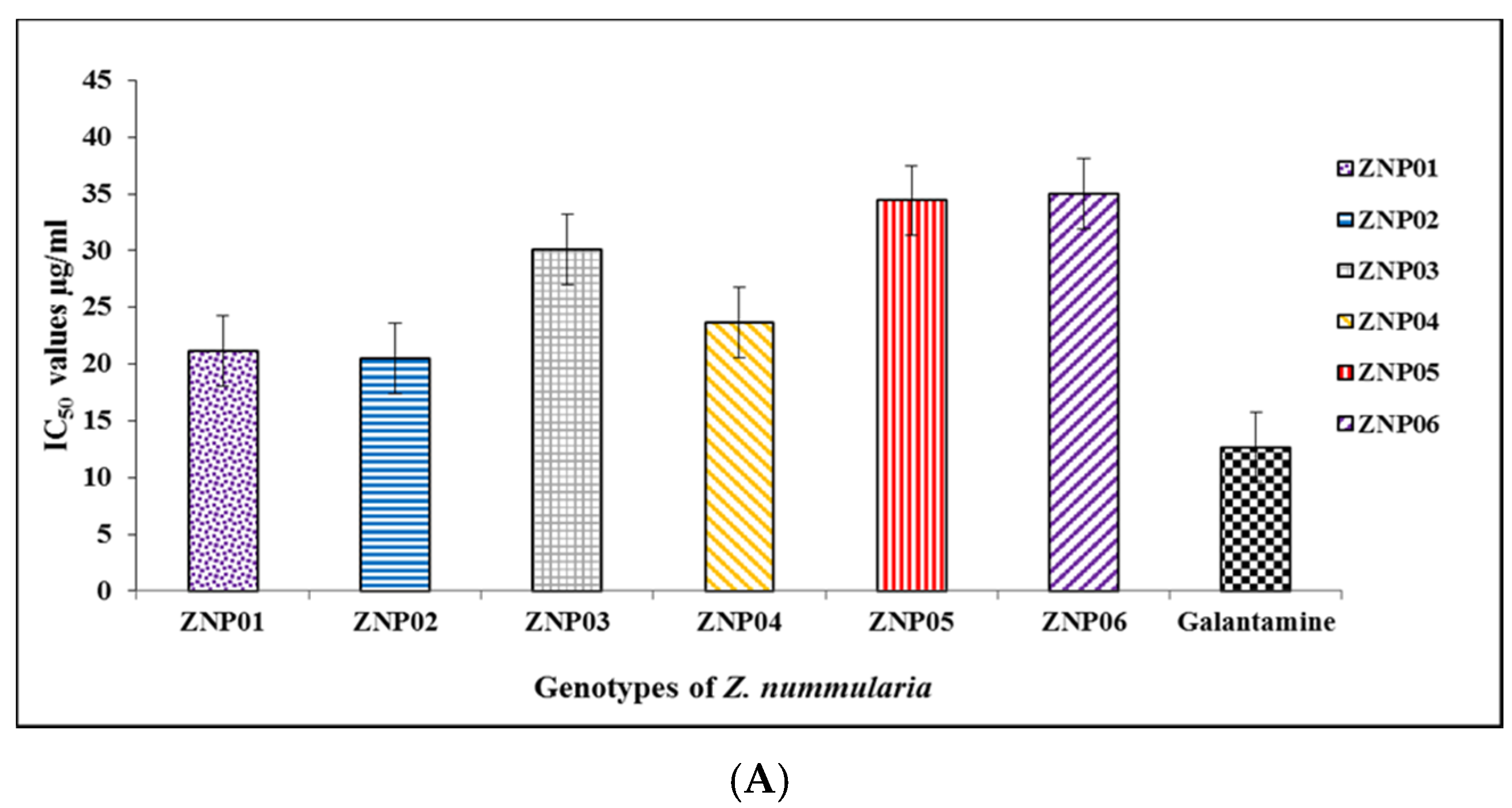
2.5. Butyryl Cholinesterase Inhibition Potential
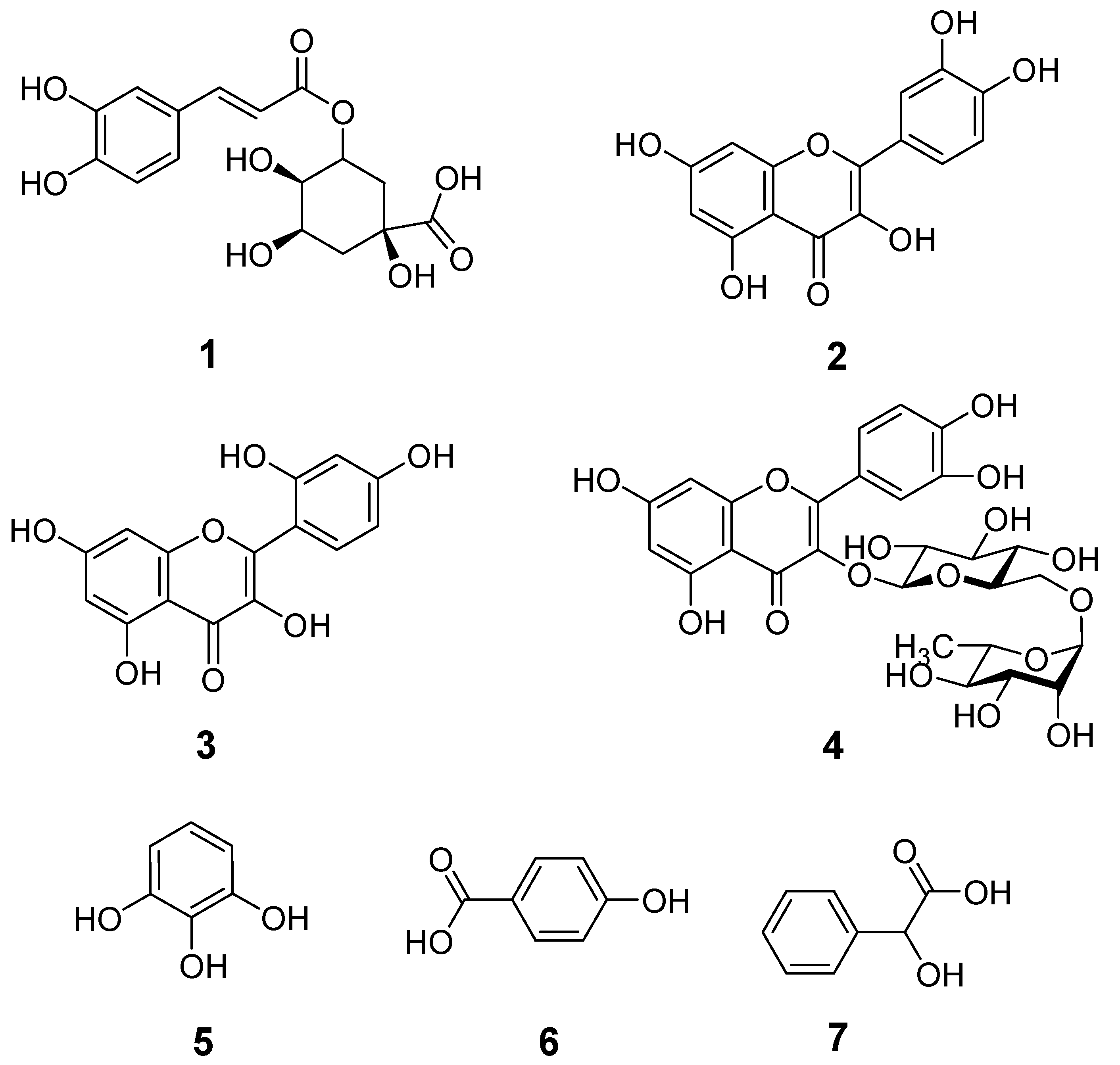
2.6. HPLC Characterization of Phenolic Compounds
2.7. Docking Studies
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Plant Collection, Extraction and Fractionation
3.3. Total Phenolic Content (TPC)
3.4. Total Flavonoid Content (TFC)
3.5. DPPH Radical Scavenging Assay
3.6. ABTS Free Radical Scavenging Assay
3.7. Anticholinesterase Assay
3.8. Samples Preparation and HPLC-UV Analysis
3.9. Docking Studies
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Javanmardi, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, H.; Hong, E.-K.; Kang, B.-H.; Kim, S.-J. Water extract of 1:1 mixture of Phellodendron cortex and Aralia cortex has inhibitory effects on oxidative stress in kidney of diabetic rats. J. Ethnopharmacol. 2000, 73, 429–436. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.; Nychas, G.-J.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Yen, G.-C. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Lai, L.-S.; Chou, S.-T.; Chao, W.-W. Studies on the antioxidative activities of Hsian-tsao (Mesona procumbensHemsl) leaf gum. J. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Howes, M.-J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals 2005, 14, 6–22. [Google Scholar] [CrossRef]
- Wimo, A.; Winblad, B.; Aguero-Torres, H.; Von Strauss, E. The magnitude of dementia occurrence in the world. Alzheimer Dis. Assoc. Disord. 2003, 17, 63–67. [Google Scholar] [CrossRef]
- Şenol, F.; Orhan, I.; Yilmaz, G.; Çiçek, M.; Şener, B. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [Google Scholar] [CrossRef]
- Eckert, G.P. Traditional used plants against cognitive decline and Alzheimer disease. Front. Pharmacol. 2010, 1, 138. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—The development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Zou, Y.-P.; Lu, Y.-H.; Wei, D.-Z. Protective effects of a flavonoid-rich extract of Hypericum perforatum L. against hydrogen peroxide-induced apoptosis in PC12 cells. Phytother. Res. 2009, 24, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Chauhan, J. Chemical examination of the whole plant of zizyphus—nummularia. Planta Medica 1977, 32, 384–387. [Google Scholar] [CrossRef]
- Pareek, O. Present status and future needs for genetic resources activities in arid zone fruits. In Plant Genetic Resources-Indian Perspective; National Bureau of Plant Genetic Resource: New Delhi, India, 1988; pp. 320–334. [Google Scholar]
- Hammer, K.; Heller, J.; Engels, J. Monographs on underutilized and neglected crops. Genet. Resour. Crop. Evol. 2001, 48, 3–5. [Google Scholar] [CrossRef]
- Gupta, D.; Mann, S.; Jain, I.; Gupta, R.K. Phytochemical, utritio al ada tioxida t activity evaluatio of fruits of ziziphus ummularia Burm. F. Int. J. Pharma. Bio. Sci. 2011, 2, 629–638. [Google Scholar]
- Pandey, A.; Singh, R.; Radhamani, J.; Bhandari, D.C. Exploring the potential of Ziziphus nummularia (Burm. f.) Wight et Arn. from drier regions of India. Genet. Resour. Crop. Evol. 2010, 57, 929–936. [Google Scholar] [CrossRef]
- Adzu, B.; Amos, S.; Wambebe, C.; Gamaniel, K. Antinociceptive activity of Zizyphus spina-christi root bark extract. Fitoterapia 2001, 72, 344–350. [Google Scholar] [CrossRef]
- Shah, A.; Tariq, M.; Al-Yahya, M. Studies on the alkaloidal fraction from the stem bark of Zizyphus nummularia. Fitoterapia 1990, 61, 46–49. [Google Scholar]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Alshalmani, S.K.; Abdellatif, A.G. Antioxidant and quantitative estimation of phenolic and flavonoids of three halophytic plants growing in Libya. J. Pharmacog. Photochem. 2013, 2, 89–94. [Google Scholar]
- Pontis, J.A.; Da Costa, L.A.M.A.; Da Silva, S.J.R.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Sohaib, M.; Butt, M.S.; Shabbir, M.A.; Shahid, M. Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α-tocopherol enriched diets. Lipids Health Dis. 2015, 14, 61. [Google Scholar] [CrossRef]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Rahman, A.; Parveen, S.; Khalid, A.; Farooq, A.; Choudhary, M.I. Acetyl and Butyrylcholine esterase-inhibiting triterpenoid alkaloids from Buxus papillosa. Phytochemistry 2001, 58, 963–968. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement. Altern. Med. 2011, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef]
- Dureja, A.; Dhiman, K. Free radical scavenging potential and total phenolic and flavonoid content of Ziziphus mauritiana and Ziziphus nummularia fruit extracts. Int. J. Green Pharm. 2012, 6, 187. [Google Scholar] [CrossRef]
- Tyrakowska, B.; Soffers, A.E.; Szymusiak, H.; Boeren, S.; Boersma, M.G.; Pawlak-Lemańska, K.; Vervoort, J.J.M.; Rietjens, I.M.C.M. TEAC antioxidant activity of 4-hydroxybenzoates. Free. Radic. Biol. Med. 1999, 27, 1427–1436. [Google Scholar] [CrossRef]
- Kim, W.-G.; Cho, K.-M.; Kil Lee, C.; Yoo, I.-D. Terreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity from Aspergillus terreus. Tetrahedron Lett. 2002, 43, 3197–3198. [Google Scholar] [CrossRef]
- Bourne, Y.; Taylor, P.; Radić, Z.; Marchot, P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003, 22, 1–12. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with A? Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Onivogui, G.; Zhang, H.; Mlyuka, E.; Diaby, M.; Song, Y. Chemical composition, nutritional properties and antioxidant activity of monkey apple (Anisophyllea laurina R. Br. ex Sabine). J. Food Nutr. Res. 2014, 2, 281–287. [Google Scholar] [CrossRef]
- Shirazi, O.U.; Khattak, M.; Shukri, N.A.M.; Nasyriq, M.N. Determination of total phenolic, flavonoid content and free radical scavenging activities of common herbs and spices. J. Pharmacogn. Phytochem. 2014, 3, 104–108. [Google Scholar]
- Kim, Y.J.; Cho, Y.A.; Lee, H.-S.; Lee, Y.T.; Gee, S.J.; Hammock, B.D. Synthesis of haptens for immunoassay of organophosphorus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity. Anal. Chim. Acta 2003, 475, 85–96. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE); Chemical Computing Group ULC: Montreal, QC, Canada, 2018.
- Iftikhar, F.; Yaqoob, F.; Tabassum, N.; Jan, M.S.; Sadiq, A.; Tahir, S.; Batool, T.; Niaz, B.; Ansari, F.L.; Choudhary, M.I.; et al. Design, synthesis, in-vitro thymidine phosphorylase inhibition, in-vivo antiangiogenic and in-silico studies of C-6 substituted dihydropyrimidines. Bioorganic Chem. 2018, 80, 99–111. [Google Scholar] [CrossRef]
- Tanoli, S.T.; Ramzan, M.; Hassan, A.; Sadiq, A.; Jan, M.S.; Khan, F.A.; Ullah, F.; Ahmad, H.; Bibi, M.; Mahmood, T.; et al. Design, synthesis and bioevaluation of tricyclic fused ring system as dual binding site acetylcholinesterase inhibitors. Bioorganic Chem. 2019, 83, 336–347. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer; Dassault Systèmes: Waltham, MA, USA, 2017. [Google Scholar]



| Samples | TPC (mg GAE/100 g) | TFC (mg QE/100 g) |
|---|---|---|
| ZNP01 | 88.893 ± 1.353 | 74.083 ± 0.601 |
| ZNP02 | 88.503 ± 1.231 | 76.023 ± 0.974 |
| ZNP03 | 82.063 ± 2.069 | 68.260 ± 1.584 |
| ZNP04 | 88.810 ± 1.336 | 74.350 ± 0.743 |
| ZNP05 | 85.487 ± 0.386 | 58.130 ± 1.041 |
| ZNP06 | 85.777 ± 0.909 | 63.350 ± 0.344 |
| Sample | No. of Peak | Retention Time (min) | Identified Phenolic Compounds | λmax (nm) | Peak Area of Sample | Peak Area of Standard | Con. (mg/100 g) |
|---|---|---|---|---|---|---|---|
| ZNP01 | 1 | 6.6 | Chlorogenic acid | 320 | 35.00 | 12.93 | 2.43 |
| 2 | 9.8 | Quercetin | 320 | 208.70 | 7089.28 | 0.03 | |
| 3 | 12.5 | Morin | 320 | 38.31 | 2.01 | 17.15 | |
| 4 | 22.3 | Rutin | 320 | 68.22 | 2241.22 | 0.01 | |
| 5 | 28.5 | Pyrogallol | 320 | 182.25 | 1.02 | 161.76 | |
| 6 | 29.7 | Mandelic acid | 320 | 116.76 | 7195.52 | 0.02 | |
| ZNP02 | 1 | 6.6 | Chlorogenic acid | 320 | 188.48 | 12.93 | 13.12 |
| 2 | 12.5 | Morin | 320 | 55.70 | 2.01 | 24.93 | |
| 3 | 22.3 | Rutin | 320 | 67.21 | 2241.22 | 0.01 | |
| 4 | 29.7 | Mandelic acid | 320 | 50.94 | 7195.52 | 0.01 | |
| ZNP03 | 1 | 12.5 | Morin | 320 | 220.18 | 2.01 | 98.55 |
| 2 | 22.3 | Rutin | 320 | 28.30 | 2241.22 | 0.01 | |
| 3 | 29.7 | Mandelic acid | 320 | 54.69 | 7195.52 | 0.01 | |
| 4 | 36.9 | Hydroxy benzoic acid | 320 | 49.69 | 40.20 | 1.12 | |
| ZNP04 | 1 | 9.8 | Quercetin | 320 | 21.93 | 7089.29 | 0.002 |
| 2 | 12.5 | Morin | 320 | 86.05 | 2.01 | 38.51 | |
| 3 | 22.3 | Rutin | 320 | 50.15 | 2241.22 | 0.01 | |
| ZNP05 | 1 | 6.6 | Chlorogenic acid | 320 | 19.51 | 12.93 | 1.36 |
| ZNP06 | 1 | 12.5 | Morin | 320 | 35.81 | 2.01 | 16.03 |
| 2 | 22.3 | Rutin | 320 | 39.08 | 2241.22 | 0.01 |
| Identified Phenolic Compounds | Binding Energy (kcal/mol) |
|---|---|
| Chlorogenic acid | −7.06 |
| Quercetin | −6.46 |
| Morin | −6.26 |
| Rutin | −9.20 |
| Pyrogallol | −4.3 |
| Mandelic acid | −4.73 |
| Hydroxy benzoic acid | −4.59 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, N.; Ali, N.; Uddin, Z.; Nazir, N.; Zahoor, M.; Rashid, U.; Ullah, R.; Alqahtani, A.S.; Alqahtani, A.M.; Nasr, F.A.; et al. Evaluation of Cholinesterase Inhibitory Potential of Different Genotypes of Ziziphus nummularia, Their HPLC-UV, and Molecular Docking Analysis. Molecules 2020, 25, 5011. https://doi.org/10.3390/molecules25215011
Uddin N, Ali N, Uddin Z, Nazir N, Zahoor M, Rashid U, Ullah R, Alqahtani AS, Alqahtani AM, Nasr FA, et al. Evaluation of Cholinesterase Inhibitory Potential of Different Genotypes of Ziziphus nummularia, Their HPLC-UV, and Molecular Docking Analysis. Molecules. 2020; 25(21):5011. https://doi.org/10.3390/molecules25215011
Chicago/Turabian StyleUddin, Nisar, Niaz Ali, Zia Uddin, Nausheen Nazir, Muhammad Zahoor, Umer Rashid, Riaz Ullah, Ali S. Alqahtani, Abdulaziz M. Alqahtani, Fahd A. Nasr, and et al. 2020. "Evaluation of Cholinesterase Inhibitory Potential of Different Genotypes of Ziziphus nummularia, Their HPLC-UV, and Molecular Docking Analysis" Molecules 25, no. 21: 5011. https://doi.org/10.3390/molecules25215011
APA StyleUddin, N., Ali, N., Uddin, Z., Nazir, N., Zahoor, M., Rashid, U., Ullah, R., Alqahtani, A. S., Alqahtani, A. M., Nasr, F. A., Liu, M., & Nisar, M. (2020). Evaluation of Cholinesterase Inhibitory Potential of Different Genotypes of Ziziphus nummularia, Their HPLC-UV, and Molecular Docking Analysis. Molecules, 25(21), 5011. https://doi.org/10.3390/molecules25215011