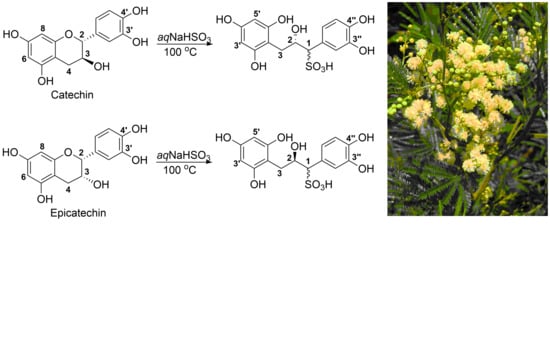
Analysis of Commercial Proanthocyanidins. Part 6: Sulfitation of Flavan-3-Ols Catechin and Epicatechin, and Procyanidin B-3
Abstract
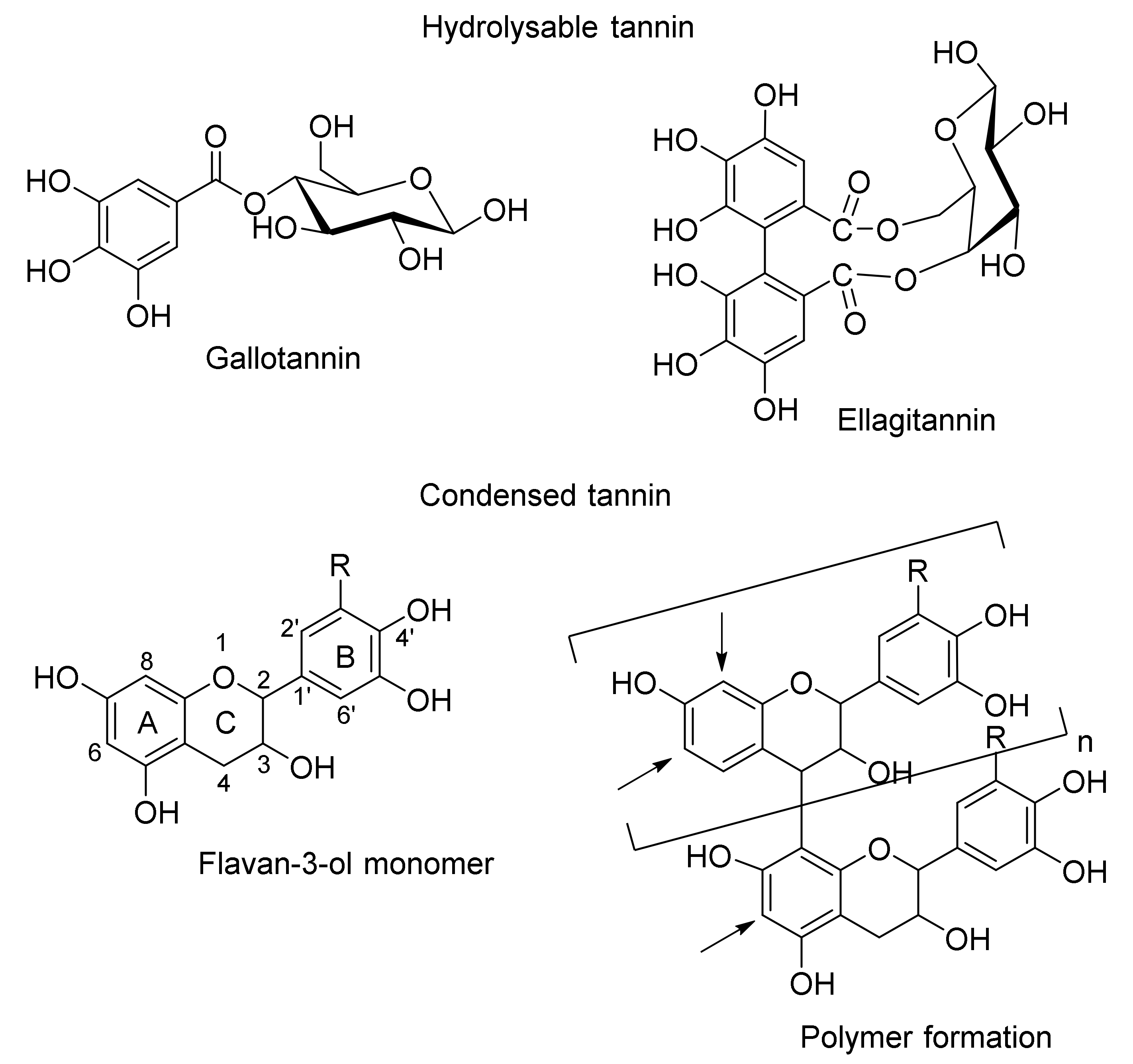
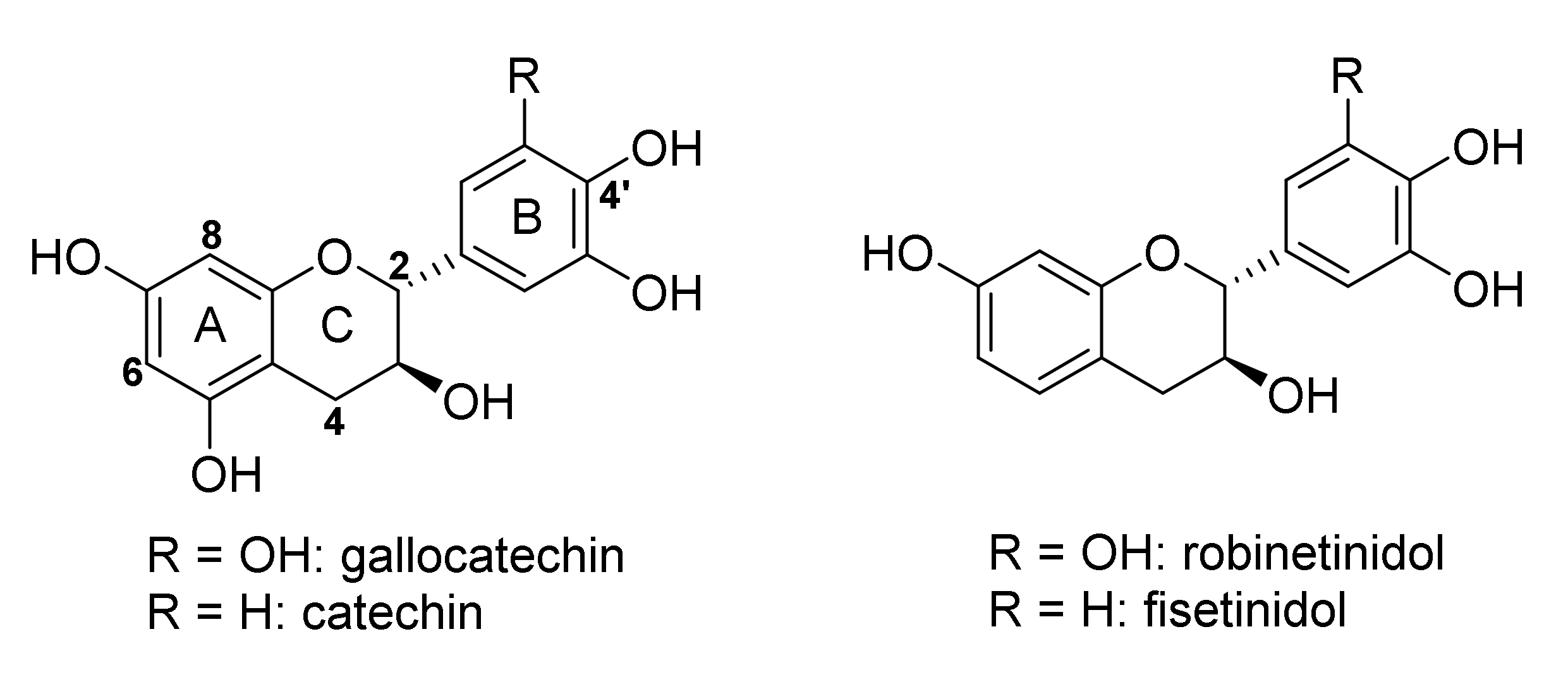
1. Introduction
2. Results and Discussion
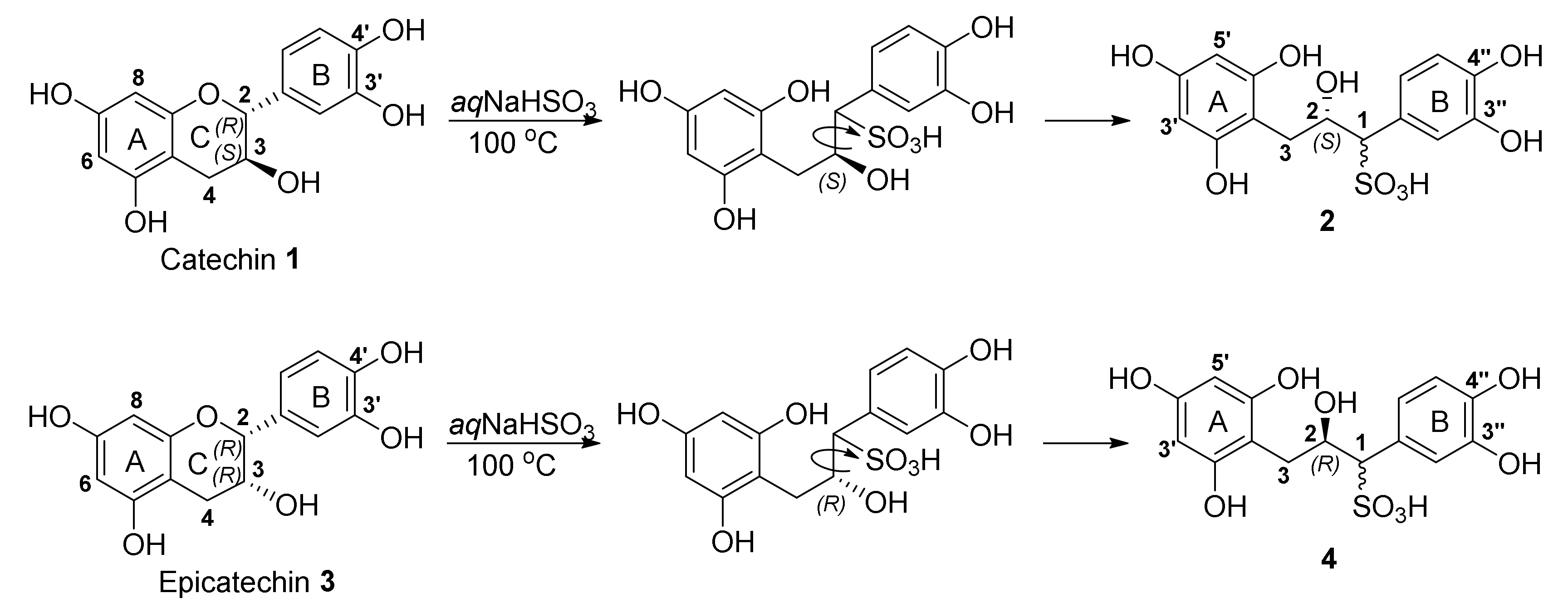
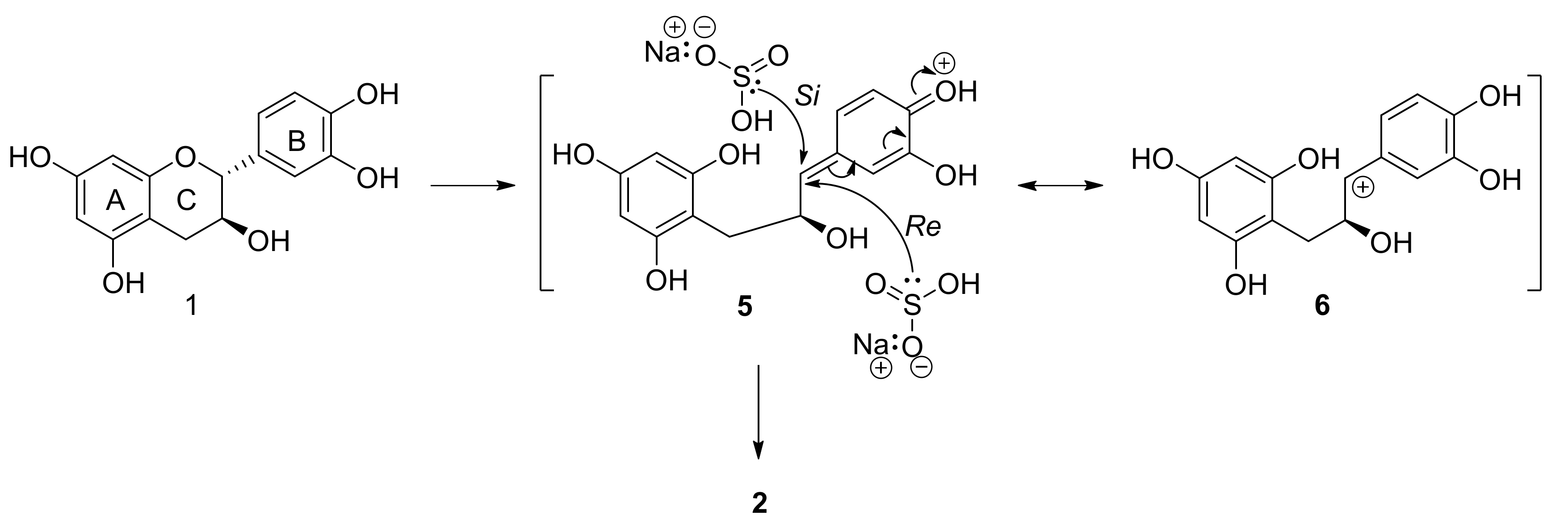
2.1. Sulfitation of Diastereomeric 2R,3S-Catechin and 2R,3R-Epicatechin
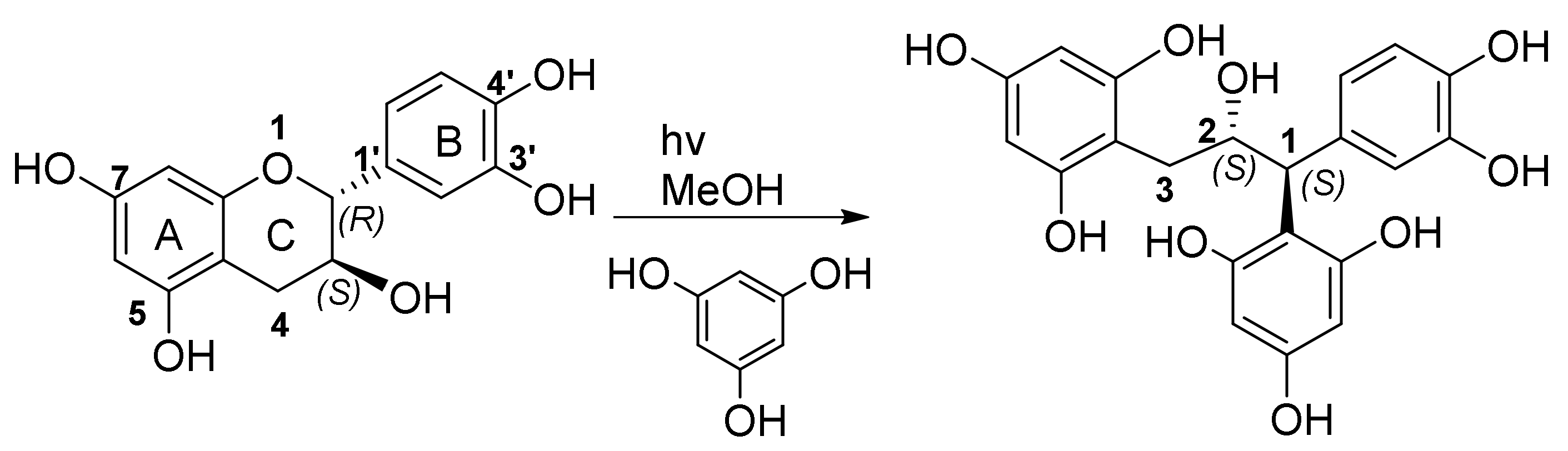
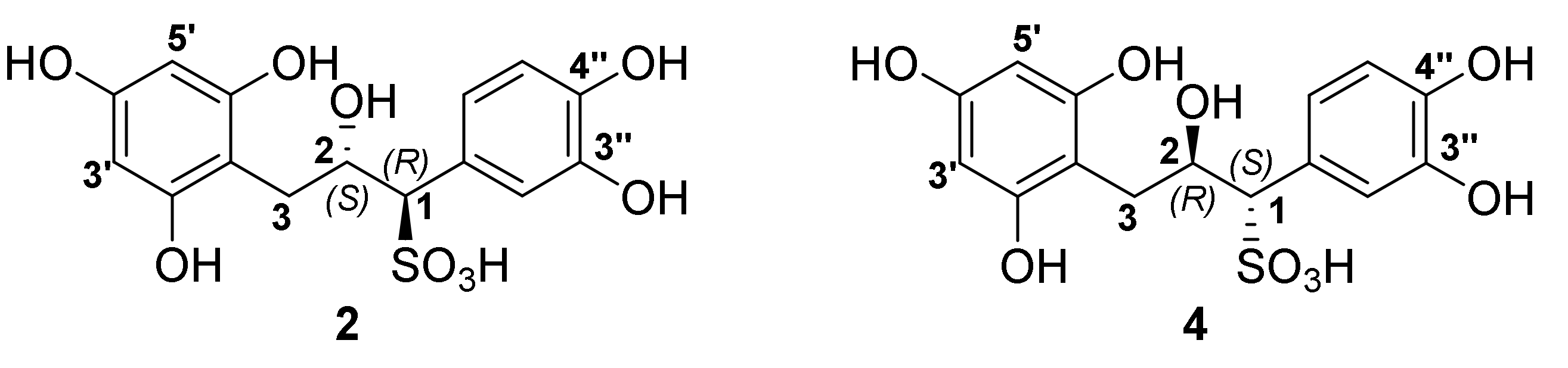
2.2. GIAO NMR Shift Calculation and DP4 Analysis
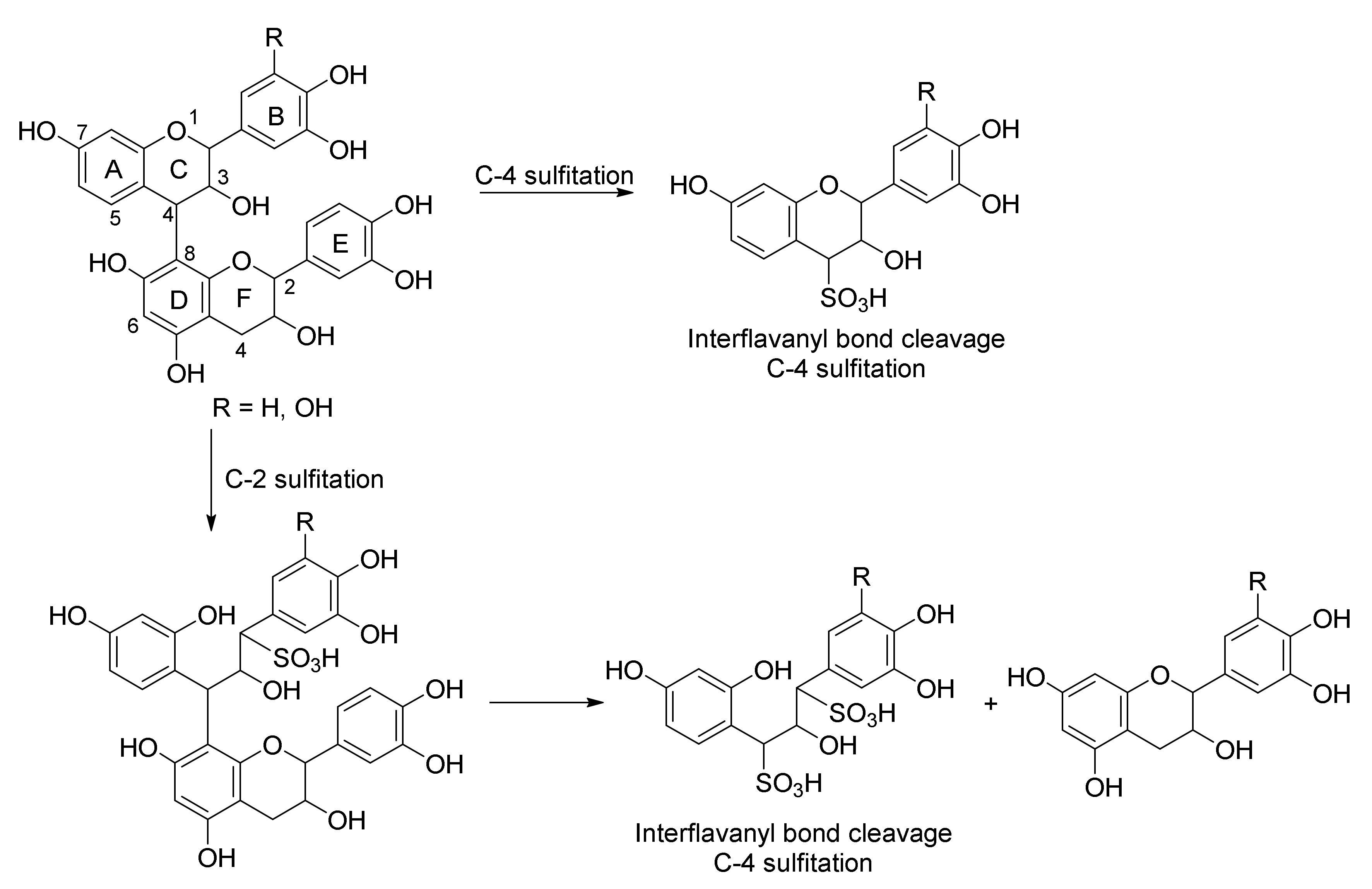
2.3. Sulfitation of Procyanidin B-3
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Covington, A.D. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-1-84973-434-9. [Google Scholar]
- Haslam, E. Polyphenols, collagen and leather. In Practical Polyphenolics. From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 2005; p. 378. [Google Scholar]
- Roux, D.G.; Paulus, E. Condensed tannis. 12. Polymeric leucofisetinidin tannins from the heartwood of Acacia mearnsii. Biochem. J. 1962, 82, 320–324. [Google Scholar] [CrossRef]
- Roux, D.G.; Evelyn, S.R. Condensed tannins 2. Biogenesis of condensed tannins based on leucoanthocyanins. Biochem. J. 1958, 70, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Robert, W.; Griffith, R.W. Chestnut wood in the tanning industry. J. For. 1924, 22, 542–545. [Google Scholar] [CrossRef]
- Garro Galvez, J.M.; Riedl, B.; Conner, A.H. Analytical studies on tara tannins. Holzforschung 1977, 51, 235–243. [Google Scholar] [CrossRef]
- Cooper, S.M.; Owen-Smith, N.; Bryant, J.P. Foliage acceptability to browsing ruminants in relation to seasonal changes in the leaf chemistry of woody plants in a South African savanna. Oecologia 1988, 75, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.R.; Mohd Norddin, M.N.A.; Latefi, N.A.S.; Oseh, J.O.; Ismail, I.; Gbadamosi, A.O.; Agi, A.J. Evaluation of a naturally derived tannin extracts biopolymer additive in drilling muds for high-temperature well applications. J. Petrol Explor. Prod. Technol. 2020, 10, 623–639. [Google Scholar] [CrossRef]
- Pizzi, A. Hot-setting tannin-urea-formaldehyde exterior wood adhesives. Adhes. Age 1977, 20, 27–29. [Google Scholar]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production: Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Parker, M.; Smith, P.A.; Birse, M.; Francis, I.L.; Kwiatkowski, M.J.; Lattey, K.A.; Liebich, B.; Herderich, M.J.L. The effect of pre- and post-ferment additions of grape derived tannin on Shiraz wine sensory properties and phenolic composition. Aust. J. Grape Wine R. 2007, 13, 30–37. [Google Scholar] [CrossRef]
- Beltran-Heredia, J.; Sanchez-Martin, J. Removing heavy metals from polluted surface water with tannin-based flocculant agent. J. Hazard. Mater. 2008, 165, 1215–1218. [Google Scholar] [CrossRef]
- Beltran-Heredia, J.; Sanchez-Martin, J. Municipal wastewater treatment by modified tannin flocculent agent. Desalination 2009, 249, 353–358. [Google Scholar] [CrossRef]
- Beltran-Heredia, J.; Sanchez-Martin, J.; Frutos-Blanco, G. Schinopsis balansae tannin-based flocculant in removing dodecyl benzene sulfonate. Sep. Purif. Technol. 2009, 67, 295–303. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Rice, R.E. Tannins as Biological Antioxidants. In Plant Polyphenols 2; Gross, G.G., Hemingway, R.W., Yoshida, T., Branham, S.J., Eds.; Springer: Boston, MA, USA, 1999; Volume 66. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini Rev. Med. Chem. 2008, 8, 436. [Google Scholar] [CrossRef]
- Gaudreault, R.; Mousseau, N. Mitigating Alzheimer’s Disease with Natural Polyphenols: A Review. Curr. Alzheimer Res. 2020, 16, 529–543. [Google Scholar] [CrossRef]
- Gollucke, A.P.; Aguiar, O., Jr.; Barbisan, L.F.; Ribeiro, D.A. Use of grape polyphenols against carcinogenesis: putative molecular mechanisms of action using in vitro and in vivo test systems. J. Med. Food 2013, 16, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; la Vecchia, C. Fruit and vegetables and cancer risk: a review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Pinent, M.; Cedó, L.; Montagut, G.; Blay, M.; Ardévol, A. Procyanidins improve some disrupted glucose homoeostatic situations: an analysis of doses and treatments according to different animal models. Crit. Rev. Food. Sci. Nutr. 2012, 52, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Sieniawska, E. Activities of Tannins - From In Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015, 10, 1877–1884. [Google Scholar]
- Mohana, T.; Navin, A.V.; Jamuna, S.; Sakeena Sadullah, M.S.; Niranjali Devaraj, S. Inhibition of differentiation of monocyte to macrophages in atherosclerosis by oligomeric proanthocyanidins -In-vivo and in-vitro study. Food. Chem. Toxicol. 2015, 82, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Heiss, C.; Kelm, M.; Keen, C.L. The potential of flavanol and procyanidin intake to influence age-related vascular disease. J. Nutr. Gerontol. Geriatr. 2012, 31, 290–323. [Google Scholar] [CrossRef] [PubMed]
- Alice Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Nicollin, A.; Zhou, X.; Pizzi, A.; Grigsby, M.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR analysis of a renewable resource additive—Thermoplastic acetylated tannins. Ind. Crops Prod. 2013, 49, 851–857. [Google Scholar] [CrossRef]
- Pizzi, A. Sulfited tannins for exterior wood adhesives. J. Org. Chem. 1972, 37, 3546–3547. [Google Scholar]
- Pizzi, A. Condensed tannins for adhesives. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 359–369. [Google Scholar] [CrossRef]
- Ferreira, D.; van Rensburg, H.; Malan, E.; Coetzee, J.; Nel, R.J.J. Recent Advances in the Chemistry of Proanthocyanidins. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense. Recent Advances in Phytochemistry, Proceedings of the Phytochemical Society of North America; Romeo, J., Ed.; Springer: Boston, MA, USA, 1999; Volume 33. [Google Scholar]
- Viviers, P.M.; Kolodziej, H.; Young, D.A.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 11. Intramolecular enantiomerism of the constituent units of tannins from the Anacardiaceae: Stoicheometric control in direct synthesis: derivation of 1H nuclear magnetic resonance parameters applicable to higher oligomers. J. Chem. Soc. Perkin Trans. 1 1983, 2555–2562. [Google Scholar] [CrossRef]
- Roux, D.G.; Ferreira, D.; Hundt, H.K.L.; Malan, E. Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Appl. Polym. Symp. 1975, 28, 335–353. [Google Scholar]
- Pizzi, A. Hardening mechanism of tannin adhesives with hexamine. Holz Roh Werkst 1994, 52, 229. [Google Scholar] [CrossRef]
- Pizzi, A.; Kueny, R.; Lecoanet, F.; Massetau, B.; Carpentier, D.; Krebs, A.; Krebs, F.; Molina, S.; Ragoubi, M. High resin content natural matrix-natural fibre biocomposites. Ind. Crops Prod. 2009, 30, 235–240. [Google Scholar] [CrossRef]
- Hoong, Y.; Paridaha, M.; Luqman, C.; Koh, M.; Loh, Y. Fortification of Sulfited Tannin from the Bark of Acacia mangium with Phenol-Formaldehyde for use as Plywood Adhesive. Ind. Crops Prod. 2009, 30, 416–421. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; van der Merwe, M.J.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: the chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Amra-Jordaan, M.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 2: An electrospray mass spectrometry investigation into the chemical composition of sulfited quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 78, 156–169. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Amra-Jordaan, M.; Bonnet, S.L.; Van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 3: The chemical composition of wattle (Acacia mearnsii) bark extract. Phytochemistry 2012, 83, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Noreljaleel, A.E.M.; Kemp, G.; Wilhelm, A.; van der Westhuizen, J.H.; Bonnet, S.L. Analysis of commercial proanthocyanidins. Part 5: A high resolution mass spectrometry investigation of the chemical composition of sulfited wattle (Acacia mearnsii De Wild.) bark extract. Phytochemistry 2019, 162, 109–120. [Google Scholar] [CrossRef]
- Wilhelm-Mouton, A.; Bonnet, S.L.; Ding, Y.; Li, X.C.; Ferreira, D.; van der Westhuizen, J.H. Photochemistry synthesis. Part 2: Enantiomerically pure polyhydroxy-1,1,3-triarylpropan-2-ols. J. Photochem. Photobiol. A Chem. 2012, 227, 18–24. [Google Scholar] [CrossRef]
- Stadler, D.; Goeppert, A.; Rasul, G.; Olag, G.A.; Surya Prakash, G.K.; Bach, T. Chiral benzylic carbocations: Low temperature NMR studies and theoretical calculations. J. Org. Chem. 2009, 74, 312–318. [Google Scholar] [CrossRef]
- Barone, G.; Gomez-Paloma, L.; Duca, D.; Silvestri, A.; Riccio, R.; Bifulco, G. Structure validation of natural products by quantum-mechanical GIAO calculations of 13C NMR chemical shifts. Chem. Eur. J. 2002, 8, 3233–3239. [Google Scholar] [CrossRef]
- Barone, G.; Duca, D.; Silvestri, A.; Gomez-Paloma, L.; Riccio, R.; Bifulco, G. Determination of the Relative Stereochemistry of Flexible Organic Compounds by Ab Initio Methods: Conformational Analysis and Boltzmann-Averaged GIAO 13C NMR Chemical Shifts. Chem. Eur. J. 2002, 8, 3240–3245. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning the Stereochemistry of Pairs of Diastereoisomers Using GIAO NMR Shift Calculation. J. Org. Chem. 2009, 74, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- Smit, S.G.; Goodman, J.M. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J. Am. Chem. Soc. 2010, 132, 12957–12959. [Google Scholar] [CrossRef]
- Van der Westhuizen, J.H. ‘n Nuwe Reeks Fotochemiese Reaksies in Flavonoïedsintese. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 1979. [Google Scholar]
Sample Availability: Samples of all of the compounds are available from the corresponding author. |


| Probability | Sulfited Catechin (2) | Sulfited Epicatechin (4) | ||
|---|---|---|---|---|
| 1R2S | 1S2S | 1R2R | 1S2R | |
| 1H NMR | 99.7% | 0.3% | 2.3% | 97.7% |
| 13C NMR | 96.1% | 3.9% | 38.9% | 61.1% |
| Combined | 100.0% | 0% | 0% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noreljaleel, A.E.M.; Wilhelm, A.; Bonnet, S.L. Analysis of Commercial Proanthocyanidins. Part 6: Sulfitation of Flavan-3-Ols Catechin and Epicatechin, and Procyanidin B-3. Molecules 2020, 25, 4980. https://doi.org/10.3390/molecules25214980
Noreljaleel AEM, Wilhelm A, Bonnet SL. Analysis of Commercial Proanthocyanidins. Part 6: Sulfitation of Flavan-3-Ols Catechin and Epicatechin, and Procyanidin B-3. Molecules. 2020; 25(21):4980. https://doi.org/10.3390/molecules25214980
Chicago/Turabian StyleNoreljaleel, Anwar E.M., Anke Wilhelm, and Susan L. Bonnet. 2020. "Analysis of Commercial Proanthocyanidins. Part 6: Sulfitation of Flavan-3-Ols Catechin and Epicatechin, and Procyanidin B-3" Molecules 25, no. 21: 4980. https://doi.org/10.3390/molecules25214980
APA StyleNoreljaleel, A. E. M., Wilhelm, A., & Bonnet, S. L. (2020). Analysis of Commercial Proanthocyanidins. Part 6: Sulfitation of Flavan-3-Ols Catechin and Epicatechin, and Procyanidin B-3. Molecules, 25(21), 4980. https://doi.org/10.3390/molecules25214980