Tannin-Based Hybrid Materials and Their Applications: A Review
Abstract
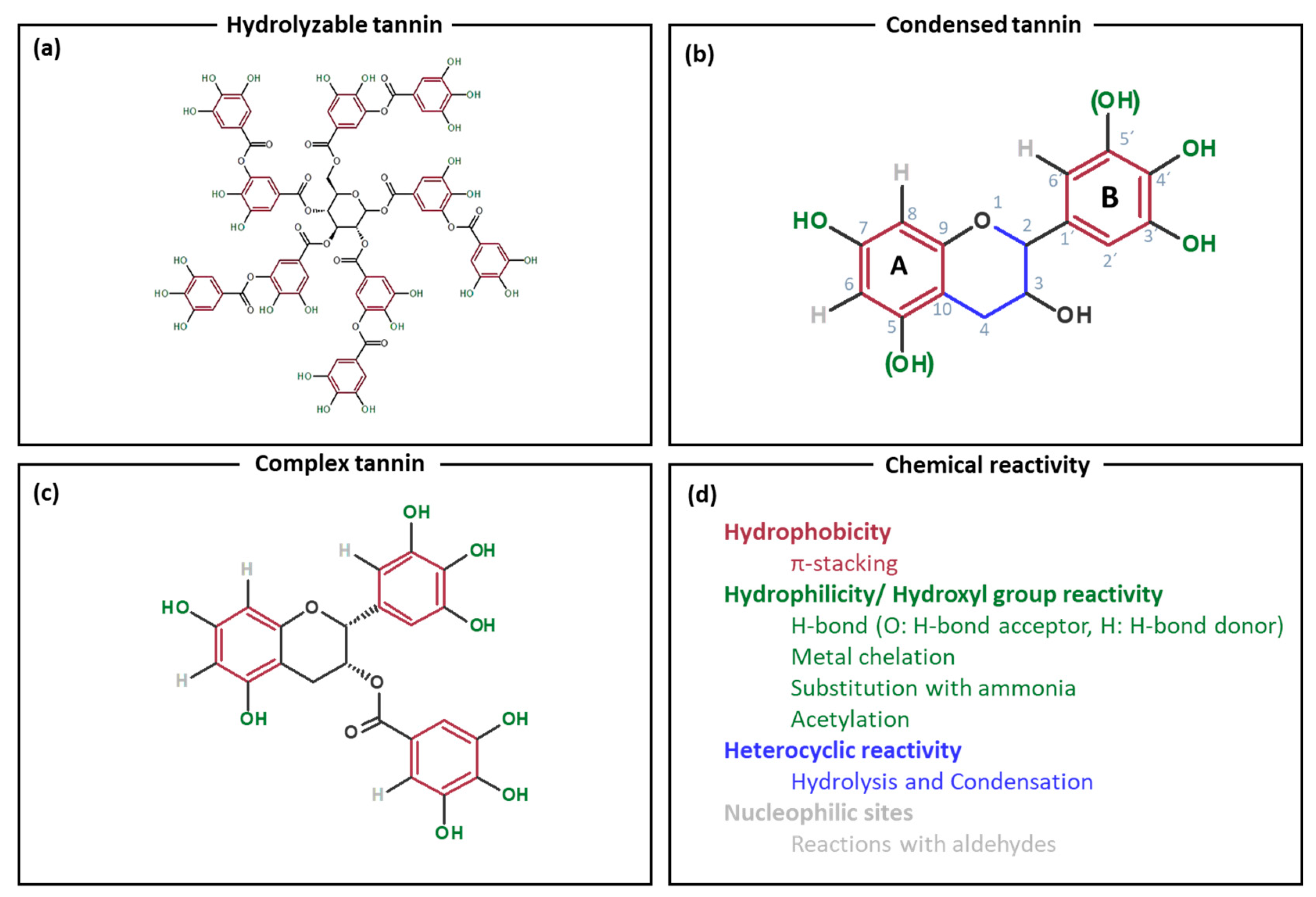
1. Introduction
2. Hybrid Materials
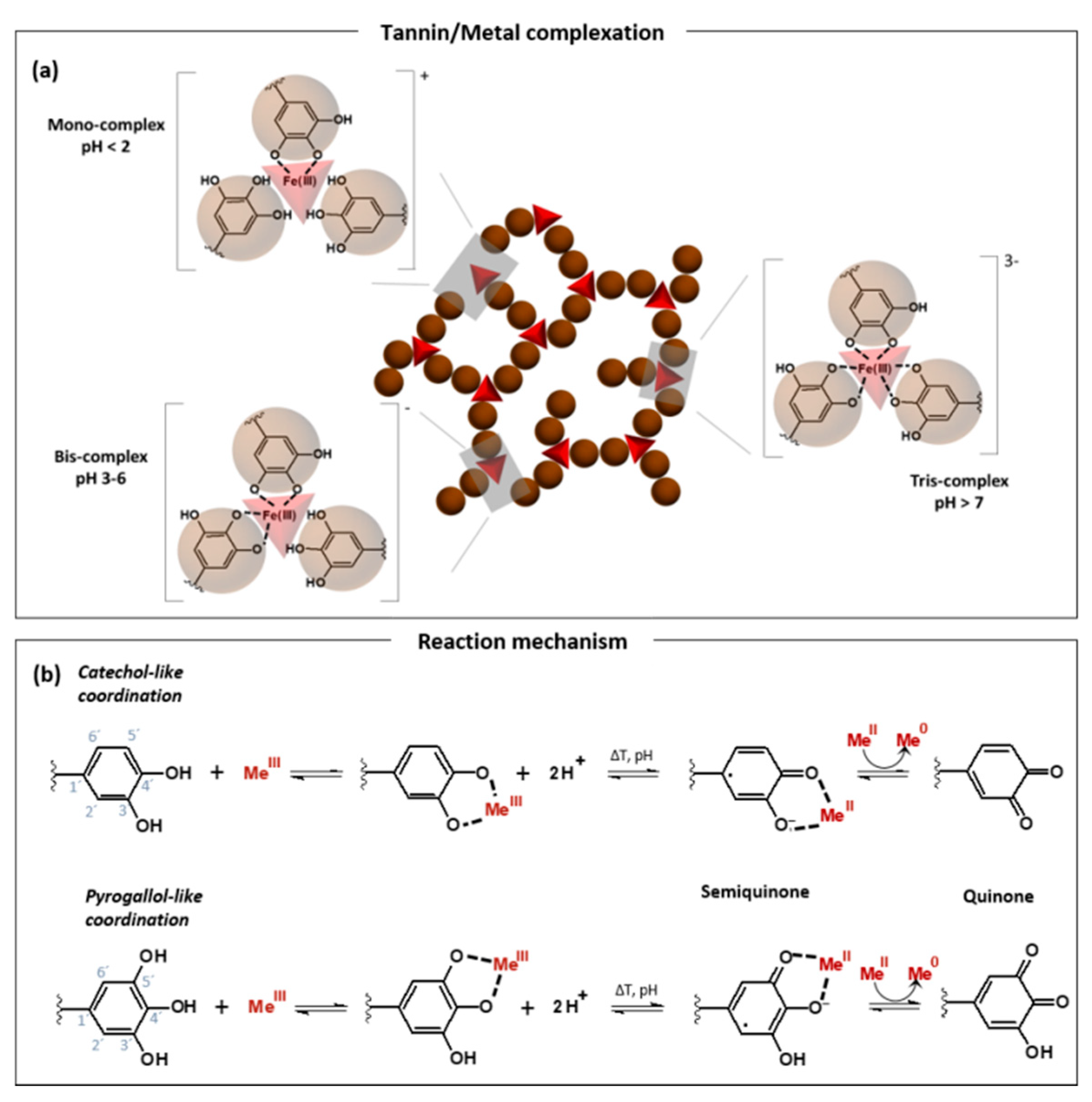
2.1. Tannin–Metal Hybrids
2.1.1. Tannin–Iron Hybrids
2.1.2. Tannin–Noble Metal Hybrids
2.1.3. Tannin-Based Metal–Carbon Hybrids
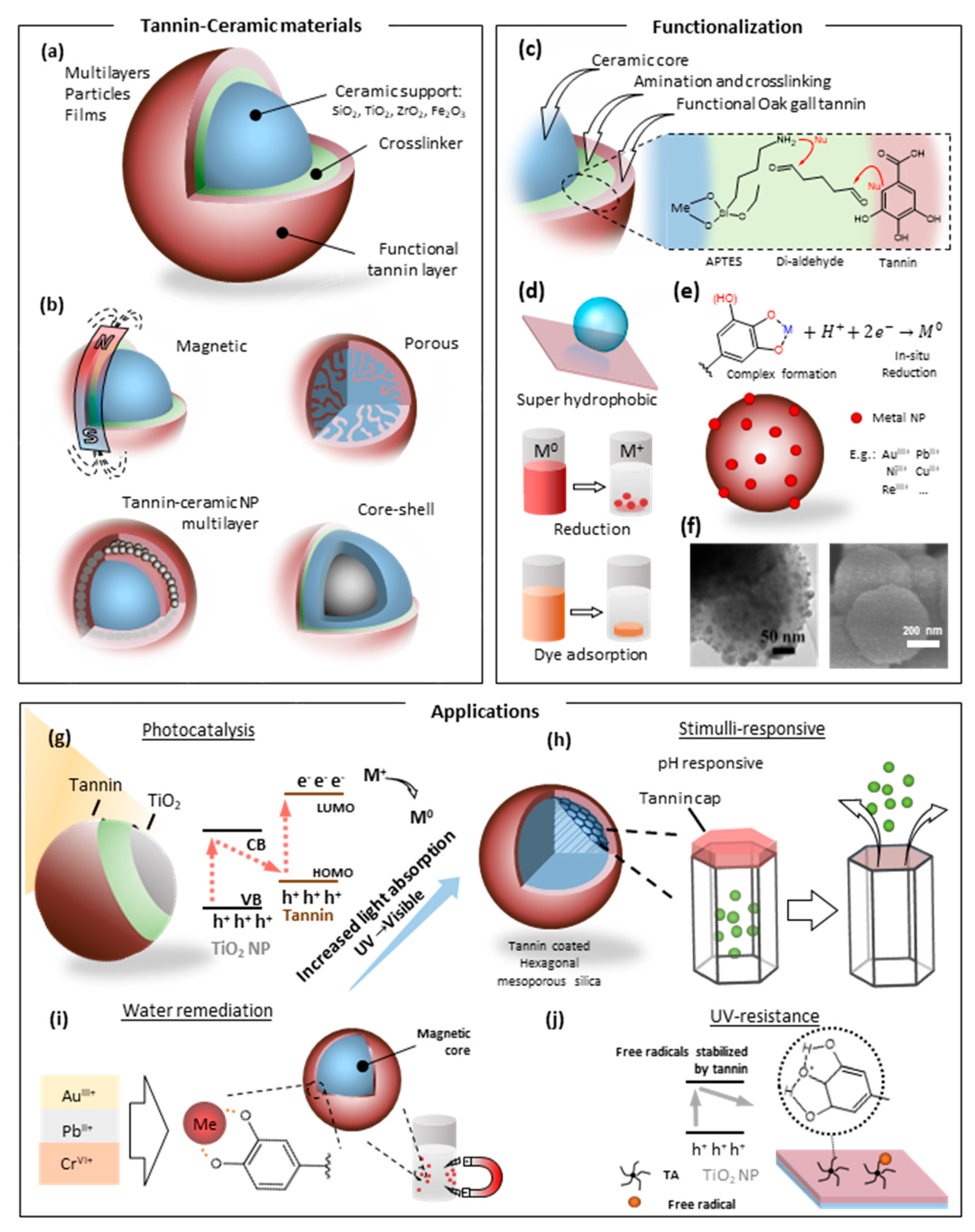
2.2. Tannin–Ceramic Hybrids
2.2.1. Tannin–Silica Hybrids
2.2.2. Tannin–Titania Hybrids
2.2.3. Tannin–Zirconia Hybrids
2.2.4. Tannin-Based Metal Oxide–Carbon Hybrids
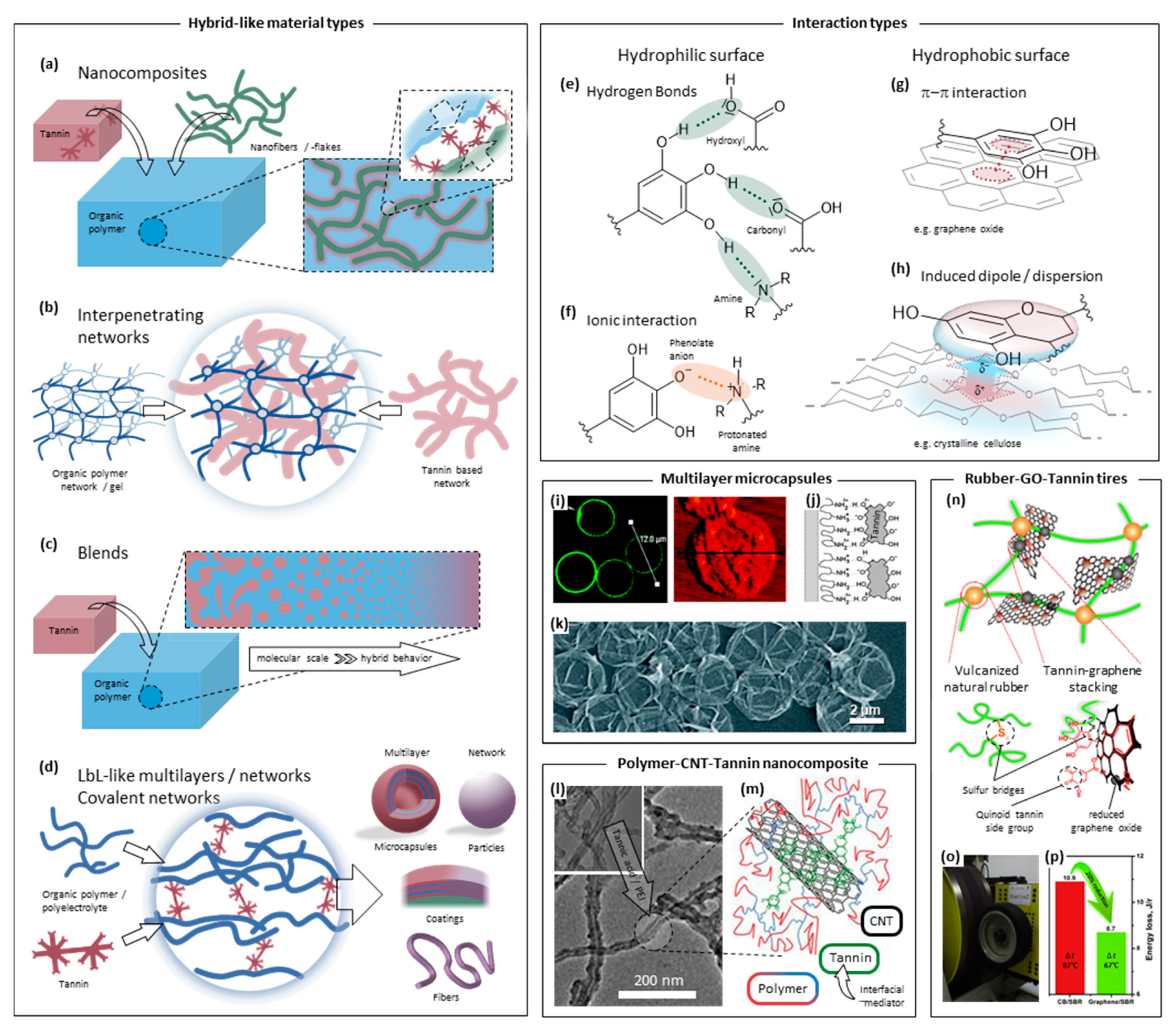
2.3. Tannin–Organic Hybrid-Like Materials
2.3.1. Tannin-Based Nanocomposites
2.3.2. Interpenetrating Networks and Polymer Blends
2.3.3. Polyelectrolyte Complexes, LbL-like Materials, and Coatings
3. Emerging Technological Opportunities
Stimuli-Responsive, Shape-Changing Materials and 4D Printing
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hernes, P.J.; Hedges, J.I. Tannin signatures of barks, needles, leaves, cones, and wood at the molecular level 1. Geochim. Cosmochim. Acta 2004, 68, 1293–1307. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Synthesis and characterization of fully biobased aromatic polyols–oxybutylation of condensed tannins towards new macromolecular architectures. RSC Adv. 2014, 4, 61564–61572. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin–lignin aerogels. Ind. Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols: Vegetable Tannin Revisited; Cambridge University Press: Cambrigde, UK, 1989; ISBN 0-521-32189-1. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M. Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests. J. Cult. Heritage 2011, 12, 149–156. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crop. Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Khanbabaee, K.; Van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Bacelo, H.A.; Santos, S.C.; Botelho, C.M. Tannin-based biosorbents for environmental applications–A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI–TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.; Pell, A. Analysis of condensed tannins: A review. Anim. Feed. Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Zhang, J.; Zhou, X. Tannin Resins for Wood Preservatives: A Review. Res. Appl. Mater. Sci. 2019, 1, 45–48. [Google Scholar]
- Ogata, T.; Morisada, S.; Oinuma, Y.; Seida, Y.; Nakano, Y. Preparation of adsorbent for phosphate recovery from aqueous solutions based on condensed tannin gel. J. Hazard. Mater. 2011, 192, 698–703. [Google Scholar] [CrossRef]
- Slabbert, N. Plant. Polyphenols. Basics of Life Sciences; Hemingway, R.F., Lakes, P., Eds.; Springer: Boston, MA, USA, 1992; ISBN 978-1-4613-6540-2. [Google Scholar]
- Santos, S.; Bacelo, H.A.M.; Boaventura, R.A.R.; Botelho, C.M.S. Tannin-Adsorbents for Water Decontamination and for the Recovery of Critical Metals: Current State and Future Perspectives. Biotechnol. J. 2019, 14, e1900060. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. [Google Scholar]
- Braghiroli, F.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2009, 63, 319–326. [Google Scholar] [CrossRef]
- Braghiroli, F.; Fierro, V.; Pizzi, A.; Rode, K.; Radke, W.; Delmotte, L.; Parmentier, J.; Celzard, A. Reaction of condensed tannins with ammonia. Ind. Crop. Prod. 2013, 44, 330–335. [Google Scholar] [CrossRef]
- Luo, C.; Grigsby, W.; Edmonds, N.; Easteal, A.; Al-Hakkak, J. Synthesis, characterization, and thermal behaviors of tannin stearates prepared from quebracho and pine bark extracts. J. Appl. Polym. Sci. 2010, 117, 352–360. [Google Scholar] [CrossRef]
- Nicollin, A.; Zhou, X.; Pizzi, A.; Grigsby, W.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR analysis of a renewable resource additive—Thermoplastic acetylated tannins. Ind. Crop. Prod. 2013, 49, 851–857. [Google Scholar] [CrossRef]
- Soto, R. Evidence of chemical reactions between di- and poly-glycidyl ether resins and tannins isolated from Pinus radiata D. Don bark. Bioresour. Technol. 2005, 96, 95–101. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Z.; Yang, C.; Ning, J.; Zhou, Z.; Li, G.-Y. Novel magnetic graphene oxide decorated with persimmon tannins for efficient adsorption of malachite green from aqueous solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 566, 48–57. [Google Scholar] [CrossRef]
- Santos, A.F.M.; Macedo, L.J.A.; Chaves, M.H.; Espinoza-Castañeda, M.; Merkoçi, A.; Lima, F.D.C.A.; Cantanhêde, W. Hybrid Self-Assembled Materials Constituted by Ferromagnetic Nanoparticles and Tannic Acid: A Theoretical and Experimental Investigation. J. Braz. Chem. Soc. 2015, 27, 727–734. [Google Scholar] [CrossRef]
- Rurack, K.; Martínez-Máñez, R. Hybrid (nano) materials meet supramolecular chemistry: A brief introduction to basic terms and concepts. In The Supramolecular Chemistry of Organic-Inorganic Hybrid Materials; John Wiley & Sons: New York, NY, USA, 2010; pp. 1–10. ISBN 978-0-470-37621-8. [Google Scholar]
- Stupp, S.I.; Palmer, L.C. Supramolecular Chemistry and Self-Assembly in Organic Materials Design. Chem. Mater. 2013, 26, 507–518. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, R.-L.; Liao, Y.; Ma, J.; Mao, H.; Zhao, S.-L. Tannin modified aminated silica as effective absorbents for removal of light rare earth ions in aqueous solution. Desalin. Water Treat. 2015, 57, 18529–18536. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Liao, X.; Shi, B. Adsorptive recovery of Au3+ from aqueous solutions using bayberry tannin-immobilized mesoporous silica. J. Hazard. Mater. 2010, 183, 793–798. [Google Scholar] [CrossRef]
- Leite, A.J.; Lima, E.C.; Dos Reis, G.S.; Thue, P.S.; Saucier, C.; Rodembusch, F.S.; Dias, S.L.; Umpierres, C.S.; Dotto, G.L. Hybrid adsorbents of tannin and APTES (3-aminopropyltriethoxysilane) and their application for the highly efficient removal of acid red 1 dye from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 4307–4318. [Google Scholar] [CrossRef]
- Binaeian, E.; Seghatoleslami, N.; Chaichi, M.J. Synthesis of oak gall tannin-immobilized hexagonal mesoporous silicate (OGT-HMS) as a new super adsorbent for the removal of anionic dye from aqueous solution. Desalin. Water Treat. 2015, 57, 8420–8436. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Li, X. Three-dimensional composite hydrogel based on polyamine zirconium oxide, alginate and tannic acid with high performance for Pb(Ⅱ), Hg(Ⅱ) and Cr(Ⅵ) trapping. J. Taiwan Inst. Chem. Eng. 2016, 65, 304–311. [Google Scholar] [CrossRef]
- Leng, Y.; Shi, L.; Du, S.; Jiang, J.; Jiang, P. A tannin-derived zirconium-containing porous hybrid for efficient Meerwein–Ponndorf–Verley reduction under mild conditions. Green Chem. 2020, 22, 180–186. [Google Scholar] [CrossRef]
- Uivarosi, V.; Munteanu, A.C.; Sharma, A. Current Aspects of Flavonoids: Their Role in Cancer Treatment; Springer: Berlin, Germany, 2019; ISBN 9789811358746. [Google Scholar]
- Cai, C.; Fu, N.; Zhou, Z.; Wu, M.; Yang, Z.; Liu, R. Mechanochemical Synthesis of Tannic Acid-Fe Coordination Compound and Its Derived Porous Carbon for CO2 Adsorption. Energy Fuels 2018, 32, 10779–10785. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Rius, J.; Zorrilla, C. Tannin biosynthesis of iron oxide nanoparticles. Appl. Phys. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Batista, F.A.; Nascimento, S.Q.; Sousa, A.B.; Júnior, E.F.; Pereira, P.I.D.A.; Fontenele, V.M.; Filho, E.C.S.; Magalhães, J.L.; Luz, R.A.; Cantanhêde, W.; et al. Synthesis, characterization and electrochemical properties of composites synthesized from silver-tannic acid hybrid nanoparticles and different clays. Appl. Clay Sci. 2019, 181, 105219. [Google Scholar] [CrossRef]
- Bartzoka, E.D.; Lange, H.; Poce, G.; Crestini, C. Stimuli-Responsive Tannin-FeIII Hybrid Microcapsules Demonstrated by the Active Release of an Anti-Tuberculosis Agent. ChemSusChem 2018, 11, 3975–3991. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Luzardo, F.H.M.; Velasco, F.G.; Correia, I.K.; Silva, P.M.; Salay, L.C. Removal of lead ions from water using a resin of mimosa tannin and carbon nanotubes. Environ. Technol. Innov. 2017, 7, 219–228. [Google Scholar] [CrossRef]
- Zhan, X.; Miyazaki, A.; Nakano, Y. Mechanisms of Lead Removal from Aqueous Solutions Using a Novel Tannin Gel Adsorbent Synthesized from Natural Condensed Tannin. J. Chem. Eng. Jpn. 2001, 34, 1204–1210. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.; Fernández-Sanjurjo, M.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine bark as bio-adsorbent for Cd, Cu, Ni, Pb and Zn: Batch-type and stirred flow chamber experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef]
- Xie, F.; Fan, R.; Yi, Q.; Zhang, Q.; Luo, Z. NaOH Modification of Persimmon Powder-formaldehyde Resin to Enhance Cu2+ and Pb2+ Removal from Aqueous Solution. Procedia Environ. Sci. 2016, 31, 817–826. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Sakane, K.K.; Simonetti, E.A.N.; Thim, G.P. Cr total removal in aqueous solution by PHENOTAN AP based tannin gel (TFC). J. Environ. Chem. Eng. 2015, 3, 725–733. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr(VI). J. Hazard. Mater. 2018, 352, 27–35. [Google Scholar] [CrossRef]
- Huang, X.; Liao, X.; Shi, B. Tannin-immobilized mesoporous silica bead (BT–SiO2) as an effective adsorbent of Cr(III) in aqueous solutions. J. Hazard. Mater. 2010, 173, 33–39. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Yi, Q.; Fan, R.; Xie, F.; Min, H.; Zhang, Q.; Luo, Z. Selective Recovery of Au(III) and Pd(II) from Waste PCBs Using Ethylenediamine Modified Persimmon Tannin Adsorbent. Procedia Environ. Sci. 2016, 31, 185–194. [Google Scholar] [CrossRef]
- Xiong, Y.; Adhikari, C.R.; Kawakita, H.; Ohto, K.; Inoue, K.; Harada, H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine. Bioresour. Technol. 2009, 100, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Adhikari, B.B.; Alam, S.; Kawakita, H.; Ohto, K.; Inoue, K. Persimmon tannin-based new sorption material for resource recycling and recovery of precious metals. Chem. Eng. J. 2013, 228, 405–414. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Kajiyama, K. Persimmon peel gel for the selective recovery of gold. Hydrometall. 2007, 87, 133–139. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. N-aminoguanidine modified persimmon tannin: A new sustainable material for selective adsorption, preconcentration and recovery of precious metals from acidic chloride solution. Bioresour. Technol. 2013, 129, 108–117. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Selective Recovery of Precious Metals from Acidic Leach Liquor of Circuit Boards of Spent Mobile Phones Using Chemically Modified Persimmon Tannin Gel. Ind. Eng. Chem. Res. 2012, 51, 11901–11913. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract. Chem. Eng. J. 2011, 174, 556–563. [Google Scholar] [CrossRef]
- Ogata, T.; Nakano, Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Water Res. 2005, 39, 4281–4286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Liang, H.; Ning, J.; Zhou, Z.; Li, G. Equilibrium, kinetics and mechanism of Au3+, Pd2+ and Ag+ ions adsorption from aqueous solutions by graphene oxide functionalized persimmon tannin. Mater. Sci. Eng. C 2017, 79, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Fan, R.; Yi, Q.; Fan, Z.; Zhang, Q.; Luo, Z. Adsorption recovery of Pd(II) from aqueous solutions by persimmon residual based bio-sorbent. Hydrometallurgy 2016, 165, 323–328. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, S.; Li, X.; Liu, X.; Xu, Q.; Liu, J.; Wang, Z. Tannic acid modified Fe3O4 core–shell nanoparticles for adsorption of Pb2+ and Hg2+. J. Taiwan Inst. Chem. Eng. 2017, 72, 163–170. [Google Scholar] [CrossRef]
- Huang, X.; Liao, X.; Shi, B. Hg(II) removal from aqueous solution by bayberry tannin-immobilized collagen fiber. J. Hazard. Mater. 2009, 170, 1141–1148. [Google Scholar] [CrossRef]
- Lopes, L.C.; Brito, L.M.; Bezerra, T.T.; Gomes, K.N.; Carvalho, F.A.D.A.; Chaves, M.H.; Cantanhêde, W. Silver and gold nanoparticles from tannic acid: Synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. Anais Acad. Brasile. Ciênci. 2018, 90, 2679–2689. [Google Scholar] [CrossRef]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [PubMed]
- Sekowski, S.; Tomaszewska, E.; Soliwoda, K.; Celichowski, G.; Grobelny, J. Interactions of hybrid gold–tannic acid nanoparticles with human serum albumin. Eur. Biophys. J. 2016, 46, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, H.; Liao, X.; Shi, B. One-step, size-controlled synthesis of gold nanoparticles at room temperature using plant tannin. Green Chem. 2010, 12, 395–399. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.V.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yang, B.J.; Jeong, K.B.; Bin Kim, C.; Lee, S.; Ku, B.-C. Signal-Induced Release of Guests from a Photolatent Metal-Phenolic Supramolecular Cage and Its Hybrid Assemblies. Angew. Chem. Int. Ed. 2017, 56, 5485–5489. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Szczurek, A.; Gadonneix, P.; Ghanbaja, J.; Parmentier, J.; Medjahdi, G.; Celzard, A. Hydrothermal Treatment of Tannin: A Route to Porous Metal Oxides and Metal/Carbon Hybrid Materials. Inorganics 2017, 5, 7. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Vidal, L.; Gadonneix, P.; Celzard, A. Hydrothermal carbons produced from tannin by modification of the reaction medium: Addition of H + and Ag +. Ind. Crop. Prod. 2015, 77, 364–374. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, M.; Yin, H.; Wei, F.; Zhang, J.; Hu, H.; Wang, S. Tannic acid-Fe coordination derived Fe/N-doped carbon hybrids for catalytic oxidation processes. Appl. Surf. Sci. 2019, 489, 44–54. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Rahim, A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic Building Blocks for the Assembly of Functional Materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Zhang, J.; He, F.; Xu, Z.; Ji, S.; Peng, S.; Yue-Xiang, L. Designing preferable functional materials based on the secondary reactions of the hierarchical tannic acid (TA)-aminopropyltriethoxysilane (APTES) coating. Chem. Eng. J. 2019, 360, 299–312. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Wang, X.; Li, R.; Cai, C.; Sun, T.; Zhao, Y.; Chen, L.; Xu, J.; Xu, J. General Surface Modification Method for Nanospheres via Tannic Acid-Fe Layer-by-Layer Deposition: Preparation of a Magnetic Nanocatalyst. ACS Appl. Nano Mater. 2019, 2, 3510–3517. [Google Scholar] [CrossRef]
- Kim, K.R.; Choi, S.; Yavuz, C.T.; Nam, Y.S. Direct Z-Scheme Tannin–TiO2 Heterostructure for Photocatalytic Gold Ion Recovery from Electronic Waste. ACS Sustain. Chem. Eng. 2020, 8, 7359–7370. [Google Scholar] [CrossRef]
- Hu, C.; Yu, L.; Jiang, Y.; Tong, G.; Zheng, Z.; Wang, J.; Liu, Y.; Zhou, Y. Tannin as a gatekeeper of pH-responsive mesoporous silica nanoparticles for drug delivery. RSC Adv. 2015, 5, 85436–85441. [Google Scholar] [CrossRef]
- Fan, R.; Min, H.; Hong, X.; Yi, Q.; Liu, W.; Zhang, Q.; Luo, Z. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J. Hazard. Mater. 2019, 364, 780–790. [Google Scholar] [CrossRef]
- Son, H.Y.; Koo, B.I.; Lee, J.B.; Kim, K.R.; Kim, W.; Jang, J.; Yoon, M.S.; Cho, J.-W.; Nam, Y.S. Tannin–Titanium Oxide Multilayer as a Photochemically Suppressed Ultraviolet Filter. ACS Appl. Mater. Interfaces 2018, 10, 27344–27354. [Google Scholar] [CrossRef]
- Binaeian, E.; Seghatoleslami, N.; Chaichi, M.J.; Tayebi, H.-A. Preparation of titanium dioxide nanoparticles supported on hexagonal mesoporous silicate (HMS) modified by oak gall tannin and its photocatalytic performance in degradation of azo dye. Adv. Powder Technol. 2016, 27, 1047–1055. [Google Scholar] [CrossRef]
- Binaeian, E.; Tayebi, H.-A.; Rad, A.S.; Payab, M. Surface modification of mesoporous silicate by tannin for immobilization of TiO2 nanoparticles: Study of photocatalytic performance. Mater. Chem. Phys. 2017, 185, 14–23. [Google Scholar] [CrossRef]
- Dai, J.; Zou, H.; Wang, R.; Wang, Y.; Shi, Z.; Qiu, S. Yolk–shell Fe3O4@SiO2@PMO: Amphiphilic magnetic nanocomposites as an adsorbent and a catalyst with high efficiency and recyclability. Green Chem. 2017, 19, 1336–1344. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Huang, L.; Shuai, Q.; Hu, S. Tannin-based magnetic porous organic polymers as robust scavengers for methylene blue and lead ions. J. Clean. Prod. 2019, 215, 280–289. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Lin, T.; Feng, Y.; Jia, S. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, W.-J.; Yin, H.-X.; Lian, H.-Z. Facile synthesis of FeIII–tannic acid film-functionalized magnetic silica microspheres for the enrichment of low-abundance peptides and proteins for MALDI-TOF MS analysis. RSC Adv. 2015, 5, 63896–63902. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Sun, H.; Yang, Z.; Du, Q.; Li, C. Improved photodynamic efficiency for methylene blue from silica-methylene blue@tannic acid-Fe(III) ions complexes in aqueous solutions. Adv. Powder Technol. 2018, 29, 341–348. [Google Scholar] [CrossRef]
- Park, T.; Kim, J.Y.; Cho, H.; Moon, H.C.; Kim, B.J.; Park, J.H.; Hong, D.; Park, J.; Choi, I.S. Artificial Spores: Immunoprotective Nanocoating of Red Blood Cells with Supramolecular Ferric Ion-Tannic Acid Complex. Polymers 2017, 9, 140. [Google Scholar] [CrossRef]
- Yang, L.; Han, L.; Ren, J.; Wei, H.; Jia, L. Coating process and stability of metal-polyphenol film. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 197–205. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, R.; Dynes, J.; Blyth, R.; Yu, G.; Kozak, L.; Huang, P. Coordination nature of aluminum (oxy)hydroxides formed under the influence of tannic acid studied by X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 2008, 72, 1959–1969. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, P.; Yang, S.; Zhu, H.; Wu, P.; Xing, H.; Dai, S. Sustainable synthesis of alkaline metal oxide-mesoporous carbons via mechanochemical coordination self-assembly. J. Mater. Chem. A 2017, 5, 23446–23452. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, S.; He, C.; Ye, C.; He, C.; Sun, S.; Xie, Y.; Zhao, C. Green Fabrication of Tannic Acid-Inspired Magnetic Composite Nanoparticles toward Cationic Dye Capture and Selective Degradation. ACS Omega 2020, 5, 6566–6575. [Google Scholar] [CrossRef]
- De Moraes, N.; De Azeredo, C.A.S.H.; Bacetto, L.A.; Da Silva, M.L.C.P.; Rodrigues, L.A. The effect of C-doping on the properties and photocatalytic activity of ZrO2 prepared via sol-gel route. Optik 2018, 165, 302–309. [Google Scholar] [CrossRef]
- Shutava, T.; Prouty, M.; Kommireddy, D.; Lvov, Y. pH Responsive Decomposable Layer-by-Layer Nanofilms and Capsules on the Basis of Tannic Acid. Macromolecules 2005, 38, 2850–2858. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Kharlampieva, E.; Drachuk, I.; Cheng, D.; Tsukruk, V.V. Responsive microcapsule reactors based on hydrogen-bonded tannic acid layer-by-layer assemblies. Soft Matter 2010, 6, 3596–3608. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, X. Noncovalent functionalization of carbon nanotubes via co-deposition of tannic acid and polyethyleneimine for reinforcement and conductivity improvement in epoxy composite. Compos. Sci. Technol. 2019, 170, 25–33. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Liu, J.; Liao, R.; Yang, G.; Wu, X.; Tang, Z.; Guo, B.; Zhang, L.; Ma, Y.; Nie, Q.; et al. Rational design of covalent interfaces for graphene/elastomer nanocomposites. Compos. Sci. Technol. 2016, 132, 68–75. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Adhesives. J. Macromol. Sci. Part. C 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Hegab, H.M.; Elmekawy, A.; Barclay, T.G.; Michelmore, A.; Zou, L.; Saint, C.P.; Ginic-Markovic, M. Single-Step Assembly of Multifunctional Poly(tannic acid)–Graphene Oxide Coating To Reduce Biofouling of Forward Osmosis Membranes. ACS Appl. Mater. Interfaces 2016, 8, 17519–17528. [Google Scholar] [CrossRef]
- Sunija, A.J.; Ilango, S.S.; Kumar, K.P.V. Thespesia populnea Reinforced Cashew Nut Husk Tannin-Based Polyurethane Composites. J. Nat. Fibers 2015, 12, 481–493. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Makino, K. Preparation and properties of biocomposites composed of epoxidized soybean oil, tannic acid, and microfibrillated cellulose. J. Appl. Polym. Sci. 2010, 120, 273–278. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Improvement of interfacial interactions using natural polyphenol-inspired tannic acid-coated nanoclay enhancement of soy protein isolate biofilms. Appl. Surf. Sci. 2017, 401, 271–282. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, H.; Zhao, S.; Zhang, W.; Zhang, S.; Li, J. Polyphenol-induced cellulose nanofibrils anchored graphene oxide as nanohybrids for strong yet tough soy protein nanocomposites. Carbohydr. Polym. 2018, 180, 354–364. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.D.F.; Magalhães, W.L.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Zhu, J.; Abhyankar, H.; Nassiopoulos, E.; Njuguna, J. Tannin-based flax fibre reinforced composites for structural applications in vehicles. IOP Conf. Series Mater. Sci. Eng. 2012, 40, 012030. [Google Scholar] [CrossRef]
- Sivasankaran, P.N.; Ward, T.A.; Salami, E.; Viyapuri, R.; Fearday, C.J.; Johan, M.R. An experimental study of elastic properties of dragonfly-like flapping wings for use in biomimetic micro air vehicles (BMAVs). Chin. J. Aeronaut. 2017, 30, 726–737. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Ge, J.; Shi, X.; Cai, M.; Wu, R.; Wang, M. A novel biodegradable antimicrobial PU foam from wattle tannin. J. Appl. Polym. Sci. 2003, 90, 2756–2763. [Google Scholar] [CrossRef]
- Segovia, C.; Sauget, A.; Besserer, A.; Kueny, R.; Pizzi, A. Evaluating mold growth in tannin-resin and flax fiber biocomposites. Ind. Crop. Prod. 2016, 83, 438–443. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P.; Salami, E.; Fearday, C. Effects of heat treatment on chitosan nanocomposite film reinforced with nanocrystalline cellulose and tannic acid. Carbohydr. Polym. 2016, 140, 202–208. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Zhang, X.; Xia, L.; Li, C.; Wu, H.; Guo, S. Achieving high-performance poly (styrene-b-ethylene-ranbutylene-b-styrene) nanocomposites with tannic acid functionalized graphene oxide. Compos. Sci. Technol. 2018, 158, 137–146. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, Q.; Lin, T.; Guo, B.; Jia, D. The use of a hybrid consisting of tubular clay and graphene as a reinforcement for elastomers. RSC Adv. 2013, 3, 17057. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, Z.; Liao, R.; Guo, B. Hydrolysable tannin as environmentally friendly reducer and stabilizer for graphene oxide. Green Chem. 2011, 13, 1655–1658. [Google Scholar] [CrossRef]
- Liao, R.; Tang, Z.; Lei, Y.; Guo, B. Polyphenol-Reduced Graphene Oxide: Mechanism and Derivatization. J. Phys. Chem. C 2011, 115, 20740–20746. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, Z.; Xu, H.; Ma, X.; Tian, M.; Yin, J. Green chemistry: Co-assembly of tannin-assisted exfoliated low-defect graphene and epoxy natural rubber latex to form soft and elastic nacre-like film with good electrical conductivity. Carbon 2017, 114, 649–660. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, Z.; Xu, H.; Ma, X.; Yin, J.; Tian, M. Fabrication of Super Extensible and Highly Tough Graphene Composite Hydrogels by Thermal Treatment Strategy for the Mixture of Tannin and Graphene Oxide. Macromol. Chem. Phys. 2017, 218, 1600549. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic Acid Induced Self-Assembly of Three-Dimensional Graphene with Good Adsorption and Antibacterial Properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, N.; Lai, J.; Liu, R.; Liu, X.-Y. Tannic acid functionalized graphene hydrogel for entrapping gold nanoparticles with high catalytic performance toward dye reduction. J. Hazard. Mater. 2015, 300, 615–623. [Google Scholar] [CrossRef]
- Fan, Y.; Cho, U.R. Influence of the hybrid materials based on carbon nanotubes and tannic acid on the rheological, thermal and mechanical performances of nitrile butadiene rubber composites. Polym. Compos. 2019, 40, 4510–4518. [Google Scholar] [CrossRef]
- Yang, S.; Wu, W.; Jiao, Y.; Cai, Z.; Fan, H. Preparation of NBR/Tannic acid composites by assembling a weak IPN structure. Compos. Sci. Technol. 2017, 153, 40–47. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Kodzuru, K.; Kawazoe, S.; Matsuo, T. Preparation of Natural Rubber/Condensed Tannin Semi-interpenetrating Polymer Network Composites by Hematin-Catalyzed Cross-Linking. J. Polym. Environ. 2010, 19, 100–105. [Google Scholar] [CrossRef]
- García, D.E.; Salazar, J.P.; Riquelme, S.; Delgado, N.; Paczkowski, S. Condensed Tannin-Based Polyurethane as Functional Modifier of PLA-Composites. Polym. Technol. Eng. 2017, 57, 709–726. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the thermal, dynamic mechanical and morphological properties of PLA-Lignin & PLA-Tannin particulate green composites. Compos. Part. B Eng. 2015, 82, 92–99. [Google Scholar] [CrossRef]
- Shutova, T.G.; Agabekov, V.E.; Lvov, Y.M. Reaction of radical cations with multilayers of tannic acid and polyelectrolytes. Russ. J. Gen. Chem. 2007, 77, 1494–1501. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Ray, S.; Ricci, A.; Kilmartin, P.A. Superior antioxidant polymer films created through the incorporation of grape tannins in ethyl cellulose. Cellulose 2014, 21, 4545–4556. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S. Dynamically Cross-Linked Tannin as a Reinforcement of Polypropylene and UV Protection Properties. Polymers 2019, 11, 102. [Google Scholar] [CrossRef]
- Grigsby, W.; Bridson, J.H.; Schrade, C. Modifying biodegradable plastics with additives based on condensed tannin esters. J. Appl. Polym. Sci. 2014, 132. [Google Scholar] [CrossRef]
- Meng, J.; Lin, X.; Zhou, J.; Zhang, R.; Chen, Y.; Long, X.; Shang, R.; Luo, X. Preparation of tannin-immobilized gelatin/PVA nanofiber band for extraction of uranium (VI) from simulated seawater. Ecotoxicol. Environ. Saf. 2019, 170, 9–17. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Yen, K.; Mandal, T.K.; Woo, E.M. Enhancement of bio-compatibility via specific interactions in polyesters modified with a bio-resourceful macromolecular ester containing polyphenol groups. J. Biomed. Mater. Res. Part. A 2008, 86, 701–712. [Google Scholar] [CrossRef]
- Yen, K.C.; Woo, E.M. Formation of dendrite crystals in poly(ethylene oxide) interacting with bioresourceful tannin. Polym. Bull. 2008, 62, 225–235. [Google Scholar] [CrossRef]
- Costa, E.; Coelho, M.; Ilharco, L.M.; Aguiar-Ricardo, A.; Hammond, P.T. Tannic Acid Mediated Suppression of PNIPAAm Microgels Thermoresponsive Behavior. Macromolecules 2011, 44, 612–621. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Induced insolubility of electrospun poly(N-vinylcaprolactam) fibres through hydrogen bonding with Tannic acid. Polymer 2016, 87, 194–201. [Google Scholar] [CrossRef]
- Chibowski, E.; Espinosa-Jiménez, M.; Ortega, A.O.; Giménez-Martin, E. Surface Free Energy, Adsorption and Zeta Potential in Leacril/Tannic Acid System. Langmuir 1998, 14, 5237–5244. [Google Scholar] [CrossRef]
- Yi, K.; Cheng, G.; Xing, F. Gelatin/tannin complex nanospheres via molecular assembly. J. Appl. Polym. Sci. 2006, 101, 3125–3130. [Google Scholar] [CrossRef]
- Wu, H.; Zhuo, L.; He, Q.; Liao, X.; Shi, B. Heterogeneous hydrogenation of nitrobenzenes over recyclable Pd(0) nanoparticle catalysts stabilized by polyphenol-grafted collagen fibers. Appl. Catal. A Gen. 2009, 366, 44–56. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Guo, J.; Wu, H.; Liao, X.; Shi, B. Facile Synthesis of Size-Controlled Silver Nanoparticles Using Plant Tannin Grafted Collagen Fiber as Reductant and Stabilizer for Microwave Absorption Application in the Whole Ku Band. J. Phys. Chem. C 2011, 115, 23688–23694. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Miao, P.; Liao, X.; Zhang, W.; Shi, B. One-step seeding growth of controllable Ag@Ni core–shell nanoparticles on skin collagen fiber with introduction of plant tannin and their application in high-performance microwave absorption. J. Mater. Chem. 2012, 22, 11933. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 101, 560–567. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, F.; Huang, Y.; Wang, Z.; Li, G.-Y. Biosorption of palladium(II) from aqueous solution by grafting chitosan on persimmon tannin extract. Int. J. Biol. Macromol. 2015, 77, 336–343. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, S.-W. Application of novel hybrid bioadsorbent, tannin/chitosan/sericite, for the removal of Pb(II) toxic ion from aqueous solution. Korean J. Chem. Eng. 2018, 35, 2198–2206. [Google Scholar] [CrossRef]
- Shutava, T.G.; Lvov, Y. Nano-engineered microcapsules of tannic acid and chitosan for protein encapsulation. J. Nanosci. Nanotechnol. 2006, 6, 1655–1661. [Google Scholar] [CrossRef]
- Erel-Unal, I.; Sukhishvili, S.A. Hydrogen-Bonded Multilayers of a Neutral Polymer and a Polyphenol. Macromolecules 2008, 41, 3962–3970. [Google Scholar] [CrossRef]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Lee, H.-I.; Min, Y.; Poon, Z.; Hammond, P.T. Hydrogen-bonded multilayer of pH-responsive polymeric micelles with tannic acid for surface drug delivery. Chem. Commun. 2009, 4194–4196. [Google Scholar] [CrossRef] [PubMed]
- Le, Z.; Chen, Y.; Han, H.; Tian, H.; Zhao, P.; Yang, C.; He, Z.; Liu, L.; Leong, K.W.; Mao, H.-Q.; et al. Hydrogen-Bonded Tannic Acid-Based Anticancer Nanoparticle for Enhancement of Oral Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 42186–42197. [Google Scholar] [CrossRef]
- Facchi, D.P.; Lima, A.C.; De Oliveira, J.H.; Lazarin-Bidóia, D.; Nakamura, C.V.; Canesin, E.A.; Bonafé, E.G.; Monteiro, J.P.; Visentainer, J.V.; Muniz, E.C.; et al. Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. Int. J. Biol. Macromol. 2017, 103, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Da Câmara, P.C.F.; Balaban, R.C.; Hedayati, M.; Popat, K.C.; Martins, A.F.; Kipper, M.J. Novel cationic tannin/glycosaminoglycan-based polyelectrolyte multilayers promote stem cells adhesion and proliferation. RSC Adv. 2019, 9, 25836–25846. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Da Câmara, P.C.; Camargo, S.E.; Camargo, C.H.; Popat, K.C.; Kipper, M.J. Novel poly(ε-caprolactone)/amino-functionalized tannin electrospun membranes as scaffolds for tissue engineering. J. Colloid Interface Sci. 2018, 525, 21–30. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Hwang, C.-H.; Kim, H.-E.; Jeong, S.-H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.; Israelachvili, J.; Waite, J. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part. A Polym. Chem. 2016, 55, 9–33. [Google Scholar] [CrossRef]
- Kim, K.; Shin, M.; Koh, M.-Y.; Ryu, J.H.; Lee, M.S.; Hong, S.; Lee, H. TAPE: A Medical Adhesive Inspired by a Ubiquitous Compound in Plants. Adv. Funct. Mater. 2015, 25, 2402–2410. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Andersen, A.; Birkedal, H. Gels and threads: Mussel-inspired one-pot route to advanced responsive materials. Chem. Commun. 2014, 50, 13278–13281. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Studart, A.R. Biologically Inspired Dynamic Material Systems. Angew. Chem. Int. Ed. 2015, 54, 3400–3416. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, W.M.; Vouga, E.; Mahadevan, L. Growth patterns for shape-shifting elastic bilayers. Proc. Natl. Acad. Sci. USA 2017, 114, 11597–11602. [Google Scholar] [CrossRef]
- Arslan, H.; Nojoomi, A.; Jeon, J.; Yum, K. 3D Printing of Anisotropic Hydrogels with Bioinspired Motion. Adv. Sci. 2018, 6, 1800703. [Google Scholar] [CrossRef]
- Clifford, D.T.; Zupan, R.J.; Brigham, J.C.; Beblow, R.V.; Whittock, M.; Davis, N. Application of the dynamic characteristics of shape-memory polymers to climate adaptive building facades. In Proceedings of the 12th Conference of Advanced Building Skins, Bern, Switzerland, 2–3 October 2017. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Yu, K.; Ritchie, A.; Mao, Y.; Dunn, M.L.; Qi, H.J. Controlled Sequential Shape Changing Components by 3D Printing of Shape Memory Polymer Multimaterials. Procedia IUTAM 2015, 12, 193–203. [Google Scholar] [CrossRef]
- Hirai, T.; Maruyama, H.; Suzuki, T.; Hayashi, S. Shape memorizing properties of a hydrogel of poly(vinyl alcohol). J. Appl. Polym. Sci. 1992, 45, 1849–1855. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Zhou, X.; Du, G. Characterization and 3D printability of poly (lactic acid)/acetylated tannin composites. Ind. Crop. Prod. 2020, 149, 112320. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. https://doi.org/10.3390/molecules25214910
Koopmann A-K, Schuster C, Torres-Rodríguez J, Kain S, Pertl-Obermeyer H, Petutschnigg A, Hüsing N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules. 2020; 25(21):4910. https://doi.org/10.3390/molecules25214910
Chicago/Turabian StyleKoopmann, Ann-Kathrin, Christian Schuster, Jorge Torres-Rodríguez, Stefan Kain, Heidi Pertl-Obermeyer, Alexander Petutschnigg, and Nicola Hüsing. 2020. "Tannin-Based Hybrid Materials and Their Applications: A Review" Molecules 25, no. 21: 4910. https://doi.org/10.3390/molecules25214910
APA StyleKoopmann, A.-K., Schuster, C., Torres-Rodríguez, J., Kain, S., Pertl-Obermeyer, H., Petutschnigg, A., & Hüsing, N. (2020). Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules, 25(21), 4910. https://doi.org/10.3390/molecules25214910





