Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids
Abstract
1. Introduction
2. Classification of Amaryllidaceae Alkaloids
3. Biosynthesis of Amaryllidaceae Alkaloids
4. Occurrence of Amaryllidaceae Alkaloids
5. Pharmacological Properties of Novel Amaryllidaceae Alkaloids
5.1. Antitumoral Cytotoxic Activity
5.2. Effects on the Central Nervous System (CNS)
5.3. Anti-Inflammatory and Antioxidant Activity
5.4. Anti-Parasitic and Antibacterial Activity
5.5. Larvicidal and Insecticidal
5.6. Others Activities
6. Production of Amaryllidaceae Alkaloids
6.1. Chemical Extraction from Amaryllidaceae Plants
6.2. Biotechnological Production of Amaryllidaceae Alkaloids
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, Z.; Yao, G. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2019, 36, 1462–1488. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Desgagné-Penix, I. Biosynthesis of the Amaryllidaceae alkaloids. Plant. Sci. Today 2014, 1, 114–120. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, A.W. The proximate principles of the Narcissus pseudonarcissus. Pharm. J. 1877, 8, 214. [Google Scholar]
- Asahina, Y.; Sugii, Y. Ueber die Identitaet des Lycorins und Narcissins. Arch. Pharm. 1913, 251, 357. [Google Scholar] [CrossRef]
- Morishima, K. Chemische und pharmakologische Untersuchungen über die Alkaloide der Lycoris radiata Herb. Arch. Exptl. Path. Pharmakol. 1897, 40, 221–240. [Google Scholar] [CrossRef]
- Hartwell, J. Plants used against cancer. A survey. Lloydia 1971, 30, 379–463. [Google Scholar]
- He, M.M.; Qu, C.R.; Gao, O.D.; Hu, X.M.; Hong, X.C. Biological and pharmacological activities of Amaryllidaceae alkaloids. Rsc. Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Hotchandani, T.; Desgagne-Penix, I. Heterocyclic Amaryllidaceae Alkaloids: Biosynthesis and Pharmacological Applications. Curr. Top. Med. Chem. 2017, 17, 418–427. [Google Scholar] [CrossRef]
- Heinrich, M. Galantamine from Galanthus and other Amaryllidaceae-chemistry and biology based on traditional use. Alkaloids Chem. Biol. 2010, 68, 157–165. [Google Scholar]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. Alkaloids Chem. Biol. 2020, 83, 113–185. [Google Scholar] [PubMed]
- Lewis, J.R. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 1992, 9, 183–191. [Google Scholar] [CrossRef]
- Lewis, J.R. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 1993, 10, 291–299. [Google Scholar] [CrossRef]
- Lewis, J.R. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 1995, 12, 339–345. [Google Scholar] [CrossRef]
- Lewis, J.R. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 1996, 13, 171–176. [Google Scholar] [CrossRef]
- Lewis, J.R. Amaryllidaceae, sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids. Nat. Prod. Rep. 2002, 19, 223–258. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, Z.; Huang, R. Muscarine, imidazole, oxazole, thiazole, Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2002, 19, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2003, 20, 606–614. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2005, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2007, 24, 886–905. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2009, 26, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2011, 28, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2013, 30, 849–868. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qu, D.; Zhang, K.M.; Cang, X.X.; Kou, Z.N.; Xiao, W.; Zhu, J.B. Phytochemical and biological investigations of Amaryllidaceae alkaloids: A review. J. Asian Nat. Prod. Res. 2017, 19, 53–100. [Google Scholar] [CrossRef] [PubMed]
- Desgagné-Penix, I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2020. [Google Scholar] [CrossRef]
- Unver, N.; Kaya, G.I.; Werner, C.; Verpoorte, R. Galanthindole: A new indole alkaloid from Galanthus plicatus ssp. byzantinus. Planta. Med. 2003, 69, 869–871. [Google Scholar]
- Bastida, J.; Berkov, S.; Torras, L.; Pigni, N.B.; de Andrade, J.P.; Martinez, V.; Codina, C.; Viladomat, F. Chemical and biological aspects of Amaryllidaceae alkaloids. Rec. Adv. Pharm. Sci. 2011, 65–100. [Google Scholar]
- Safratova, M.; Hostalkova, A.; Hulcova, D.; Breiterova, K.; Hrabcova, V.; Machado, M.; Fontinha, D.; Prudencio, M.; Kunes, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018, 41, 208–218. [Google Scholar] [CrossRef]
- Zhan, G.; Liu, J.; Zhou, J.; Sun, B.; Aisa, H.A.; Yao, G. Amaryllidaceae alkaloids with new framework types from Zephyranthes candida as potent acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2017, 127, 771–780. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qu, S.M.; Wang, Y.; Wang, H.T. Cytotoxic and anti-inflammatory active plicamine alkaloids from Zephyranthes grandiflora. Fitoterapia 2018, 130, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Desgagné-Penix, I. Chapter 3: Biosynthesis of Amaryllidaceae Alkaloids: A Biochemical Outlook. In Alkaloids: Biosynthesis, Biological Roles and Health Benefits; Sobarzo-Sanchez, E., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015. [Google Scholar]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2016, 15, 317–337. [Google Scholar] [CrossRef] [PubMed]
- El Tahchy, A.; Ptak, A.; Boisbrun, M.; Barre, E.; Guillou, C.; Dupire, F.; Chretien, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Kinetic study of the rearrangement of deuterium-labeled 4′-O-methylnorbelladine in Leucojum aestivum shoot cultures by mass spectrometry. Influence of precursor feeding on amaryllidaceae alkaloid accumulation. J. Nat. Prod. 2011, 74, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Ptak, A.; Laurain-Mattar, D. 4′-O-Methylnorbelladine feeding enhances galantamine and lycorine production by Leucojum aestivum L. shoot cultures. Eng. Life Sci. 2015, 15, 640–645. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Kirby, G.W. Phenol oxidation and biosynthesis. Part V. The synthesis of galantamine. J. Chem. Soc. (Resumed) 1962, 153, 806–817. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Kirby, G.W.; Taylor, J.B.; Thomas, G.M. Phenol oxidation and biosynthesis. Part VI. The biogenesis of Amaryllidaceae alkaloids. J. Chem. Soc. (Resumed) 1963, 866, 4545–4558. [Google Scholar] [CrossRef]
- Eichhorn, J.; Takada, T.; Kita, Y.; Zenk, M.H. Biosynthesis of the Amaryllidaceae alkaloid galantamine. Phytochemistry 1998, 49, 1037–1047. [Google Scholar] [CrossRef]
- El Tahchy, A. Étude de la voie de biosynthèse de la galantamine chez Leucojum aestivum L.—Criblage phytochimique de quelques Amaryllidaceae. Ph.D. Thesis, Nancy Université Henri Poincaré, Nancy, France, 2010. [Google Scholar]
- El Tahchy, A.; Boisbrun, M.; Ptak, A.; Dupire, F.; Chretien, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. New method for the study of Amaryllidaceae alkaloid biosynthesis using biotransformation of deuterium-labeled precursor in tissue cultures. Acta. Biochim. Pol. 2010, 57, 75–82. [Google Scholar] [CrossRef]
- Singh, A.; Desgagne-Penix, I. Transcriptome and metabolome profiling of Narcissus pseudonarcissus ‘King Alfred’ reveal components of Amaryllidaceae alkaloid metabolism. Sci. Rep. 2017, 7, 17356. [Google Scholar] [CrossRef]
- Hotchandani, T.; de Villers, J.; Desgagne-Penix, I. Developmental Regulation of the Expression of Amaryllidaceae Alkaloid Biosynthetic Genes in Narcissus papyraceus. Genes 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Baek, S.A.; Kim, J.K.; Park, S.U. Transcriptome Analysis and Metabolic Profiling of Lycoris radiata. Biology 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, X.; Xu, S.; Xia, B.; Jiang, Y.; Xue, Y.; Wang, R. Cloning and characterization of a tyrosine decarboxylase involved in the biosynthesis of galantamine in Lycoris aurea. PeerJ 2019, 7, e6729. [Google Scholar] [CrossRef] [PubMed]
- Suhadolnik, R.J.; Fischer, A.G.; Zulalian, J. Biogenesis of the Amaryllidaceae alkaloids. II. Studies with whole plants, floral primordia and cell free extracts. Biochem. Biophys. Res. Commun. 1963, 11, 208–212. [Google Scholar] [CrossRef]
- Wildman, W.; Battersby, A.; Breuer, S. Biosynthesis in the Amaryllidaceae. Incorporation of 3-C14-Tyrosine and Phenylalanine in Nerine Bowdenii W. Wats. J. Am. Chem. Soc. 1962, 84, 4599–4600. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, N.; Li, X.; Shen, W.; Liang, L.; Wang, C.; Wang, R.; Peng, F.; Xia, B. Molecular cloning and characterization of a phenylalanine ammonia-lyase gene (LrPAL) from Lycoris radiata. Mol. Biol. Rep. 2011, 38, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, B.; Liang, L.; Li, X.; Xu, S.; Peng, F.; Wang, R. Molecular and analysis of a phenylalanine ammonia-lyase gene (LrPAL2) from Lycoris radiata. Mol. Biol. Rep. 2013, 40, 2293–2300. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Qiao, C.; Zhang, G.; Luo, Y. Functional characterization of phenylalanine ammonia-lyase- and cinnamate 4-hydroxylase-encoding genes from Lycoris radiata, a galantamine-producing plant. Int. J. Biol. Macromol. 2018, 117, 1264–1279. [Google Scholar] [CrossRef]
- Fahrendorf, T.; Dixon, R.A. Stress responses in alfalfa (Medicago sativa L.). XVIII: Molecular cloning and expression of the elicitor-inducible cinnamic acid 4-hydroxylase cytochrome P450. Arch. Biochem. Biophys. 1993, 305, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Teutsch, H.G.; Hasenfratz, M.P.; Lesot, A.; Stoltz, C.; Garnier, J.M.; Jeltsch, J.M.; Durst, F.; Werck-Reichhart, D. Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate 4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc. Natl. Acad. Sci. USA 1993, 90, 4102–4106. [Google Scholar] [CrossRef]
- Nikolova, M.; Gevrenova, R. Determination of phenolic acids in amaryllidaceae species by high performance liquid chromatography. Pharm. Biol. 2005, 43, 289–291. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Gheldiu, A.M.; Puscas, C.; Silaghi-Dumitrescu, R.; Duma, M.; Tiperciuc, B.; Varban, R.; Vlase, L. Sources for developing new medicinal products: Biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement. Altern Med. 2018, 18, 226. [Google Scholar] [CrossRef] [PubMed]
- Ferdausi, A.; Chang, X.M.; Hall, A.; Jones, M. Galantamine production in tissue culture and metabolomic study on Amaryllidaceae alkaloids in Narcissus pseudonarcissus cv. Carlton. Ind. Crops Prod. 2020, 144, 112058. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef]
- Singh, A.; Massicotte, M.A.; Garand, A.; Tousignant, L.; Ouellette, V.; Berube, G.; Desgagne-Penix, I. Cloning and characterization of norbelladine synthase catalyzing the first committed reaction in Amaryllidaceae alkaloid biosynthesis. BMC Plant. Biol. 2018, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, M.B.; Holland, C.K.; Jez, J.M.; Kutchan, T.M. Identification of a Noroxomaritidine Reductase with Amaryllidaceae Alkaloid Biosynthesis Related Activities. J. Biol. Chem. 2016, 291, 16740–16752. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Augustin, M.M.; Starks, C.M.; O’Neil-Johnson, M.; May, G.D.; Crow, J.A.; Kutchan, T.M. Cloning and characterization of a norbelladine 4′-O-methyltransferase involved in the biosynthesis of the Alzheimer’s drug galantamine in Narcissus sp. aff. pseudonarcissus. PLoS ONE 2014, 9, e103223. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Augustin, M.M.; May, G.D.; Crow, J.A.; Kutchan, T.M. CYP96T1 of Narcissus sp. aff. pseudonarcissus Catalyzes Formation of the Para-Para’ C-C Phenol Couple in the Amaryllidaceae Alkaloids. Front. Plant. Sci. 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Vaneckova, N.; Host’alkova, A.; Safratova, M.; Kunes, J.; Hulcova, D.; Hrabinova, M.; Doskocil, I.; Stepankova, S.; Opletal, L.; Novakova, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. Rsc. Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- N’Tamon, A.D.; Okpekon, A.T.; Bony, N.F.; Bernadat, G.; Gallard, J.-F.; Kouamé, T.; Séon-Méniel, B.; Leblanc, K.; Rharrabti, S.; Mouray, E. Streamlined targeting of Amaryllidaceae alkaloids from the bulbs of Crinum scillifolium using spectrometric and taxonomically-informed scoring metabolite annotations. Phytochemistry 2020, 179, 112485. [Google Scholar] [CrossRef]
- Al Mamun, A.; Maříková, J.; Hulcová, D.; Janoušek, J.; Šafratová, M.; Nováková, L.; Kučera, T.; Hrabinová, M.; Kuneš, J.; Korábečný, J. Amaryllidaceae Alkaloids of Belladine-Type from Narcissus pseudonarcissus cv. Carlton as New Selective Inhibitors of Butyrylcholinesterase. Biomolecules 2020, 10, 800. [Google Scholar] [CrossRef]
- Ka, S.; Masi, M.; Merindol, N.; Di Lecce, R.; Plourde, M.B.; Seck, M.; Gorecki, M.; Pescitelli, G.; Desgagne-Penix, I.; Evidente, A. Gigantelline, gigantellinine and gigancrinine, cherylline- and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity. Phytochemistry 2020, 175, 112390. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.; Liu, X.M.; Huang, X.J.; Zhang, D.M.; Zhang, W.; Wang, L.; Ye, W.C. Four New Amaryllidaceae Alkaloids from Lycoris radiata and Their Cytotoxicity. Planta Med. 2015, 81, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Sugiura, Y.; Funasaki, M.; Kagechika, H.; Ishibashi, M.; Ohsaki, A. Two new alkaloids from Crinum asiaticum var. japonicum. J. Nat. Med. 2019, 73, 648–652. [Google Scholar] [CrossRef]
- Zhan, G.; Zhou, J.; Liu, R.; Liu, T.; Guo, G.; Wang, J.; Xiang, M.; Xue, Y.; Luo, Z.; Zhang, Y.; et al. Galantamine, Plicamine, and Secoplicamine Alkaloids from Zephyranthes candida and Their Anti-acetylcholinesterase and Anti-inflammatory Activities. J. Nat. Prod. 2016, 79, 760–766. [Google Scholar] [CrossRef]
- Liu, Z.M.; Huang, X.Y.; Cui, M.R.; Zhang, X.D.; Chen, Z.; Yang, B.S.; Zhao, X.K. Amaryllidaceae alkaloids from the bulbs of Lycoris radiata with cytotoxic and anti-inflammatory activities. Fitoterapia 2015, 101, 188–193. [Google Scholar] [CrossRef]
- Tallini, L.R.; Osorio, E.H.; Santos, V.D.D.; Borges, W.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. Hippeastrum reticulatum (Amaryllidaceae): Alkaloid Profiling, Biological Activities and Molecular Docking. Molecules 2017, 22, 2191. [Google Scholar] [CrossRef]
- Zhan, G.; Zhou, J.; Liu, J.; Huang, J.; Zhang, H.; Liu, R.; Yao, G. Acetylcholinesterase Inhibitory Alkaloids from the Whole Plants of Zephyranthes carinata. J. Nat. Prod. 2017, 80, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Emir, A.; Emir, C.; Bozkurt, B.; Onur, M.A.; Bastida, J.; Somer, N.U. Alkaloids from Galanthus fosteri. Phytochem. Lett. 2016, 17, 167–172. [Google Scholar] [CrossRef]
- Breiterova, K.; Koutova, D.; Marikova, J.; Havelek, R.; Kunes, J.; Majorosova, M.; Opletal, L.; Hostalkova, A.; Jenco, J.; Rezacova, M.; et al. Amaryllidaceae Alkaloids of Different Structural Types from Narcissus, L. cv. Professor Einstein and Their Cytotoxic Activity. Plants 2020, 9, 137. [Google Scholar] [CrossRef]
- Katoch, D.; Kumar, D.; Padwad, Y.S.; Singh, B.; Sharma, U. Pseudolycorine N-oxide, a new N-oxide from Narcissus tazetta. Nat. Prod. Res. 2020, 34, 2051–2058. [Google Scholar] [CrossRef]
- Carvalho, K.R.; Silva, A.B.; Torres, M.C.M.; Pinto, F.C.L.; Guimaraes, L.A.; Rocha, D.D.; Silveira, E.R.; Costa-Lotufo, L.V.; Braz, R.; Pessoa, O.D.L. Cytotoxic Alkaloids from Hippeastrum solandriflorum Lindl. J. Braz. Chem. Soc. 2015, 26, 1976–1980. [Google Scholar]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and Their Cholinesterase-Inhibitory Activities: An in Vitro and in Silico Study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Huong, P.T.T.; Van Thanh, N.; Trung, N.Q.; Van Cuong, T.; Mai, N.T.; Cuong, N.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C. Crinane, augustamine, and β-carboline alkaloids from Crinum latifolium. Phytochem. Lett. 2018, 24, 27–30. [Google Scholar] [CrossRef]
- Cho, N.; Du, Y.; Valenciano, A.L.; Fernandez-Murga, M.L.; Goetz, M.; Clement, J.; Cassera, M.B.; Kingston, D.G.I. Antiplasmodial alkaloids from bulbs of Amaryllis belladonna Steud. Bioorg. Med. Chem. Lett. 2018, 28, 40–42. [Google Scholar] [CrossRef]
- Tallini, L.R.; Torras-Claveria, L.; Borges, W.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. N-oxide alkaloids from Crinum amabile (Amaryllidaceae). Molecules 2018, 23, 1277. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cala, A.; Tabanca, N.; Cimmino, A.; Green, I.R.; Bloomquist, J.R.; van Otterlo, W.A.; Macias, F.A.; Evidente, A. Alkaloids with Activity against the Zika Virus Vector Aedes aegypti (L.)-Crinsarnine and Sarniensinol, Two New Crinine and Mesembrine Type Alkaloids Isolated from the South African Plant Nerine sarniensis. Molecules 2016, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Bessa, C.D.P.B.; de Andrade, J.P.; de Oliveira, R.S.; Domingos, E.; Santos, H.; Romao, W.; Bastida, J.; Borges, W.S. Identification of Alkaloids from Hippeastrum aulicum (Ker Gawl.) Herb. (Amaryllidaceae) Using CGC-MS and Ambient Ionization Mass Spectrometry (PS-MS and LS-MS). J. Braz. Chem. Soc. 2017, 28, 819–830. [Google Scholar] [CrossRef]
- Chaichompoo, W.; Chokchaisiri, R.; Sangkaew, A.; Pabuprapap, W.; Yompakdee, C.; Suksamrarn, A. Alkaloids with anti-human carbonic anhydrase isozyme II activity from the bulbs of Crinum asiaticum L. var. asiaticum. Phytochem. Lett. 2020, 37, 101–105. [Google Scholar] [CrossRef]
- Moodley, N.; Crouch, N.; Bastida, J.; Mulholland, D. Novel alkaloids and a ceramide from Brunsvigia natalensis (Amaryllidaceae) and their anti-neoplastic activity. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Katoch, D.; Kumar, D.; Padwad, Y.S.; Singh, B.; Sharma, U. Narciclasine-4-O-beta-d-xylopyranoside, a new narciclasine glycoside from Zephyranthes minuta. Nat. Prod. Res. 2020, 34, 233–240. [Google Scholar] [CrossRef]
- Masi, M.; Frolova, L.V.; Yu, X.; Mathieu, V.; Cimmino, A.; De Carvalho, A.; Kiss, R.; Rogelj, S.; Pertsemlidis, A.; Kornienko, A.; et al. Jonquailine, a new pretazettine-type alkaloid isolated from Narcissus jonquilla quail, with activity against drug-resistant cancer. Fitoterapia 2015, 102, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Huo, J.M.; Hu, J.; Xu, Z.P.; Zhang, X. Amaryllidaceae alkaloids from Crinum latifolium with cytotoxic, antimicrobial, antioxidant, and anti-inflammatory activities. Fitoterapia 2018, 130, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.C.; Krai, P.; Dalal, S.; Su, Q.; Cassera, M.; Goetz, M.; Kingston, D.G.I. New potently bioactive alkaloids from Crinum erubescens. Bioorg. Med. Chem. 2016, 24, 5418–5422. [Google Scholar] [CrossRef]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae, with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2017, 116, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Qu, X.; Liu, J.; Tong, Q.; Zhou, J.; Sun, B.; Yao, G. Zephycandidine A, the First Naturally Occurring Imidazo[1,2-f]phenanthridine Alkaloid from Zephyranthes candida, Exhibits Significant Anti-tumor and Anti-acetylcholinesterase Activities. Sci. Rep. 2016, 6, 33990. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Ji, Y.B.; Zhang, W.G.; Xu, Y.; Yan, X.J.; Sun, Y.F.; Song, H.; Xu, C.R.; Cai, L.P.; Zheng, H.X.; et al. Chemical Constituents from Hymenocallis littoralis. Lett. Org. Chem. 2016, 13, 536–539. [Google Scholar] [CrossRef]
- Hulcova, D.; Marikova, J.; Korabecny, J.; Hostalkova, A.; Jun, D.; Kunes, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Novakova, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Zhang, F.J.; Shu, X.C.; Wang, T.; Zhuang, W.B.; Wang, Z. The complete chloroplast genome sequence of Lycoris radiata. Mitochondrial DNA Part B-Resour. 2019, 4, 2886–2887. [Google Scholar] [CrossRef]
- Erenler, R.; Nusret, G.; Elmastaş, M.; Eminağaoğlu, Ö. Evaluation of antioxidant capacity with total phenolic content of Galanthus krasnovii (Amaryllidaceae). Turk. J. Biod. 2019, 2, 13–17. [Google Scholar] [CrossRef]
- Costa, G.G.P.d.; Silva, C.A.G.; Gomes, J.V.D.; Torres, A.G.; Santos, I.R.I.; Almeida, F.T.C.d.; Fagg, C.W.; Simeoni, L.A.; Silveira, D.; Gomes-Copeland, K.K.P. Influence of in vitro micropropagation on lycorine biosynthesis and anticholinesterase activity in Hippeastrum goianum. Rev. Bras. Farm. 2019, 29, 262–265. [Google Scholar] [CrossRef]
- Cahlikova, L.; Vaneckova, N.; Safratova, M.; Breiterova, K.; Blunden, G.; Hulcova, D.; Opletal, L. The Genus Nerine Herb. (Amaryllidaceae): Ethnobotany, Phytochemistry, and Biological Activity. Molecules 2019, 24, 4238. [Google Scholar] [CrossRef] [PubMed]
- El Mokni, R.; Pasta, S.; Pacifico, D. Amaryllis belladonna L. (Amaryllidaceae; Amaryllidoideae), first record as naturalised geophyte in Tunisia and North Africa. Hacquetia 2020, 19, 331–336. [Google Scholar] [CrossRef]
- Balmford, B.; Balmford, J.; Balmford, A.; Blakeman, S.; Manica, A.; Cowling, R.M. Diurnal versus nocturnal pollination of Brunsvigia gregaria RA Dyer (Amaryllidaceae) at a coastal site. S. Afr. J. Bot. 2006, 72, 291–294. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calve, B.; Wauthoz, N.; Megalizzi, V.; Gras, T.; Bruyere, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Decaestecker, C.; Mathieu, V.; Dubois, J.; Kornienko, A.; Kiss, R.; Evidente, A.; Pottier, L. Lycorine and its derivatives for anticancer drug design. Mini. Rev. Med. Chem. 2010, 10, 41–50. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Bastida, J.; Pandey, S.; Griffin, C. Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells. Phytochemistry 2009, 70, 913–919. [Google Scholar] [CrossRef]
- Brimijoin, S. Molecular forms of acetylcholinesterase in brain, nerve and muscle: Nature, localization and dynamics. Prog. Neurobiol. 1983, 21, 291–322. [Google Scholar] [CrossRef]
- Heller, M.; Hanahan, D.J. Human erythrocyte membrane bound enzyme acetylcholinesterase. Biochim. Biophys. Acta. 1972, 255, 251–272. [Google Scholar] [CrossRef]
- Szelenyi, J.G.; Bartha, E.; Hollan, S.R. Acetylcholinesterase activity of lymphocytes: An enzyme characteristic of T-cells. Br. J. Haematol. 1982, 50, 241–245. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Sereno, L.; Coma, M.; Rodriguez, M.; Sanchez-Ferrer, P.; Sanchez, M.B.; Gich, I.; Agullo, J.M.; Perez, M.; Avila, J.; Guardia-Laguarta, C.; et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 2009, 35, 359–367. [Google Scholar] [CrossRef]
- Garcia-Horsman, J.A.; Mannisto, P.T.; Venalainen, J.I. On the role of prolyl oligopeptidase in health and disease. Neuropeptides 2007, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Polgar, L. The prolyl oligopeptidase family. Cell Mol. Life Sci. 2002, 59, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Current concepts on selected plant secondary metabolites with promising inhibitory effects against enzymes linked to Alzheimer’s disease. Curr. Med. Chem. 2012, 19, 2252–2261. [Google Scholar] [CrossRef]
- Babkova, K.; Korabecny, J.; Soukup, O.; Nepovimova, E.; Jun, D.; Kuca, K. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors. Future Med. Chem. 2017, 9, 1015–1038. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Farlow, M.R.; Greig, N.H.; Sambamurti, K. Current drug targets for Alzheimer’s disease treatment. Drug Dev. Res. 2002, 56, 267–281. [Google Scholar] [CrossRef]
- Galimberti, D.; Scarpini, E. Old and new acetylcholinesterase inhibitors for Alzheimer’s disease. Expert Opin. Investig. Drugs 2016, 25, 1181–1187. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Elgorashi, E.E.; Zschocke, S.; van Staden, J. The anti-inflammatory and antibacterial activities of Amaryllidaceae alkaloids. S. Afr. J. Bot. 2003, 69, 448–449. [Google Scholar] [CrossRef]
- Osorio, E.J.; Robledo, S.M.; Bastida, J. Alkaloids with antiprotozoal activity. Alkaloids Chem. Biol. 2008, 66, 113–190. [Google Scholar]
- Nair, J.J.; van Staden, J. The Amaryllidaceae as a source of antiplasmodial crinane alkaloid constituents. Fitoterapia 2019, 134, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Antiplasmodial constituents in the minor alkaloid groups of the Amaryllidaceae. S. Afr. J. Bot 2019, 126, 362–370. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Amaryllidaceae alkaloids: Absolute configuration and biological activity. Chirality 2017, 29, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Rook, F. Towards a molecular understanding of the biosynthesis of Amaryllidaceae alkaloids in support of their expanding medical use. Int. J. Mol. Sci. 2013, 14, 11713–11741. [Google Scholar] [CrossRef] [PubMed]
- Emir, A.; Onur, M.A. Simultaneous Quantification of Galantamine and Lycorine in Galanthus fosteri by HPLC-DAD. Marmara Pharm. J. 2016, 20, 320–324. [Google Scholar] [CrossRef]
- Pavlov, A.; Berkov, S.; Courot, E.; Gocheva, T.; Tuneva, D.; Pandova, B.; Georgiev, M.; Georgiev, V.; Yanev, S.; Burrus, M.; et al. Galantamine production by Leucojum aestivum in vitro systems. Process. Biochem. 2007, 42, 734–739. [Google Scholar] [CrossRef]
- Diop, M.; Hehn, A.; Ptak, A.; Chrétien, F.; Doerper, S.; Gontier, E.; Bourgaud, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Hairy root and tissue cultures of Leucojum aestivum L.—Relationships to galantamine content. Phytochem. Rev. 2007, 6, 137–141. [Google Scholar] [CrossRef]
- Berkov, S.; Pavlov, A.; Georgiev, V.; Bastida, J.; Burrus, M.; Ilieva, M.; Codina, C. Alkaloid synthesis and accumulation in Leucojum aestivum in vitro cultures. Nat. Prod. Commun. 2009, 4, 359–364. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Diop, M.F.; Ptak, A.; Chretien, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Galantamine content of bulbs and in vitro cultures of Leucojum aestivum L. Nat. Prod. Commun. 2006, 1, 475–479. [Google Scholar]
- Zhou, J.; Liu, Z.; Wang, S.; Li, J.; Li, Y.; Chen, W.K.; Wang, R. Fungal endophytes promote the accumulation of Amaryllidaceae alkaloids in Lycoris radiata. Environ. Microbiol. 2020, 22, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pandey, S.S.; Shanker, K.; Kalra, A. Endophytes enhance the production of root alkaloids ajmalicine and serpentine by modulating the terpenoid indole alkaloid pathway in Catharanthus roseus roots. J. Appl. Microbiol. 2020, 128, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Georgiev, V.; Pavlov, A. Elicitation of galantamine biosynthesis by Leucojum aestivum liquid shoot cultures. J. Plant Physiol. 2013, 170, 1122–1129. [Google Scholar] [CrossRef]
- Diamond, A.; Desgagne-Penix, I. Metabolic engineering for the production of plant isoquinoline alkaloids. Plant Biotechnol. J. 2016, 14, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Facchini, P.J.; Bohlmann, J.; Covello, P.S.; De Luca, V.; Mahadevan, R.; Page, J.E.; Ro, D.K.; Sensen, C.W.; Storms, R.; Martin, V.J. Synthetic biosystems for the production of high-value plant metabolites. Trends Biotechnol. 2012, 30, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fossati, E.; Narcross, L.; Ekins, A.; Falgueyret, J.P.; Martin, V.J. Synthesis of Morphinan Alkaloids in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0124459. [Google Scholar] [CrossRef]
- Hawkins, K.M.; Smolke, C.D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat. Chem. Biol. 2008, 4, 564–573. [Google Scholar] [CrossRef]
- Matsumura, E.; Nakagawa, A.; Tomabechi, Y.; Ikushiro, S.; Sakaki, T.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Sato, F.; Minami, H. Microbial production of novel sulphated alkaloids for drug discovery. Sci. Rep. 2018, 8, 7980. [Google Scholar] [CrossRef]
- Narcross, L.; Fossati, E.; Bourgeois, L.; Dueber, J.E.; Martin, V.J.J. Microbial Factories for the Production of Benzylisoquinoline Alkaloids. Trends Biotechnol. 2016, 34, 228–241. [Google Scholar] [CrossRef]
- Slattery, S.S.; Diamond, A.; Wang, H.; Therrien, J.A.; Lant, J.T.; Jazey, T.; Lee, K.; Klassen, Z.; Desgagne-Penix, I.; Karas, B.J.; et al. An Expanded Plasmid-Based Genetic Toolbox Enables Cas9 Genome Editing and Stable Maintenance of Synthetic Pathways in Phaeodactylum tricornutum. ACS Synth. Biol. 2018, 7, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.; de Vos, R.C.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Farhi, M.; Marhevka, E.; Ben-Ari, J.; Algamas-Dimantov, A.; Liang, Z.; Zeevi, V.; Edelbaum, O.; Spitzer-Rimon, B.; Abeliovich, H.; Schwartz, B.; et al. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat. Biotechnol. 2011, 29, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hahn, F.M.; Baidoo, E.; Kahlon, T.S.; Wood, D.F.; McMahan, C.M.; Cornish, K.; Keasling, J.D.; Daniell, H.; Whalen, M.C. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab. Eng. 2012, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, Z.; Kempinski, C.; Eric Nybo, S.; Husodo, S.; Williams, R.; Chappell, J. Engineering triterpene metabolism in tobacco. Planta 2012, 236, 867–877. [Google Scholar] [CrossRef] [PubMed]
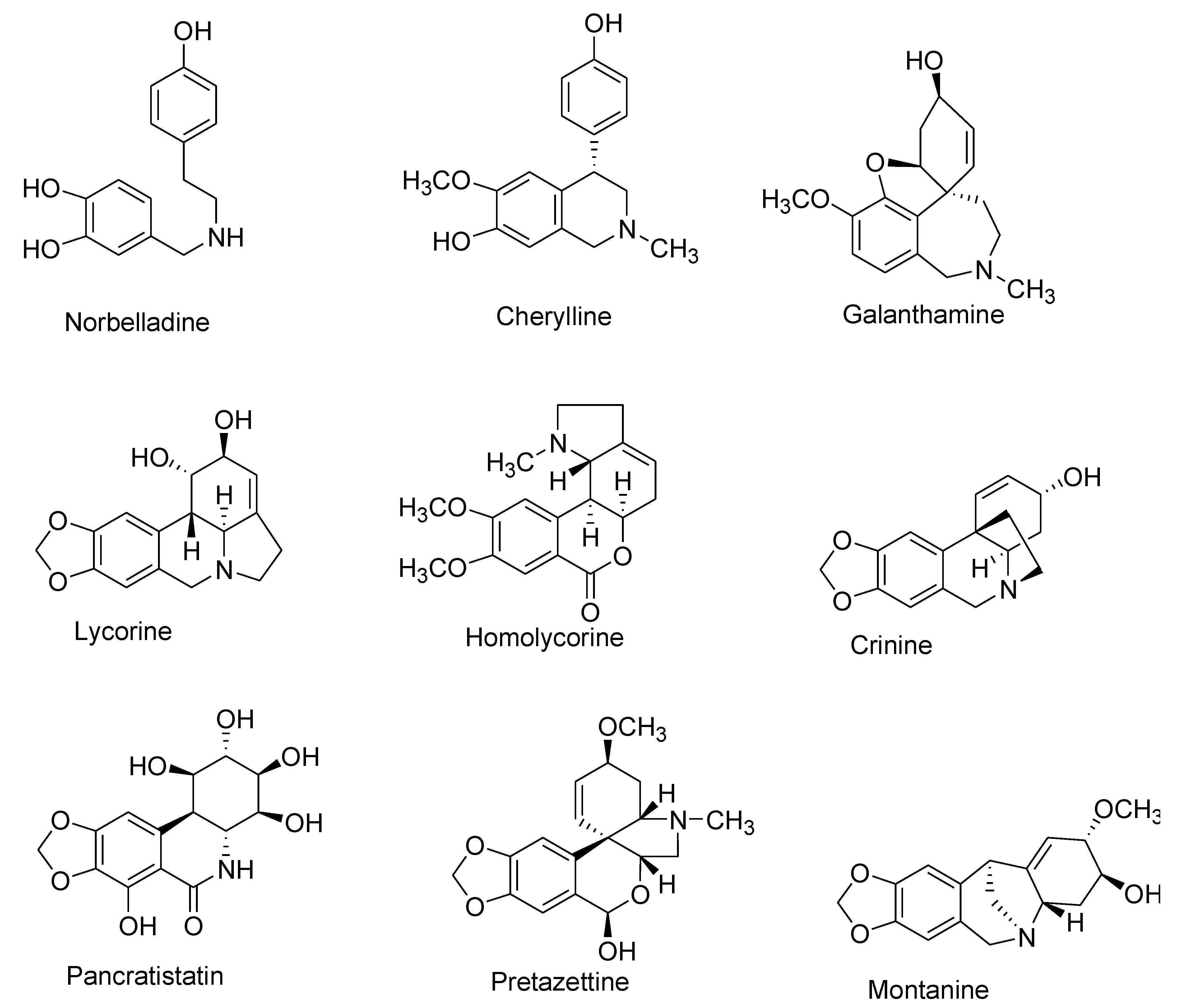
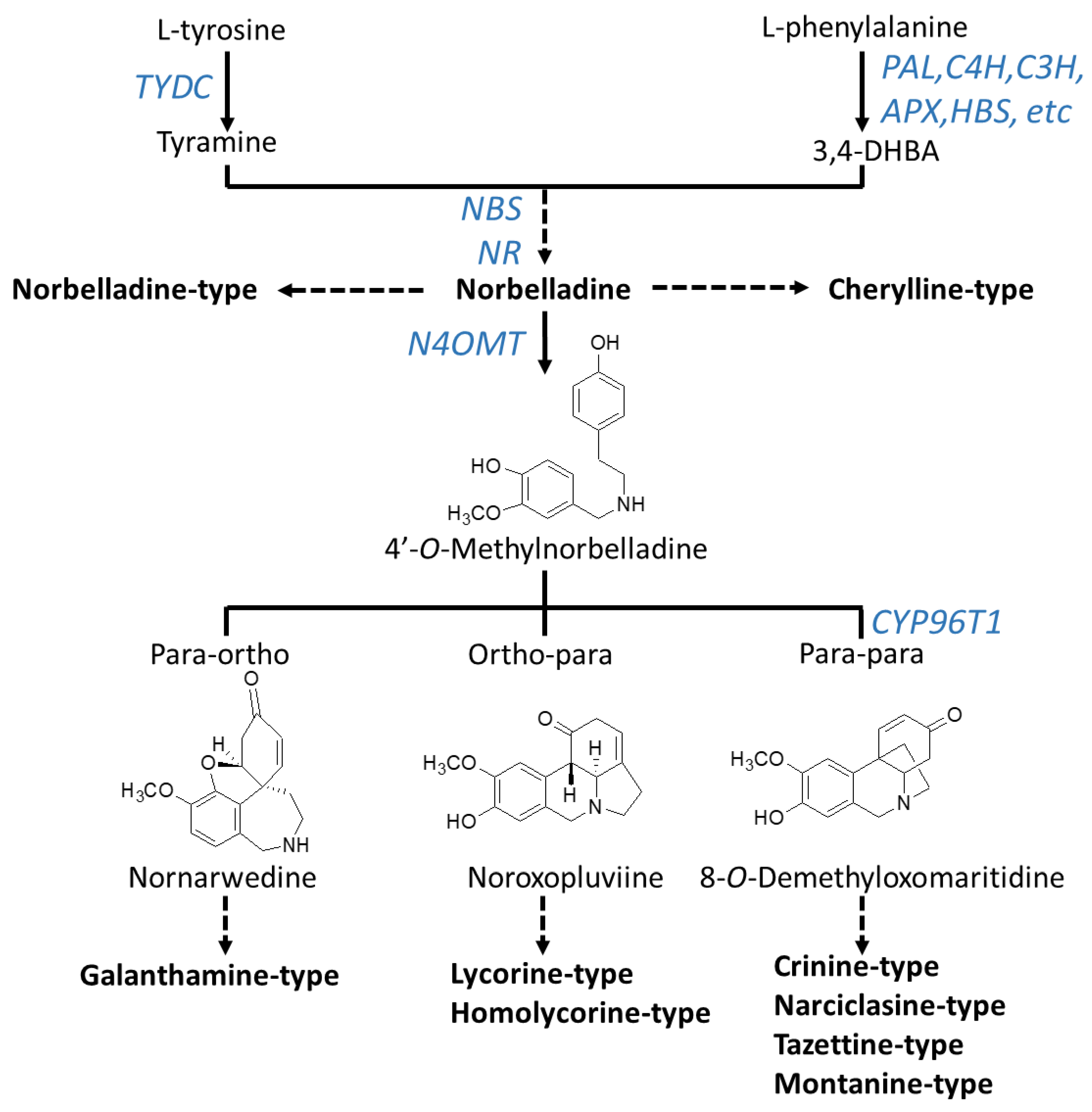
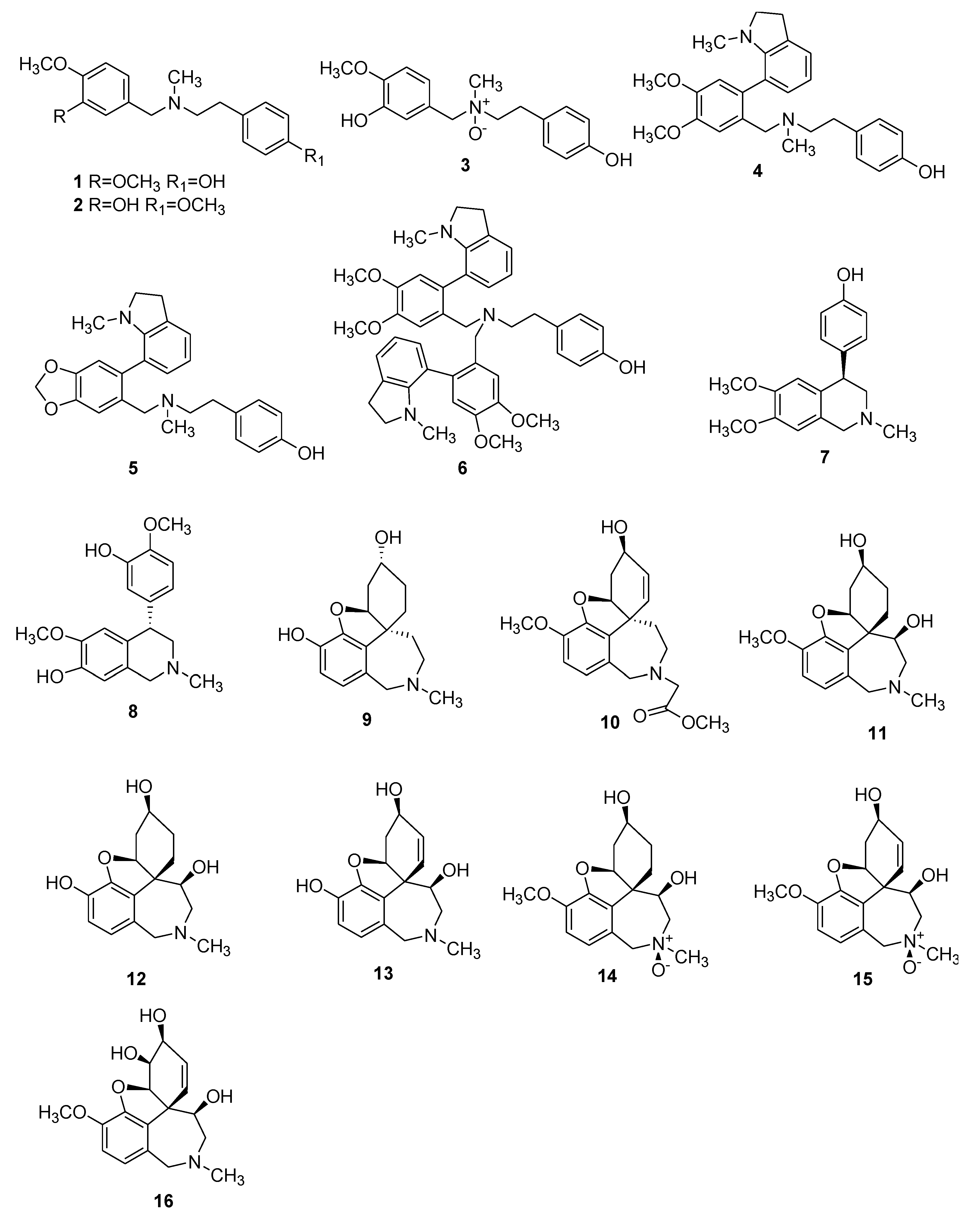
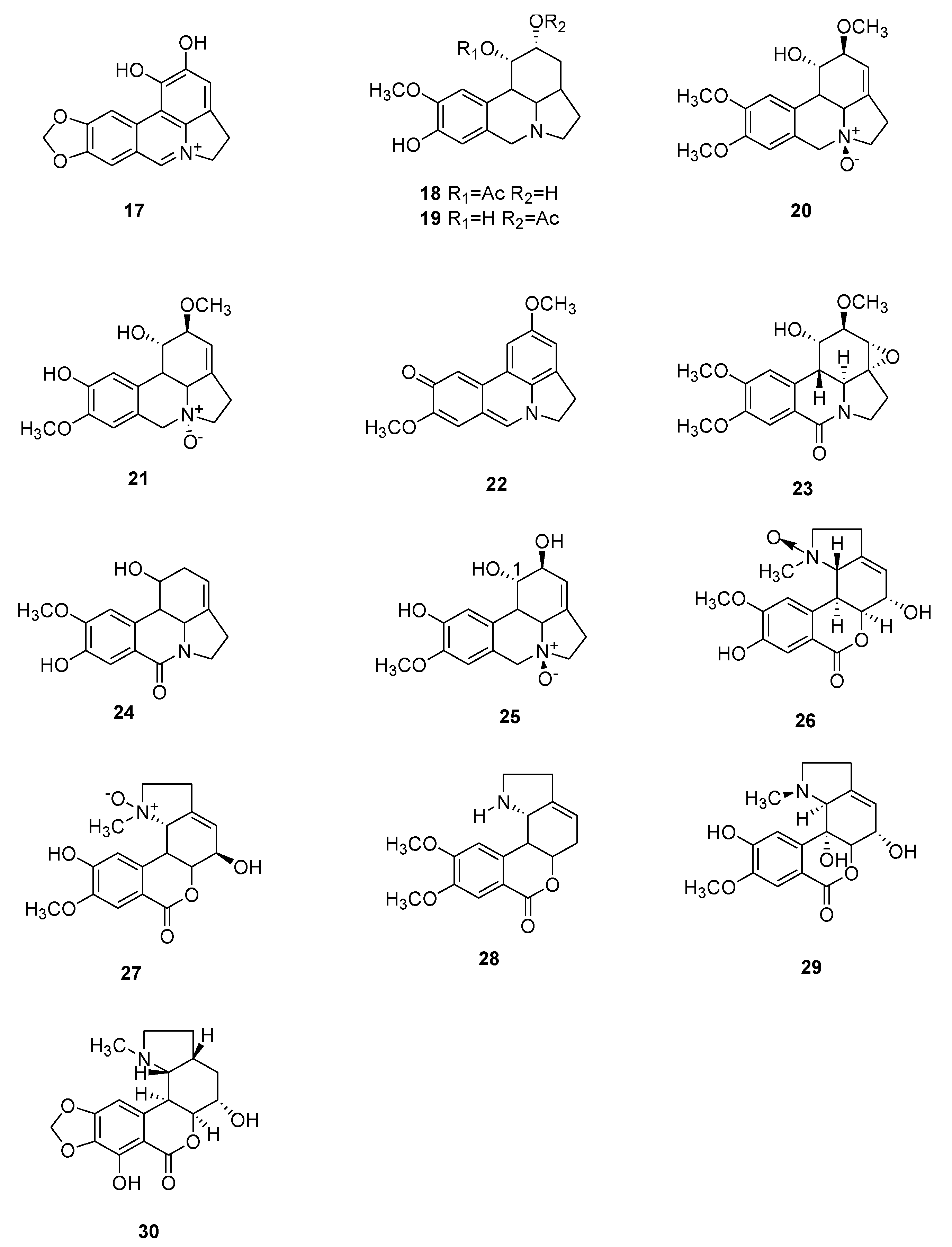
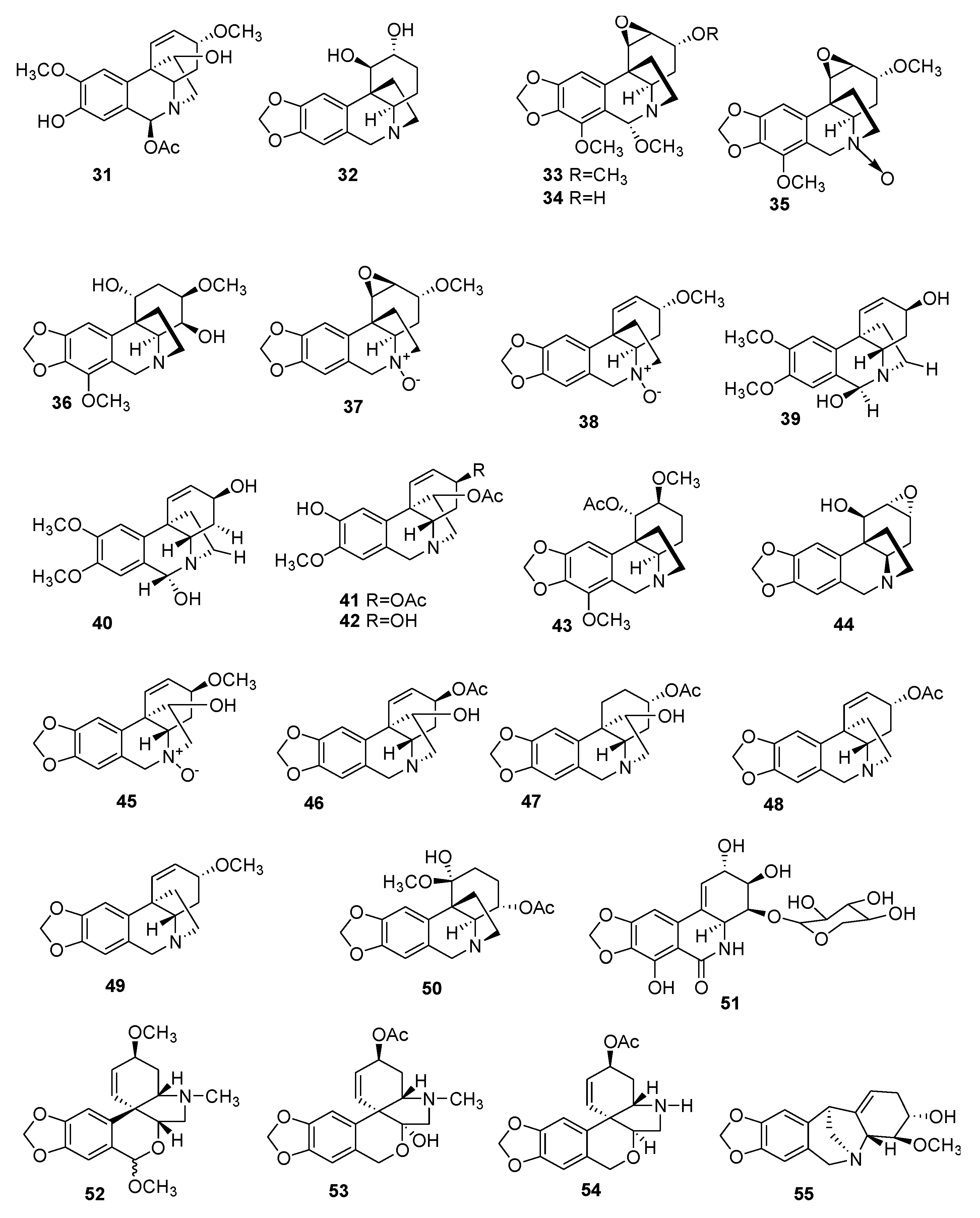
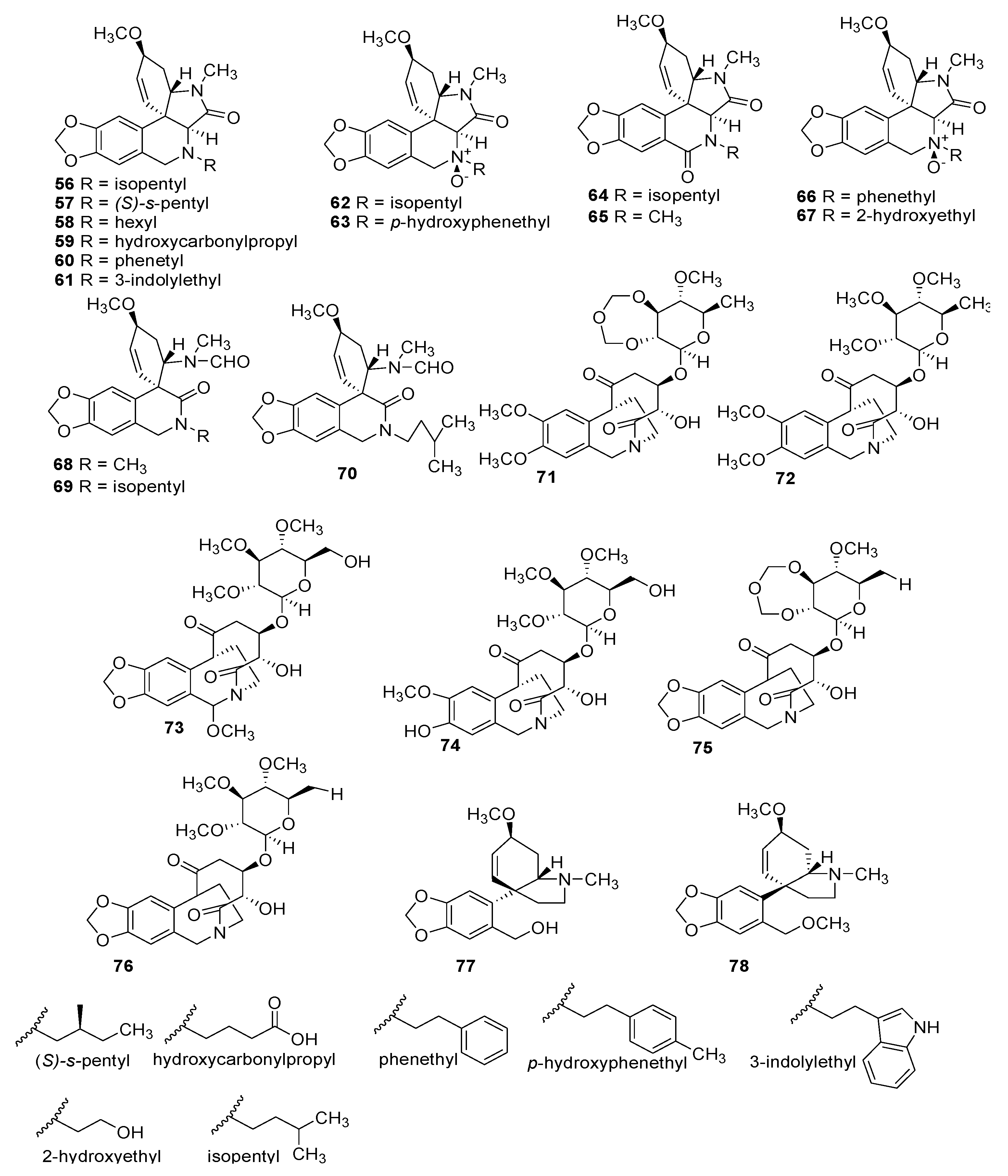

| Number | Type Name | Ring-Type |
|---|---|---|
| I | Norbelladine | N-(3,4-Dioxybenzyl)-4-oxyphenethylamine |
| II | Cherylline | Tetrahydroisoquinoline |
| III | Galantamine | 6H-Benzof,f]-2-benzazepine |
| IV | Lycorine | Pyrrolo[d,e]phenanthridine |
| V | Homolycorine | 2-Benzopyrano-[3,4-g]indole |
| VI | Crinine | 5,10b-Ethanophenanthridine |
| VII | Narciclasine | Lycoricidine |
| VIII | Pretazettine | 2-Benzopyrano [3,4-c]indole |
| IX | Montanine | 5,11-Methanomorphanthridine |
| X | Other | Different ring types and biogenetic origin |
| No | Alkaloid | Activity | Formula | Organ | Ref. |
|---|---|---|---|---|---|
| I—NORBELLADINE-TYPE | |||||
| 1 | 6-O-Demethylbelladine | CNS | C18H23NO3 | B | [60] |
| 2 | 4′-O-Demethylbelladine | CNS | C18H23NO3 | B | [60] |
| 3 | 4′-O,N-dimethylnorbelladine N-oxide | Tum | C17H22NO4 | B | [61] |
| 4 | Carltonine A | CNS | C27H32N2O3 | B | [62] |
| 5 | Carltonine B | CNS | C26H28N2O3 | B | [62] |
| 6 | Carltonine C | CNS | C44H49N3O5 | B | [62] |
| II—CHERYLLINE-TYPE | |||||
| 7 | Gigantelline | CNS, Tum | C18H21NO3 | B | [63] |
| 8 | Gigantellinine | CNS, Tum | C18H21NO4 | B | [63] |
| III—GALANTAMINE-TYPE | |||||
| 9 | Lycoranine C | Tum | C16H21NO3 | B | [64] |
| 10 | Crijaponine B | CNS, Tum | C19H23NO5 | R, F | [65] |
| 11 | 11β-Hydroxylycoramine | Inf | C17H23NO4 | B, L, F | [66] |
| 12 | 9-De-O-methyl-11β-hydroxylycoramine | Inf | C16H21NO4 | B, L, F | [66] |
| 13 | 9-De-O-methyl-11β-hydroxygalantamine | CNS, Inf | C16H19NO4 | B, L, F | [66] |
| 14 | 11β-Hydroxylycoramine N-oxide | Inf | C17H23NO5 | B, L, F | [66] |
| 15 | 11β-Hydroxygalantamine N-oxide | Inf | C17H21NO5 | B, L, F | [66] |
| 16 | 2β,11β-Dihydroxygalantamine | Inf | C17H21NO5 | B, L, F | [66] |
| IV—LYCORINE-TYPE | |||||
| 17 | (+)-1-Hydroxy-ungeremine | Inf, Tum | C16H12NO4+ | B | [67] |
| 18 | Reticulinine | CNS | C17H21NO4 | B, L | [68] |
| 19 | Isoreticulinine | CNS | C17H21NO4 | B, L | [68] |
| 20 | Galanthine N-β-oxide | CNS | C18H23NO5 | B, L, F | [69] |
| 21 | Carinatine N-α-oxide | CNS | C17H21NO5 | B, L, F | [69] |
| 22 | Zephycarinatine I | CNS | C17H15NO3 | B, L, F | [69] |
| 23 | Oxoincartine | CNS | C18H21NO6 | B, L, F | [70] |
| 24 | 7-Oxonorpluviine | nm | C16H17NO4 | B | [71] |
| 25 | pseudolycorine N-oxide | Tum | C16H19NO5 | B, L, F | [72] |
| V—HOMOLYCORINE-TYPE | |||||
| 26 | (+)-2-Hydroxy-8-demethyl-homolycorine-α-N-oxide | Inf, Tum | C17H19NO6 | B | [67] |
| 27 | Lycoranine E | Tum | C17H19NO6 | B | [64] |
| 28 | Lycoranine F | Tum | C17H19NO4 | B | [64] |
| 29 | 2α-10bα-Dihydroxy-9-O-demethylhomolycorine | Tum | C17H19NO6 | B | [73] |
| 30 | 7-Hydroxyclivonine | CNS | C17H19NO6 | B | [74] |
| VI—CRININE-TYPE | |||||
| 31 | (+)-6β-Acetyl-8-hydroxy-9-methoxy-crinamine | Inf, Tum | C19H23NO6 | B | [67] |
| 32 | Crijaponine A | CNS, Tum | C16 H19 NO4 | R, F | [65] |
| 33 | 6α-Methoxyundulatine | Tum | C19H23NO6 | L | [75] |
| 34 | 6α-Methoxycrinamidine | Tum | C18H21NO6 | L | [75] |
| 35 | Undulatine N-oxide | Tum | C18H21NO6 | L | [75] |
| 36 | 1,4-Dihydroxy-3-methoxy powellan | Par, Tum | C18H23NO6 | B | [76] |
| 37 | Augustine N-oxide | CNS, Par | C17 H19NO5 | B, L | [77] |
| 38 | Buphanisine N-oxide | CNS, Par | C17 H19NO4 | B, L | [77] |
| 39 | 6α-Hydroxymaritidine | CNS, Par | C17H21NO4 | B, L | [68] |
| 40 | 6β-Hydroxymaritidine | CNS, Par | C17H21NO4 | B, L | [68] |
| 41 | 3,11-O-Diacetyl-9-O-demethylmaritidine | CNS | C20H22NO6 | B, L, F | [70] |
| 42 | 11-O-Acetyl-9-O-demethylmaritidine | CNS | C18H20NO5 | B, L, F | [70] |
| 43 | Crinsarnine | Ins, Lar | C20H25NO6 | B | [78] |
| 44 | Gigancrinine | CNS, Tum | C16H17NO4 | B | [63] |
| 45 | Haemanthamine N-oxide | nm | C17H19NO5 | B, L | [79] |
| 46 | Crinasiaticine A | hCAII | C18H19NO5 | B | [80] |
| 47 | Crinasiaticine B | hCAII | C18H21NO5 | B | [80] |
| 48 | 3-O-Acetylvittatine | Tum | C18H19NO4 | B | [61] |
| 49 | 3-O-Methyl-epi-vittatine | Tum | C17H19NO3 | B | [81] |
| 50 | Crouchinine | nm | C19H23NO6 | B | [81] |
| VIII—NARCICLASINE-TYPE | |||||
| 51 | Narciclasine-4-O-β-d-xylopyranoside | Tum | C19H21NO11 | B, L, F | [82] |
| VIII—TAZETTINE-TYPE | |||||
| 52 | Jonquailine | Tum | C19H23NO5 | B | [83] |
| 53 | Scillitazettine | Par, Tum | C19H21NO6 | B | [61] |
| 54 | Scilli-N-desmethylpretazettine | Par, Tum | C18H19NO5 | B | [61] |
| IX—MONTANINE-TYPE | |||||
| 55 | 4-O-Methylnangustine | CNS | C17H19NO4 | B | [74] |
| X—OTHER-TYPES | |||||
| PLICAMINE | |||||
| 56 | N-Isopentyl-5,6-dihydroplicane | Inf | C23H30N2O4 | B, L, F | [66] |
| 57 | N-(S)-s-Pentyl-5,6-dihydroplicane | Inf | C23H30N2O4 | B, L, F | [66] |
| 58 | N-Hexyl-5,6-dihydroplicane | Inf | C24H32N2O4 | B, L, F | [66] |
| 59 | N-Hydroxycarbonylpropyl-5,6-dihydroplicane | Inf | C22H26N2O6 | B, L, F | [66] |
| 60 | N-Phenethyl-5,6-dihydroplicane | Inf | C26H28N2O4 | B, L, F | [66] |
| 61 | N-3-Indolylethyl-5,6-dihydroplicane | CNS, Inf | C28H29N3O4 | B, L, F | [66] |
| 62 | N-Isopentyl-5,6-dihydroplicane N-oxide | Inf | C23H30N2O5 | B, L, F | [66] |
| 63 | Bliquine N-oxide | CNS | C26H28N2O6 | B, L, F | [66] |
| 64 | Zephycarinatine C | Inf | C23H28N2O5 | B, L, F | [69] |
| 65 | Zephycarinatine D | Inf | C19H20N2O5 | B, L, F | [69] |
| 66 | Zephycarinatine E | Inf | C23H28N2O5 | B, L, F | [69] |
| 67 | Zephycarinatine F | CNS, Inf | C20H24N2O6 | B, L, F | [69] |
| SECO-PLICAMINE | |||||
| 68 | N-Methyl-11,12-seco-5,6-dihydroplicane | Inf | C19H22N2O5 | B, L, F | [66] |
| 69 | N-Isopentyl-11,12-seco-5,6-dihydroplicane | Inf | C23H30N2O5 | B, L, F | [66] |
| 70 | Zephycarinatine H | CNS | C23H32N2O4 | B, L, F | [69] |
| CRIPOWELLIN | |||||
| 71 | 4,8-Dimethoxy-cripowellin C | Inf, Mic, Oxi, Tum | C26H35NO11 | B | [84] |
| 72 | 4,8-Dimethoxy-cripowellin D | Inf, Mic, Oxi, Tum | C26H37NO10 | B | [84] |
| 73 | 9-Methoxy-cripowellin B | Inf, Mic, Oxi, Tum | C26H35NO12 | B | [84] |
| 74 | 4-Methoxy-8-hydroxy-cripowellin B | Inf, Mic, Oxi, Tum | C25H35NO11 | B | [84] |
| 75 | Cripowellin C | Tum | C25H31NO11 | L | [85] |
| 76 | Cripowellin D | Tum | C25H33NO10 | L | [85] |
| MESEMBRINE | |||||
| 77 | Sarniensinol | Ins, Lar | C18H23NO4 | B | [78] |
| 78 | Sarniensine | Ins, Lar | C19H25NO4 | B | [86] |
| OTHERS | |||||
| 79 | (+)-N-Methoxylcarbonyl-2-demethyl-isocorydione | Inf, Tum | C20H17NO7 | B | [67] |
| 80 | Lycoranine D | Tum | C15H15NO3 | B | [64] |
| 81 | Zephycandidine A | Tum | C16H10N2O2 | B, L, F | [87] |
| 82 | Hymenolitatine | Tum | C17H15NO4 | B | [88] |
| 83 | Zephycandidine I | CNS | C18H25NO4 | B, L, F | [30] |
| 84 | Zephycandidine II | CNS | C15H19NO2 | B, L, F | [30] |
| 85 | Zephycandidine III | CNS | C17H19NO4 | B, L, F | [30] |
| 86 | Narcipavline | CNS | C33H34N2O5 | B | [29] |
| 87 | Narcikachnine | nm | C33H36N2O5 | B | [29] |
| 88 | Narcimatuline | CNS | C33H34N2O5 | B | [89] |
| 89 | Zephycarinatine A | CNS | C25H32N2O5 | B, L, F | [69] |
| 90 | Zephycarinatine B | CNS | C22H30N2O4 | B, L, F | [69] |
| 91 | Zephycarinatine G | CNS | C23H32N2O3 | B, L, F | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ka, S.; Koirala, M.; Mérindol, N.; Desgagné-Penix, I. Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids. Molecules 2020, 25, 4901. https://doi.org/10.3390/molecules25214901
Ka S, Koirala M, Mérindol N, Desgagné-Penix I. Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids. Molecules. 2020; 25(21):4901. https://doi.org/10.3390/molecules25214901
Chicago/Turabian StyleKa, Seydou, Manoj Koirala, Natacha Mérindol, and Isabel Desgagné-Penix. 2020. "Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids" Molecules 25, no. 21: 4901. https://doi.org/10.3390/molecules25214901
APA StyleKa, S., Koirala, M., Mérindol, N., & Desgagné-Penix, I. (2020). Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids. Molecules, 25(21), 4901. https://doi.org/10.3390/molecules25214901





