Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects
Abstract
1. Introduction
2. Limonene
2.1. Microbiological Biotransformation of Limonene
2.2. The Biotransformation of Limonene by Insects
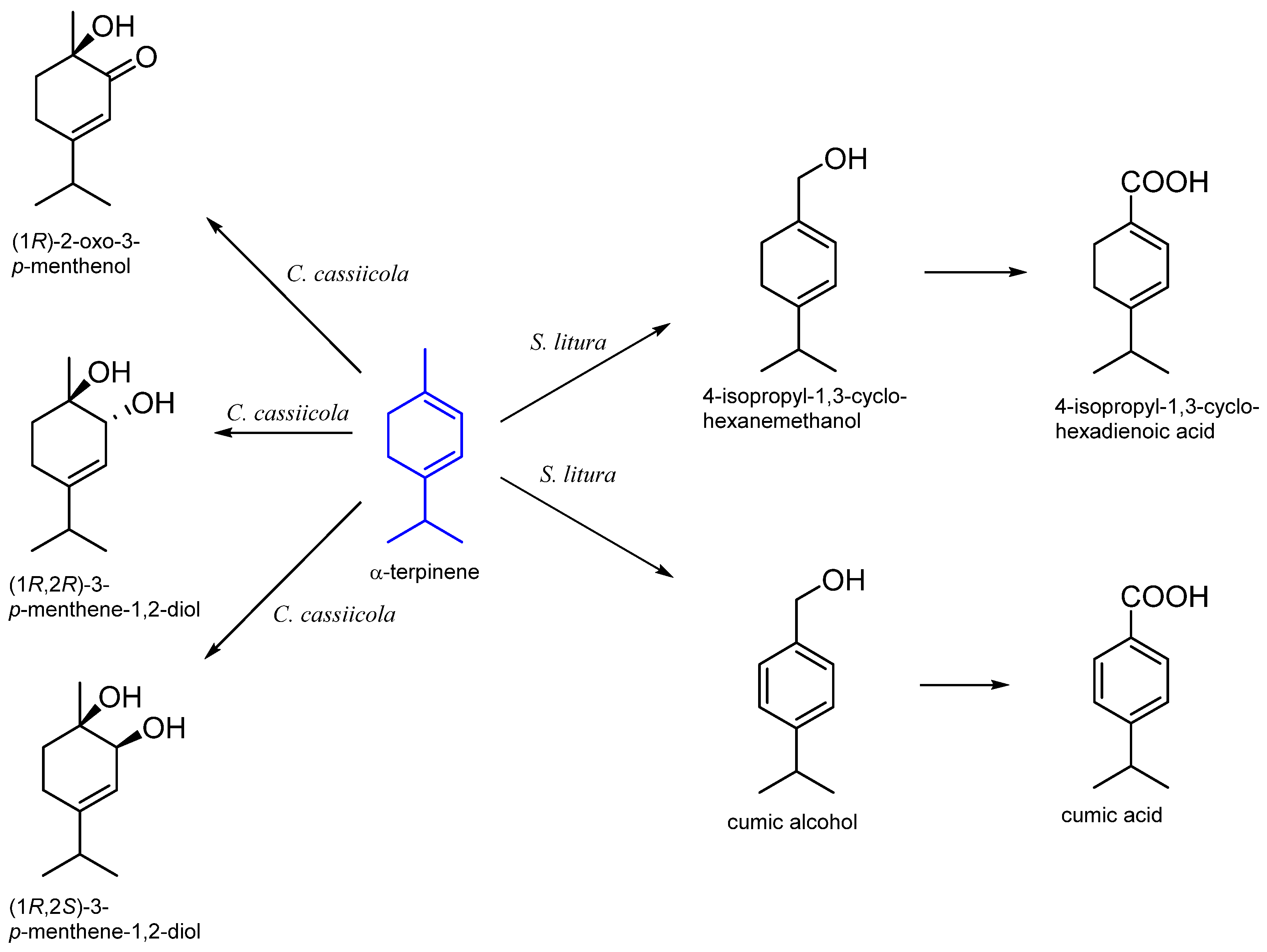
3. α-Terpinene
4. γ-Terpinene
5. Terpinen-4-ol
6. α-Terpineol
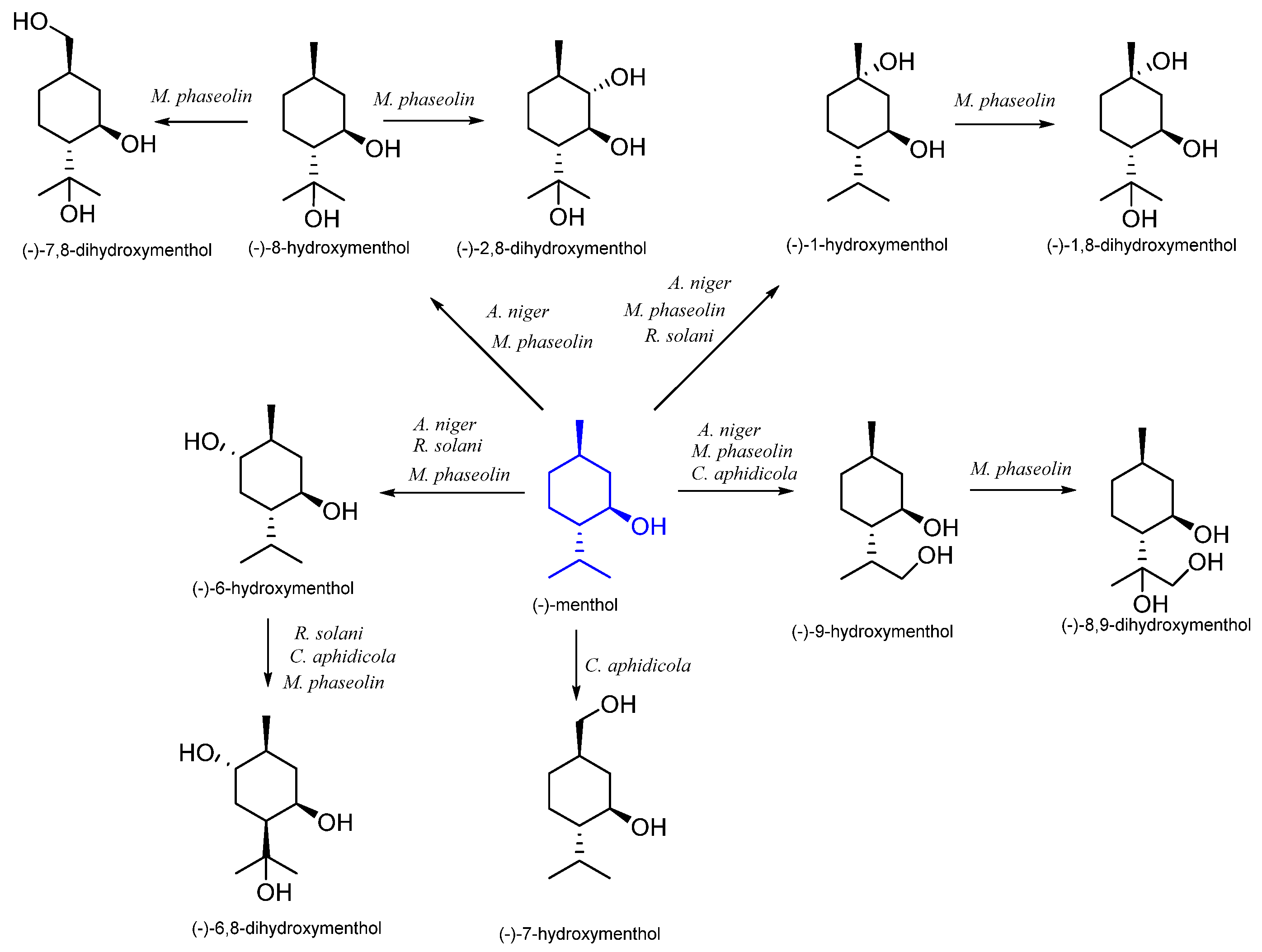
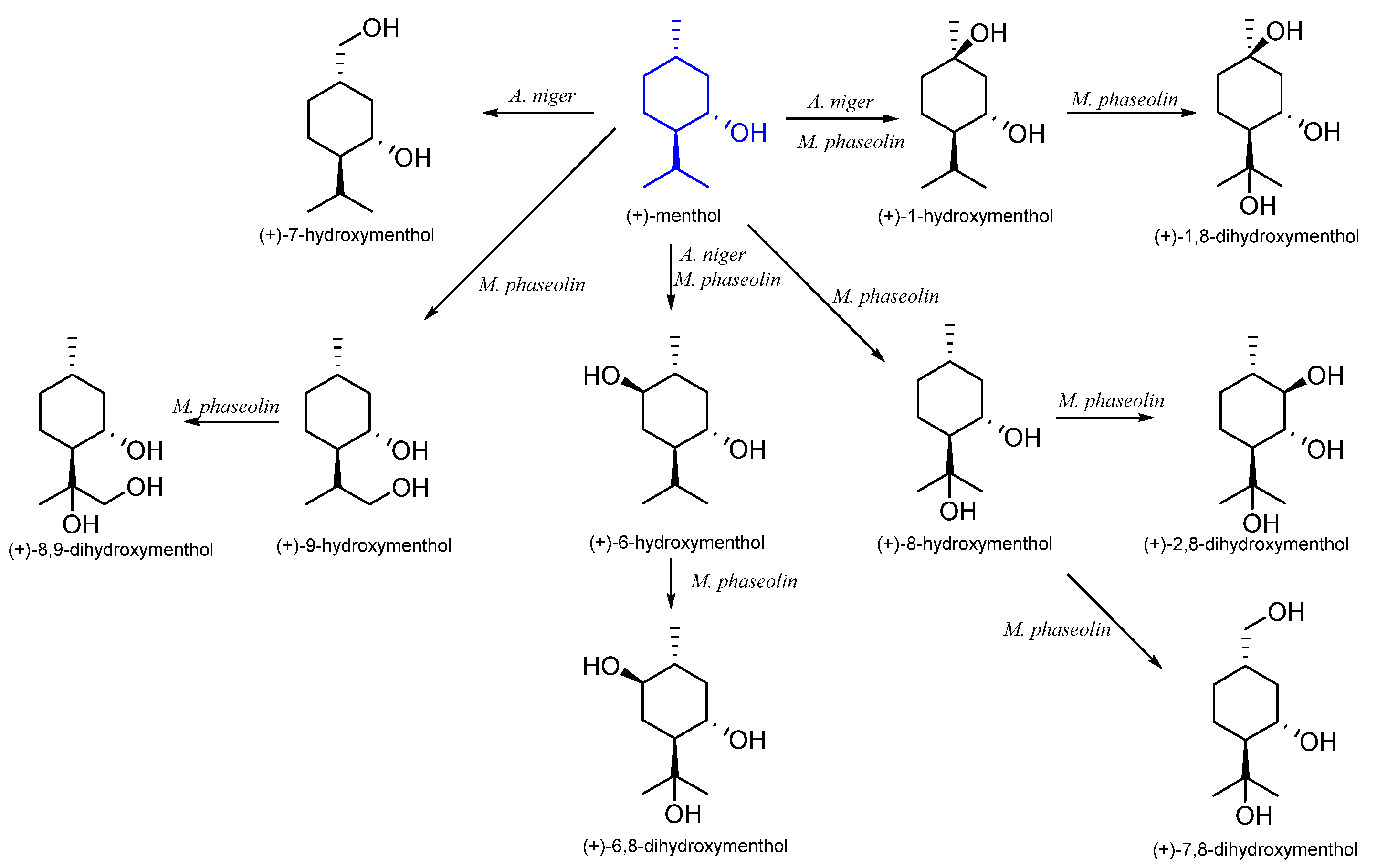
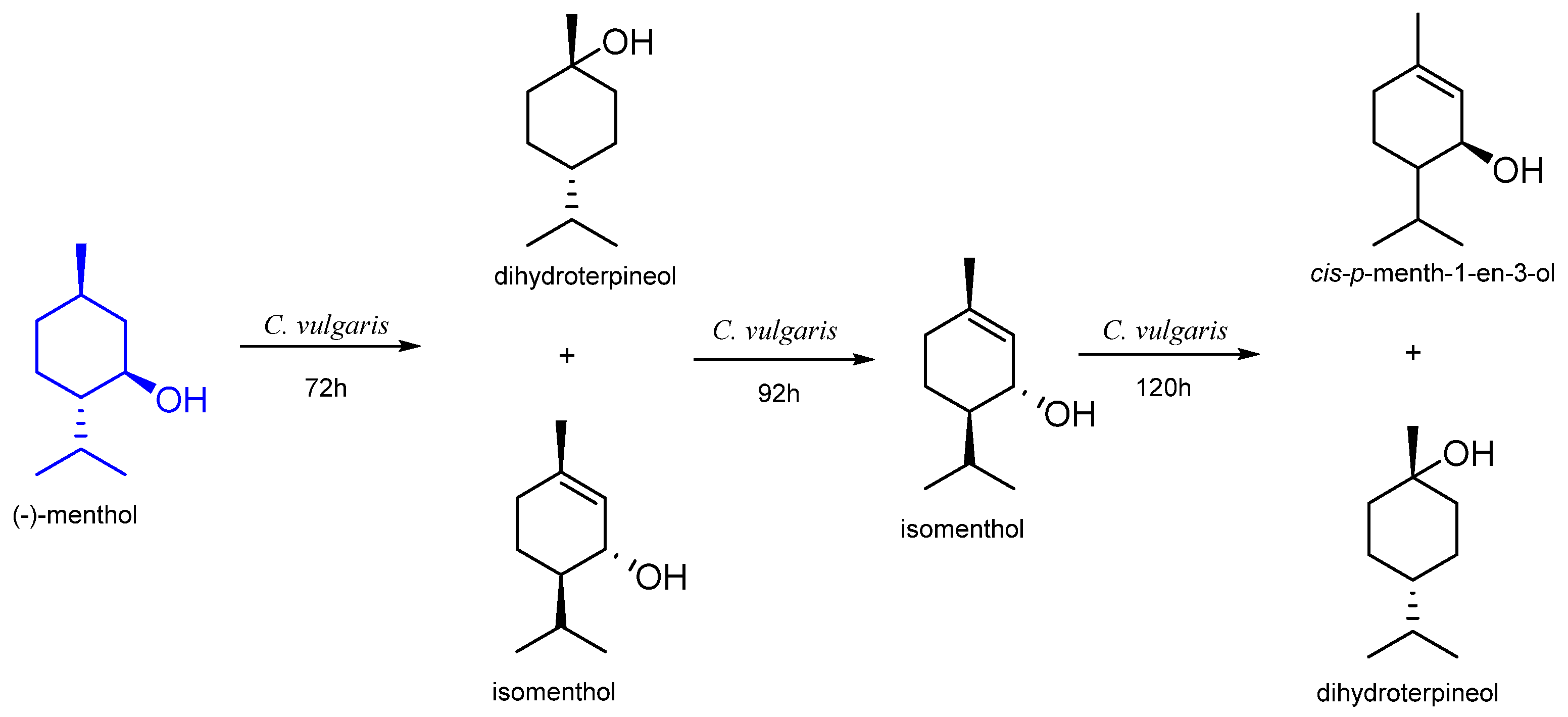
7. Menthol
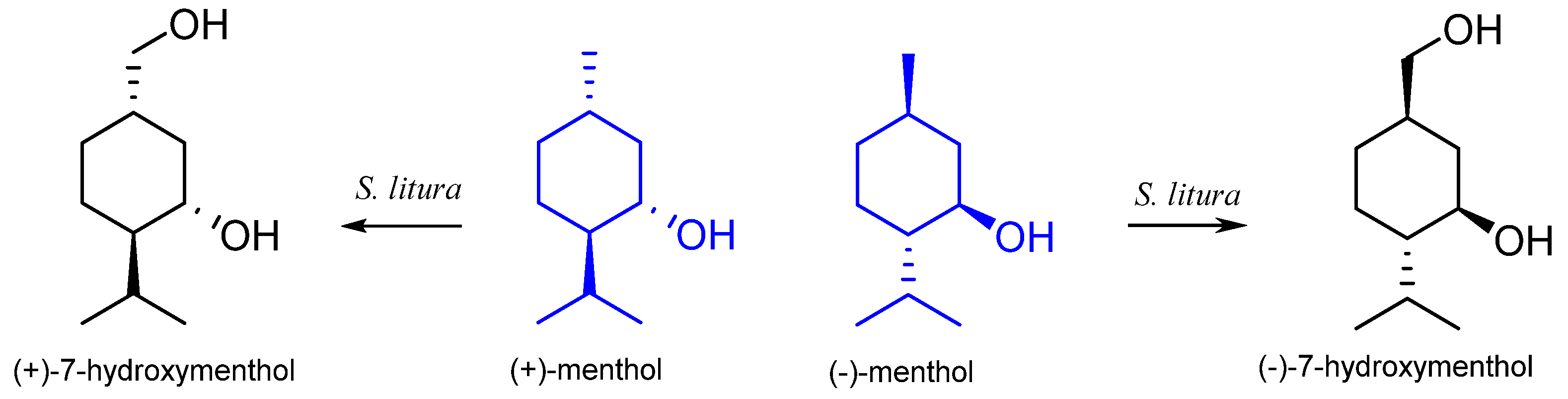
7.1. Microbiological Biotransformation of Menthol
7.2. The Biotransformation of Menthol by Insects
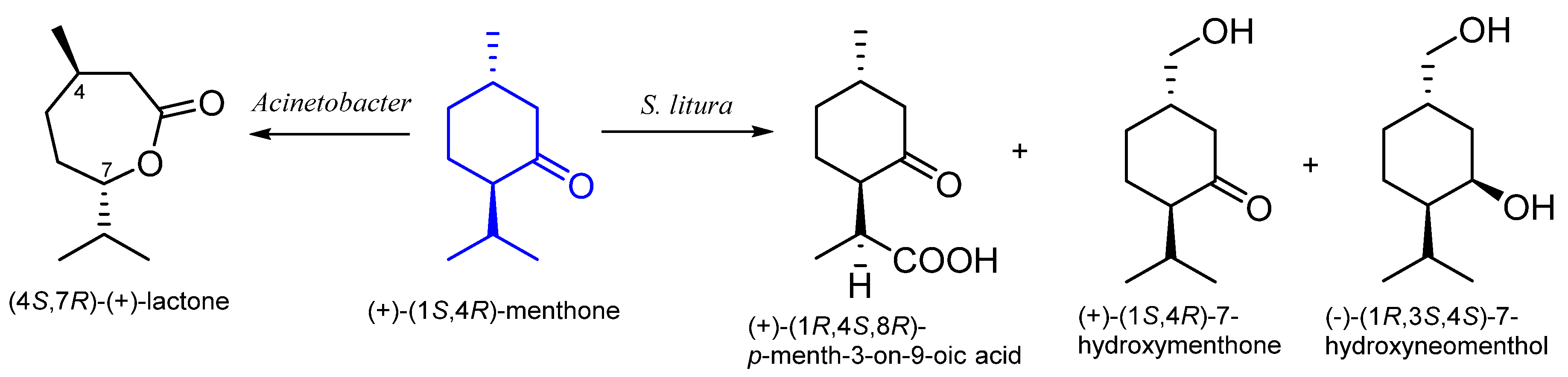
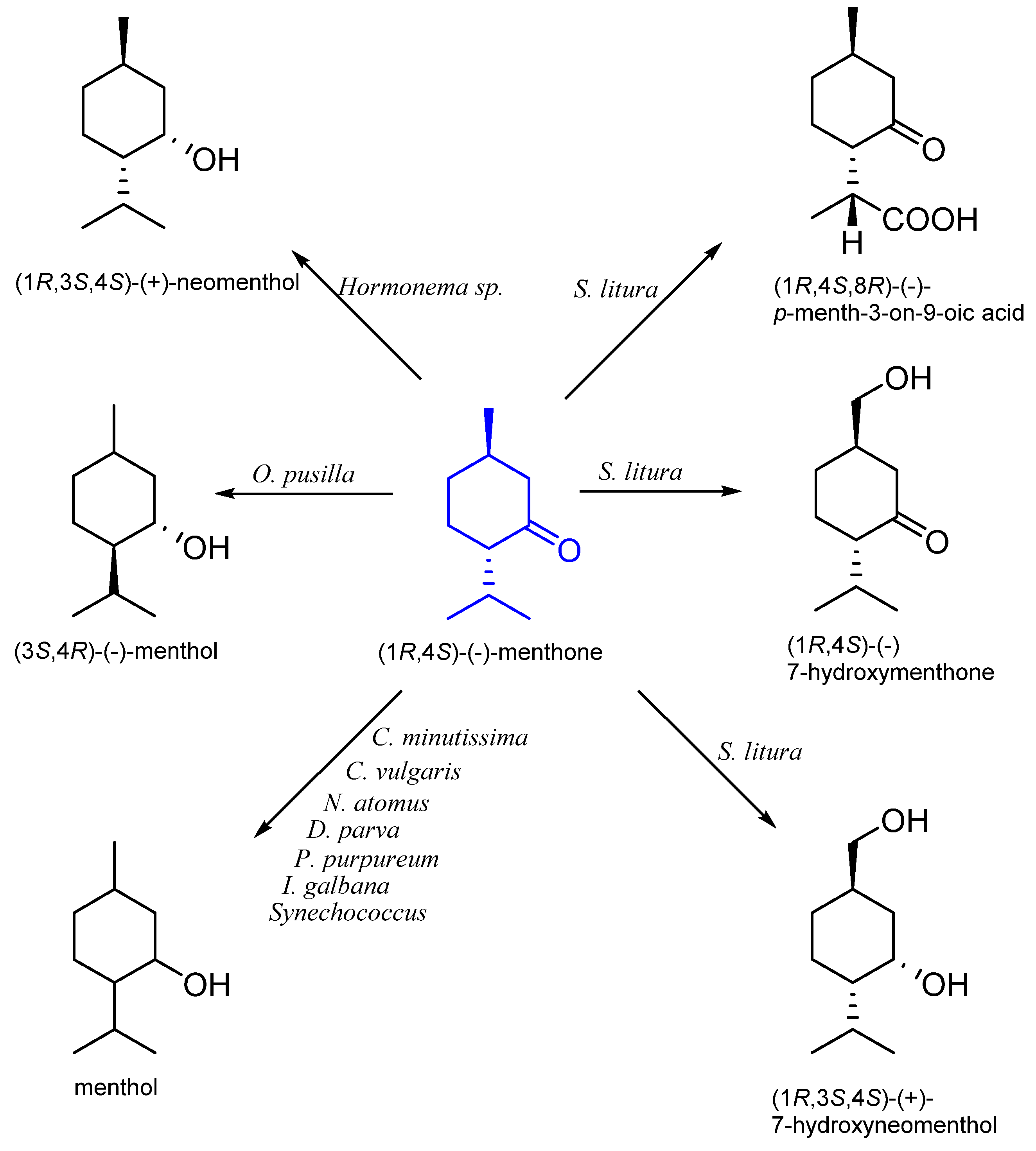
8. Menthone
8.1. Microbiological Biotransformation of Menthone
8.2. The Biotransformation of Menthone by Insects
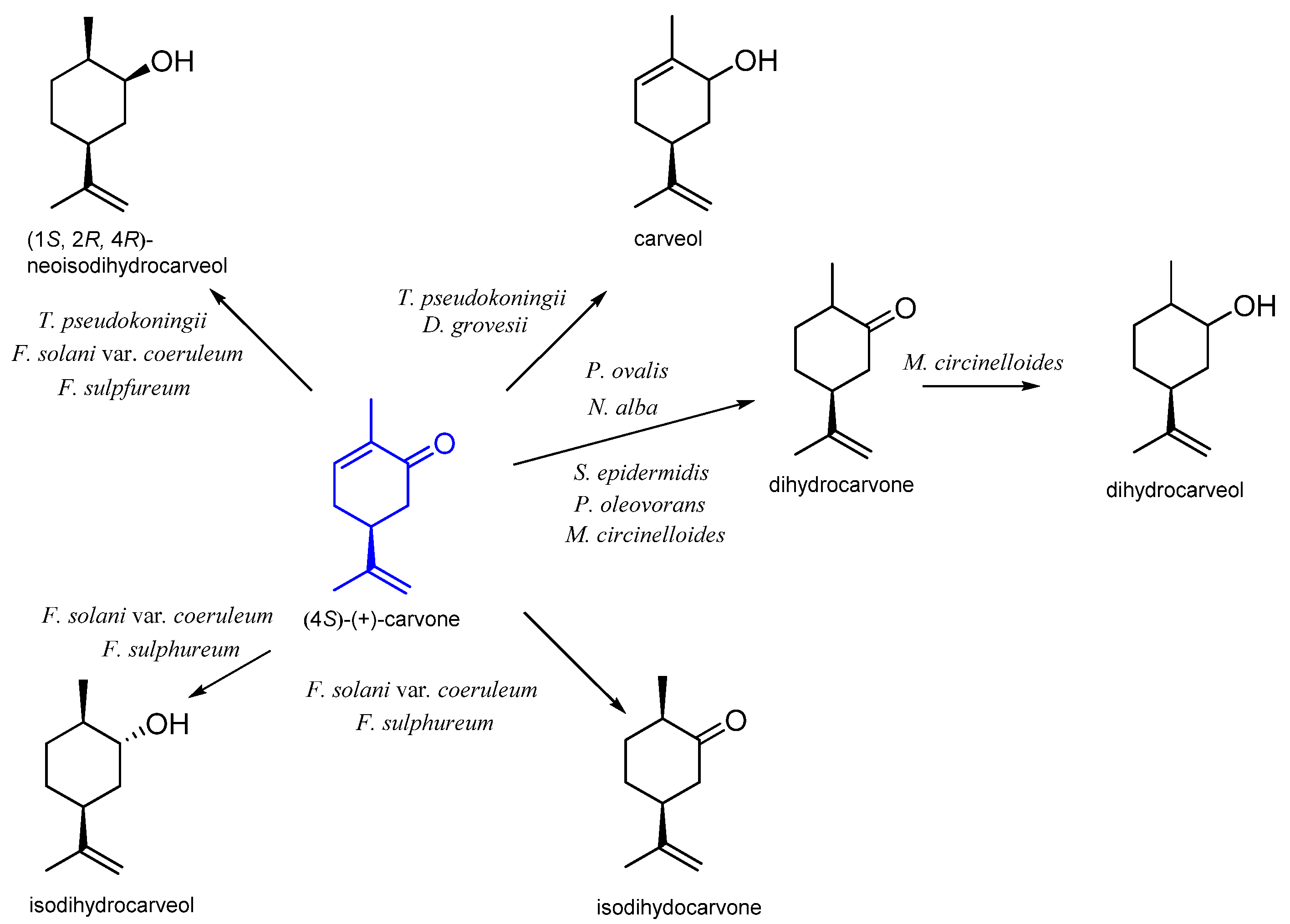
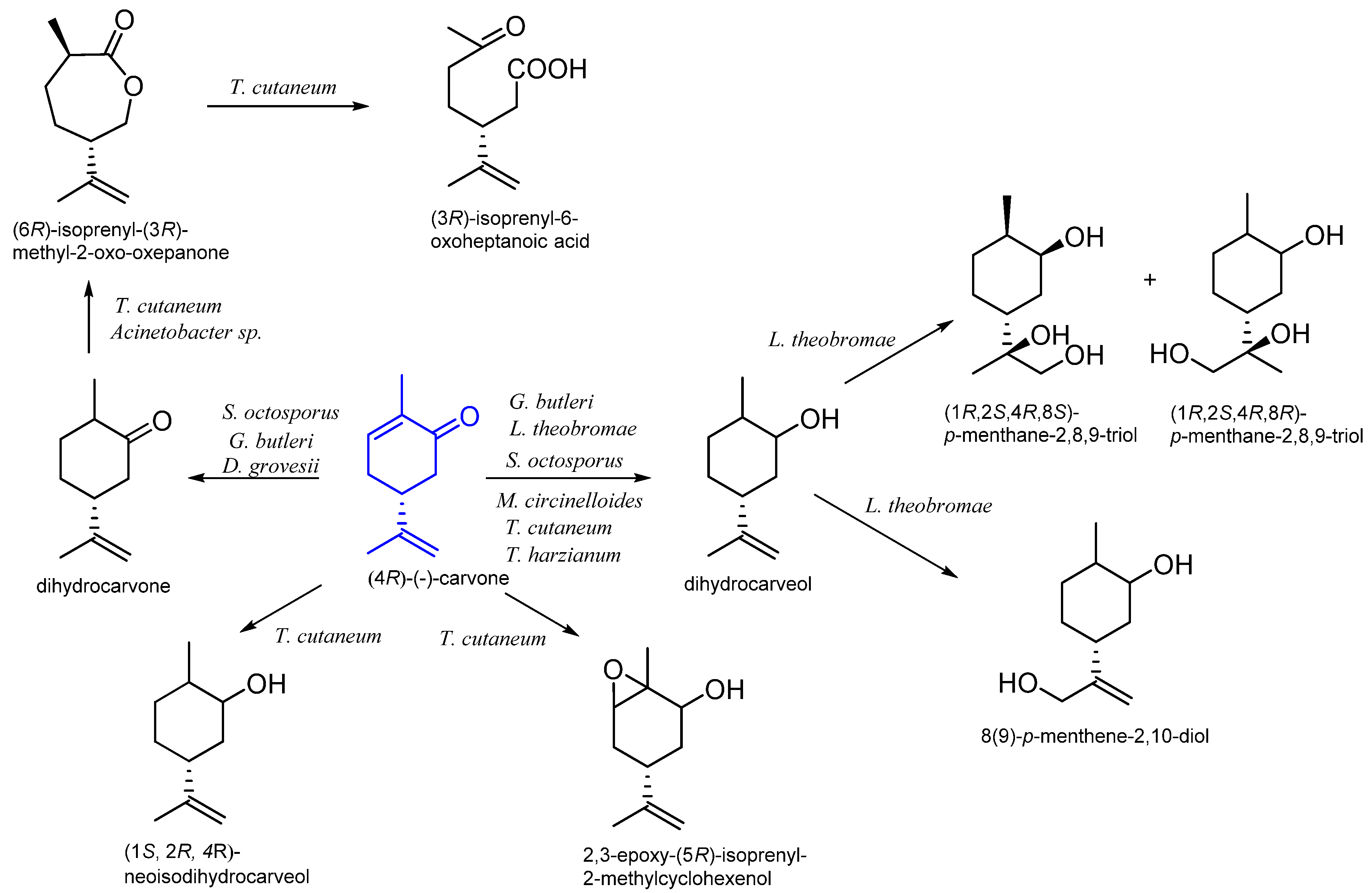
9. Carvone
9.1. Microbiological Biotransformation of Carvone
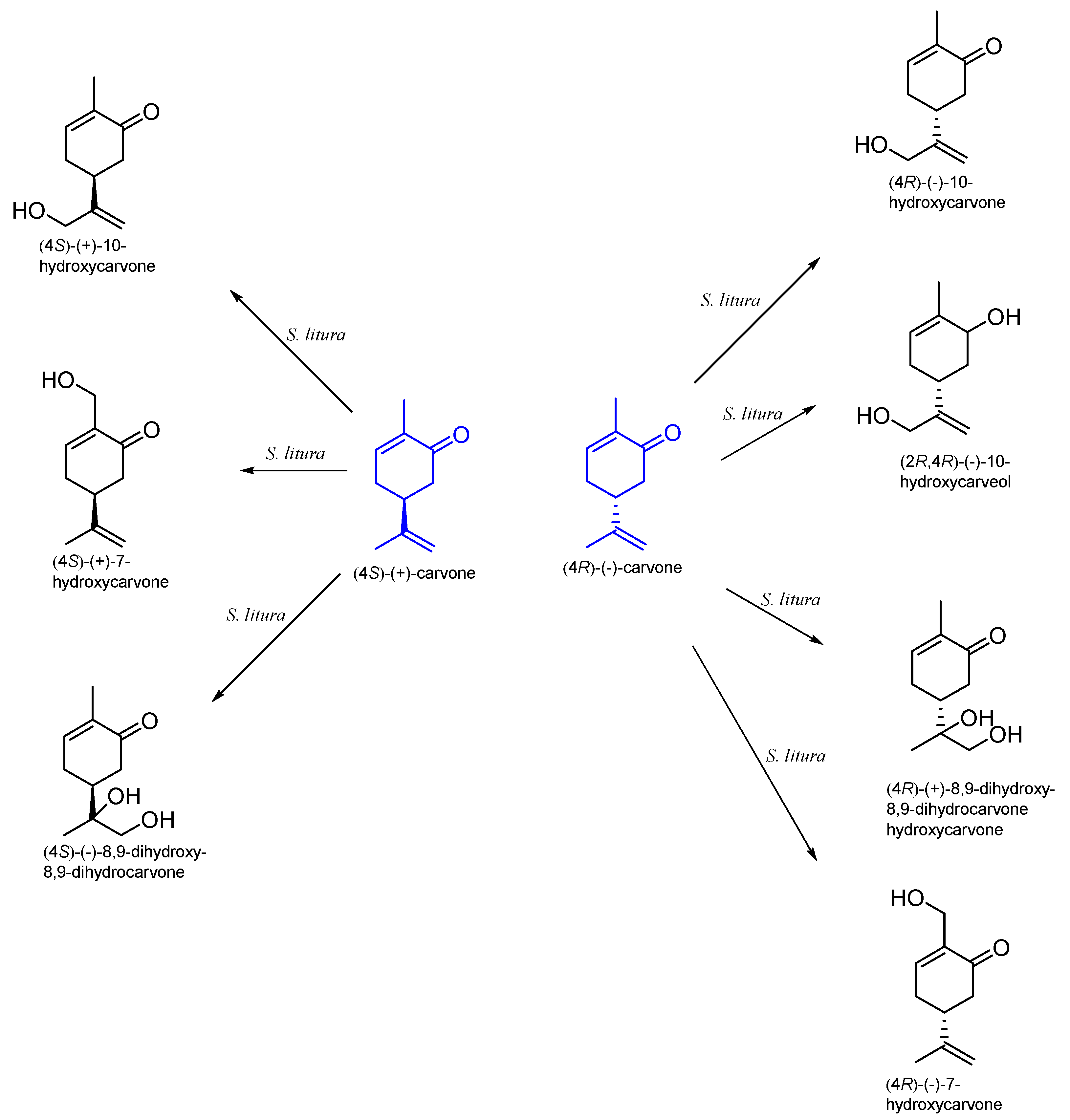
9.2. The Biotransformation of Carvone by Insects
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Biotransformation of terpenes. Biotechnol. Adv. 2006, 24, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Terpenes; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006; pp. 1–3. ISBN 3-527-31786-4. [Google Scholar]
- Yazaki, K.; Arimura, G.; Ohnishi, T. ‘Hidden’ Terpenoids in Plants: Their Biosynthesis, Localization and Ecological Roles Plant. Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Schauvinhold, I.; Larson, M.; Xu, R.; Charbonneau, A.L.; Schmidt, A.; Wilkerson, C.; Last, R.L.; Pichersky, E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 10865–10870. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 3, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.; Feeny, P. Toxicity of angular furanocoumarins to swallowtail butterflies: Escalation in a coevolutionary arms race? Science 1981, 212, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.V.; Schillheim, B.; Mahr, S.; Reufer, Y.; Sanjoyo, S.; Conrath, U.; Buchs, J. Oxygen transfer rate identifies priming compounds in parsley cells. BMC Plant Biol. 2015, 15, 282. [Google Scholar] [CrossRef]
- Harbrone, J.B. Ekologia Biochemiczna; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1997; ISBN 8301122420. [Google Scholar]
- Guengerich, F.P. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–78. [Google Scholar] [CrossRef]
- Vasiliou, V.; Pappa, A.; Estey, T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab. Rev. 2004, 36, 279–299. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Effects of rosemary, thyme and lemongrass oils and their major constituents on detoxifying enzyme activity and insecticidal activity in Trichoplusia ni. Pestic. Biochem. Physiol. 2017, 140, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Gull, S. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. Can. Entomol. 2017, 149, 649–661. [Google Scholar] [CrossRef]
- Shu, Y.; Du, Y.; Chen, J.; Wie, J.; Wang, J. Responses of the cutworm Spodoptera litura (Lepidoptera: Noctuidae) to two Bt corn hybrids expressing Cry1Ab. Sci. Rep. 2017, 7, 41577. [Google Scholar]
- Qin, H.G.; Zheng-Xiang, Y.E.; Huang, S.J.; Ding, J.; Luo, R.H. The correlations of the different host plants with preference level, life duration and survival rate of Spodoptera litura Fabricius. Chin. J. Eco-Agric. 2004, 12, 40–42. [Google Scholar]
- Cheema, H.K.; Kang, B.K.; Jindal, V.; Kaur, S.; Gupta, V.K. Biochemical mechanisms and molecular analysis of fenvalerate resistant population of Spodoptera litura (Fabricius). Crop Prot. 2020, 127, 104951. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Miret, J.A.J.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest categorisation of Spodoptera litura. EFSA J. 2019, 17, 5765. [Google Scholar]
- Karuppaiah, V.; Srivastava, C.; Padaria, J.C.; Subramanian, S. Quantitative changes of the carboxylesterase associated with pyrethroid susceptibility in Spodoptera litura (Lepidoptera: Noctuidae). Afr. Entomol. 2017, 25, 175–182. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhang, Y.; Liao, X. A systemic study of indoxacarb resistance in Spodoptera litura revealed complex expression profiles and regulatory mechanism. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.J.; Mccarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Marmulla, R.; Harder, J. Microbial monoterpene transformations—A review. Front. Microbiol. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Takechi, H. Biotransformation of Monoterpenoids by the Larvae of Common Cutworm (Spodoptera litura). Nat. Prod. Commun. 2007, 2, 435–443. [Google Scholar] [CrossRef]
- Speelmans, G.; Bijlsma, A.; Eggink, G. Limonene bioconversion to high concentrations of a single and stable product, perillic acid, by a solvent-resistant Pseudomonas putida strain. Appl. Microbiol. Biotechnol. 1998, 50, 538–544. [Google Scholar] [CrossRef]
- van Rensburg, E.; Moleleki, N.; van der Walt, J.P.; Botes, P.J.; van Dyk, M.S. Biotransformation of (+)limonene and (–)piperitone by yeasts and yeast-like fungi. Biotechnol. Lett. 1997, 19, 779–782. [Google Scholar] [CrossRef]
- Noma, Y.; Yamasaki, S.; Asakawa, Y. Biotransformation of limonene and related compounds by Aspergillus cellulosae. Phytochemistry 1992, 31, 2725–2727. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; De Kimpe, N.; Soccol, C.R. Evaluation of antioxidant activity of the fermented product from the biotransformation of R-(+)-limonene in solid-state fermentation of orange waste by Diaporthe sp. Biotechnol. Res. Innov. 2019, 3, 168–176. [Google Scholar] [CrossRef]
- Molina, G.; Bution, M.L.; Bicas, J.L.; Dolder, M.A.H.; Pastore, G.M. Comparative study of the bioconversion process using R-(+)- and S-(–)-limonene as substrates for Fusarium oxysporum 152B. Food Chem. 2015, 174, 606–613. [Google Scholar] [CrossRef]
- Molina, G.; Pessôa, M.G.; Bicas, J.L.; Fontanille, P.; Larroche, C.; Pastore, G.M. Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresour. Technol. 2019, 294, 122180. [Google Scholar] [CrossRef]
- Sales, A.; Moreira, R.C.; Pastore, G.M.; Bicas, J.L. Establishment of culture conditions for bio-transformation of R-(+)-limonene to limonene-1,2-diol by Colletotrichum nymphaeae CBMAI 0864. Process Biochem. 2019, 78, 8–14. [Google Scholar] [CrossRef]
- Marostica, M.R., Jr.; Pastore, G.M. Production of R-(+)-a-terpineol by the biotransformation of limonene from orange essential oil, using cassava, waste water as medium. Food Chem. 2007, 101, 345–350. [Google Scholar] [CrossRef]
- Duetz, W.A.; Fjallman, A.H.M.; Ren, S.; Jourdat, C.; Withol, B. Biotransformation of D-Limonene to (+) trans-Carveol by Toluene-Grown Rhodococcus opacus PWD4 Cells. Appl. Environ. Microbiol. 2001, 67, 2829–2832. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.; Berger, R.G. Effects of R-(+)-limonene on submerged cultures of the terpene transforming basidiomycete Pleurotus sapidus. J Biotechnol. 1999, 69, 163–168. [Google Scholar] [CrossRef]
- Hamada, H.; Kondo, Y.; Ishihara, K.; Nakajima, N.; Hamada, H.; Kurihara, R.; Hirata, T. Stereoselective biotransformation of limonene and limonene oxide by cyanobacterium, Synechococcus sp. PCC 7942. J. Biosci. Bioeng. 2003, 96, 581–584. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Holtackers, R.; Lüscher, D.; Bauer, U.; Witholt, B.; Duetz, W.A. Biocatalytic production of perillyl alkohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl. Environ. Microbiol. 2005, 71, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.A.; Almeida, D.S.; Siani, A.C.; Lucchetti, L.; Lacerda, P.S.B.; Freitas, A.; Tappin, M.R.R.; Bon, E.P.S. Bioconversion of R-(+)-limonene to perillic acid by the yeast Yarrowia lipolytica. Braz. J. Microbiol. 2013, 44, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Jan Fiedurek, J.; Skowronek, M. Biotransformation of (R)-(+)-Limonene by the Psychrotrophic Fungus Mortierella minutissima in H2O2-Oxygenated Culture. Food Technol. Biotechnol. 2009, 47, 131–136. [Google Scholar]
- Demyttenaere, J.C.R.; Van Belleghem, K.; De Kimpe, N. Biotransformation of (R)-(+)- and (S)-(−)-limonene by fungi and the use of solid phase microextraction for screening. Phytochemistry 2001, 57, 199–208. [Google Scholar] [CrossRef]
- Miyazawa, M.; Wada, T.; Kameoka, H. Biotransformation of (+)- and (−)-Limonene by the Larvae of Common Cutworm (Spodoptera litura). J. Agric. Food Chem. 1998, 46, 300–303. [Google Scholar] [CrossRef]
- Fickers, P.; Benetti, P.H.; Wache, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Khan, S.S.; Khan, A.; Rani, M.; Ahmad, V.U.; Choudhary, M.I. Biotransformation of monoterpenoids and their antimicrobial activities. Phytomedicine 2014, 21, 1597–1626. [Google Scholar] [CrossRef]
- Chang, D.; Zhang, J.; Witholt, B.; Li, Z. Chemical and enzymatic synthetic methods for asymmetric oxidation of the C–C double bond. Biocatal. Biotransform. 2004, 22, 113–131. [Google Scholar] [CrossRef]
- Miyazawa, M.; Wada, T.; Kameoka, H. Biotransformation of α-terpinene in common cutworm larvae (Spodoptera litura Fabricius). J. Agric. Food Chem. 1996, 44, 2889–2893. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Brauer, B.; Kaspera, R.; Berger, R.G. Biotransformation of g-terpinene using Stemphylium botryosum (Wallroth) yields p-mentha-1,4-dien-9-ol, a novel odorous Monoterpenol. Biocatal. Biotransform. 2005, 23, 457–463. [Google Scholar] [CrossRef]
- Miyazawa, M.; Wada, T. Biotransformation of γ-terpinene and (−)-α-phellandrene by the larvae of common cutworm (Spodoptera litura). J. Agric. Food Chem. 2000, 48, 2893–2895. [Google Scholar] [CrossRef]
- Draczyńska-Łusiak, B.; Siewiński, A. Enantioselectivity of the metabolism of some monoterpenic components of coniferous tree resin by Armillariella mellea (honey fungus). J. Basic Microbiol. 1989, 29, 269–275. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kumagae, S. Biotransformation of (R)-and (S)-terpinen-4-ol by the larvae of common cutworm (Spodoptera litura). J. Agric. Food Chem. 2001, 49, 4312–4314. [Google Scholar] [CrossRef]
- Miyazawa, M.; Ohsawa, M. Biotransformation of α-terpineol by the larvae of common cutworm (Spodoptera litura). J. Agric. Food Chem. 2002, 50, 4916–4918. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takahashi, H.; Toyota, M.; Noma, Y. Biotransformation of monoterpenoids, (-)- and (+)-menthols, terpinolene and carvotanacetone by Aspergillus species. Phytochemistry 1991, 30, 3981–3987. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Yaqoob, M.; Farooq, A.; Shazia, S.; Asif, F.; Choudhary, M.I. Fungal Transformation of (1R,2S,5R)-(−)-Menthol by Cephalosporium aphidicola. J. Nat. Prod. 1998, 61, 1340–1342. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kawazoe, H.; Hyakumachi, M. Biotransformation of l-menthol by twelve isolates of soil-borne plant pathogenic fungi (Rhizoctonia solani) and classification of fungi. J. Chem. Technol. Biotechnol. 2003, 78, 620–625. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kumagae, S.; Kameoka, H. Biotransformation of (+)- and (−)-Menthol by the Larvae of Common Cutworm (Spodoptera litura). J. Agric. Food Chem. 1999, 47, 3938–3940. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Ahmed, M.A.; Ali, R.A.; Choudhary, M.I. Hydroxylation of (+)-menthol by Macrophomina phaseolina. Biocatal. Biotransform. 2011, 29, 77–82. [Google Scholar] [CrossRef]
- Esmaeili, A.; Mirmhadi, H.R.; Rafiee, F. Biotransformation of menthol by Chlorella vulgaris and study of the pathways involved. Chem. Nat. Compd. 2014, 49, 1160–1161. [Google Scholar] [CrossRef]
- Alphand, V.; Furstoss, R. Microbiological Transformations. 23. A Surprising Regioselectivity of Microbiological Baeyer-Villiger Oxidations of Menthone and Dihydrocarvone. Tetrahedron Asymmetry 1992, 3, 379–382. [Google Scholar] [CrossRef]
- Marumoto, S.; Okuno, Y.; Hagiwara, Y.; Miyazawa, M. Biotransformation of (–)-(1R, 4S)-menthone and (+)-(1S, 4R)-menthone by the common cutworm Spodoptera litura larvae. J. Oleo Sci. 2017, 66, 883–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Dyk, M.S.; van Rensburg, E.; Rensburg, I.P.B.; Moleleki, N. Biotransformation of monoterpenoid ketones by yeasts and yeast-like fungi. J. Mol. Catal. B Enzym. 1998, 5, 149–154. [Google Scholar] [CrossRef]
- Hook, I.L.; Ryan, S.; Sheridan, H. Biotransformation of aliphatic and aromatic ketones, including several monoterpenoid ketones and their derivatives by five species of marine microalgae. Phytochemistry 2003, 63, 31–36. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Mohagheghzadeh, A.; Moshavash, M.; Ostovan, Z.; Rasoul-Amini, S.; Morowvat, M.H.; Ghoshoon, M.B.; Raee, M.J.; Mosavi-Azam, S.B. Biotransformation of monoterpenes by Oocystis pusilla. World J. Microbiol. Biotechnol. 2009, 25, 1301–1304. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Mohagheghzadeh, A.; Ostovan, Z.; Moshavash, M.; Rasoul-Amini, S.; Morowvat, M.H. Biotransformation of some monoterpenoid ketones by Chlorella vulgaris MCCS 012. Chem. Nat. Compd. 2010, 46, 734–737. [Google Scholar] [CrossRef]
- Jiittner, F.; Hans, R. The reducing capacities of cyanobacteria for aldehydes and ketones. Appl. Microbiol. Biotechnol. 1986, 25, 52–54. [Google Scholar] [CrossRef]
- Demyttenaere, J.C.R. Biotransformation of terpenoids by microorganisms. Stud. Nat. Prod. Chem. 2001, 25, 125–178. [Google Scholar] [CrossRef]
- Verstegen-Haaksma, A.A.; Swarts, H.J.; Jansen, B.J.M.; de Groot, A.; BottemaMacGillavry, N.; Witholt, B. Application of S-(+)-carvone in the synthesis of biologically active natural products using chemical transformations and bioconversions. Ind. Crop. Prod. 1995, 4, 15–21. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Leitao, A.C.; Gorris, L.G.M.; Smid, E.J. Comparative study on the action of S-(+)-carvone, in situ, on the potato storage fungi Fusarium solani var. coeruleum and F. sulphureum. J. Appl. Bacteriol. 1996, 80, 535–539. [Google Scholar] [CrossRef]
- Carballeira, J.D.; Alvarez, E.; Campillo, M.; Pardo, L.; Sinisterra, J.V. Diplogelasinospora grovesii IMI 171018, a new whole cell biocatalyst for the stereoselective reduction of ketones. Tetrahedron: Asymmetry 2004, 15, 951–962. [Google Scholar] [CrossRef]
- Nunes, F.M.; dos Santos, G.F.; Saraiva, N.N.; Trapp, M.A.; de Mattos, M.C.; Maria da Conceição, F.O.; Rodrigues-Filho, E. New fungi for whole-cell biotransformation of carvone enantiomers. Novel p-menthane-2, 8, 9-triols production. Appl. Catal. A Gen. 2013, 468, 88–94. [Google Scholar] [CrossRef]
- Carballeira, J.D.; Valmaseda, M.; Alvarez, E.; Gago, J.S. Gongronella butleri, Schizosaccharomyces octosporus and Diplogelasinospora grovesii: Novel microorganisms useful for the stereoselective reduction of ketones. Enzyme Microb. Technol. 2004, 34, 611–623. [Google Scholar] [CrossRef]
- Pinheiro, L.; Marsaioli, A.J. Microbial monooxygenases applied to fragrance compounds. J. Mol. Catal. B Enzymatic 2007, 44, 78–86. [Google Scholar] [CrossRef]
- Marumoto, S.; Okuno, Y.; Hagiwara, Y.; Miyazawa, M. Biotransformation of (+)-Carvone and (-)-Carvone by the Common Cutworm Spodoptera litura Larvae. J. Oleo Sci. 2018, 67, 1253–1257. [Google Scholar] [CrossRef]
- Ottolina, G.; Carrea, G.; Colonna, S.; Rückemann, A. A predictive active site model for cyclohexanone monooxygenase catalyzed Baeyer-Villiger oxidations. Tetrahedron Asymmetry 1996, 7, 1123–1136. [Google Scholar] [CrossRef]
- Kuriata-Adamusiak, R.; Strub, D.; Lochyński, S. Application of microorganisms towards synthesis of chiral terpenoid derivatives. Appl. Microbiol. Biotechnol. 2012, 95, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Volcho, K.P.; Salakhutdinov, N.F. Synthesis of hydroxy derivatives of limonene. Rus. Chem. Rev. 2014, 83, 281. [Google Scholar] [CrossRef]
- Alifano, P.; Talà, A. Microbes at work in perfumery. Suppl. Househ. Pers. Care Today 2010, 1, 20–29. [Google Scholar]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2016. [Google Scholar]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as a antimictobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
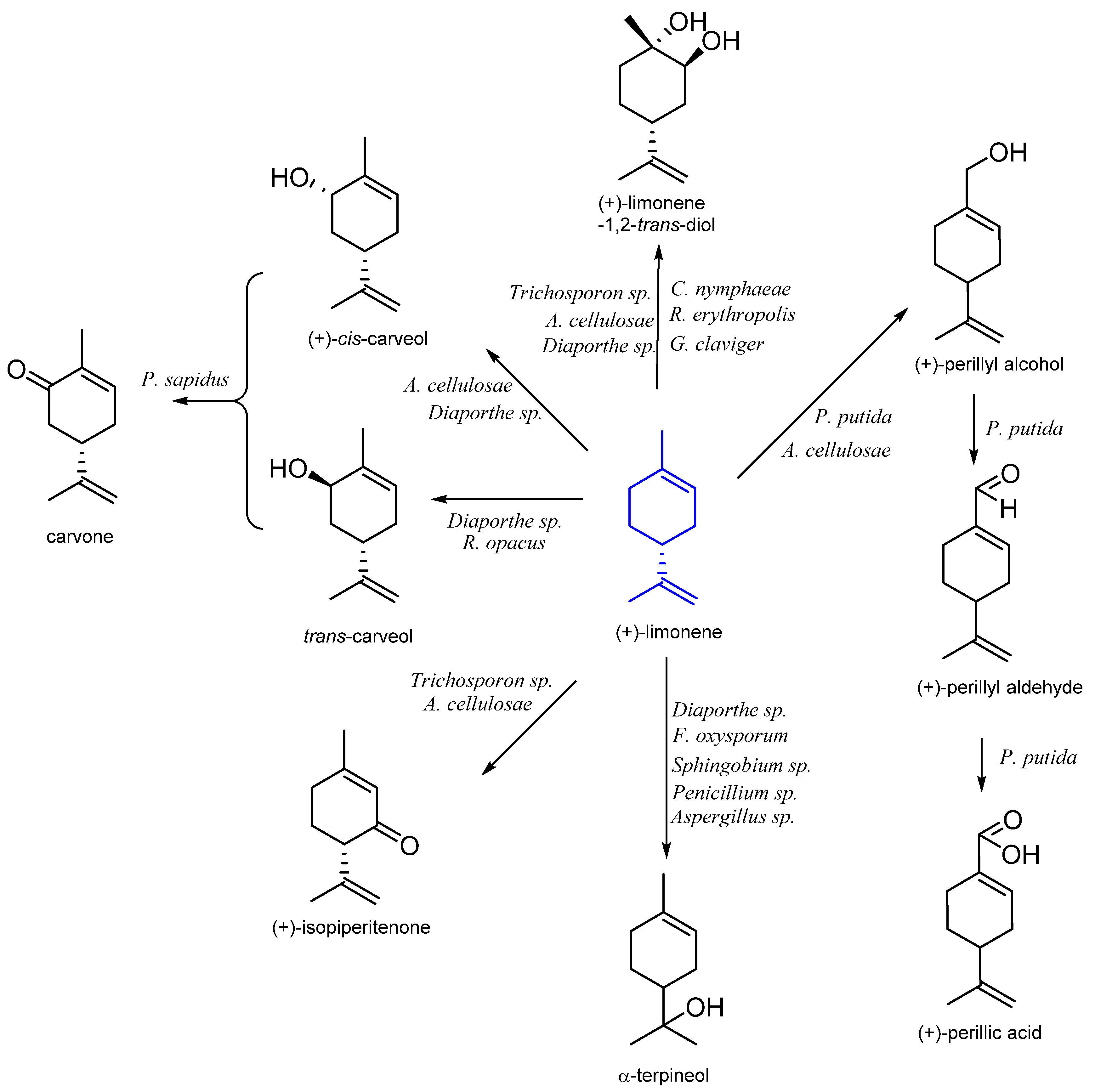
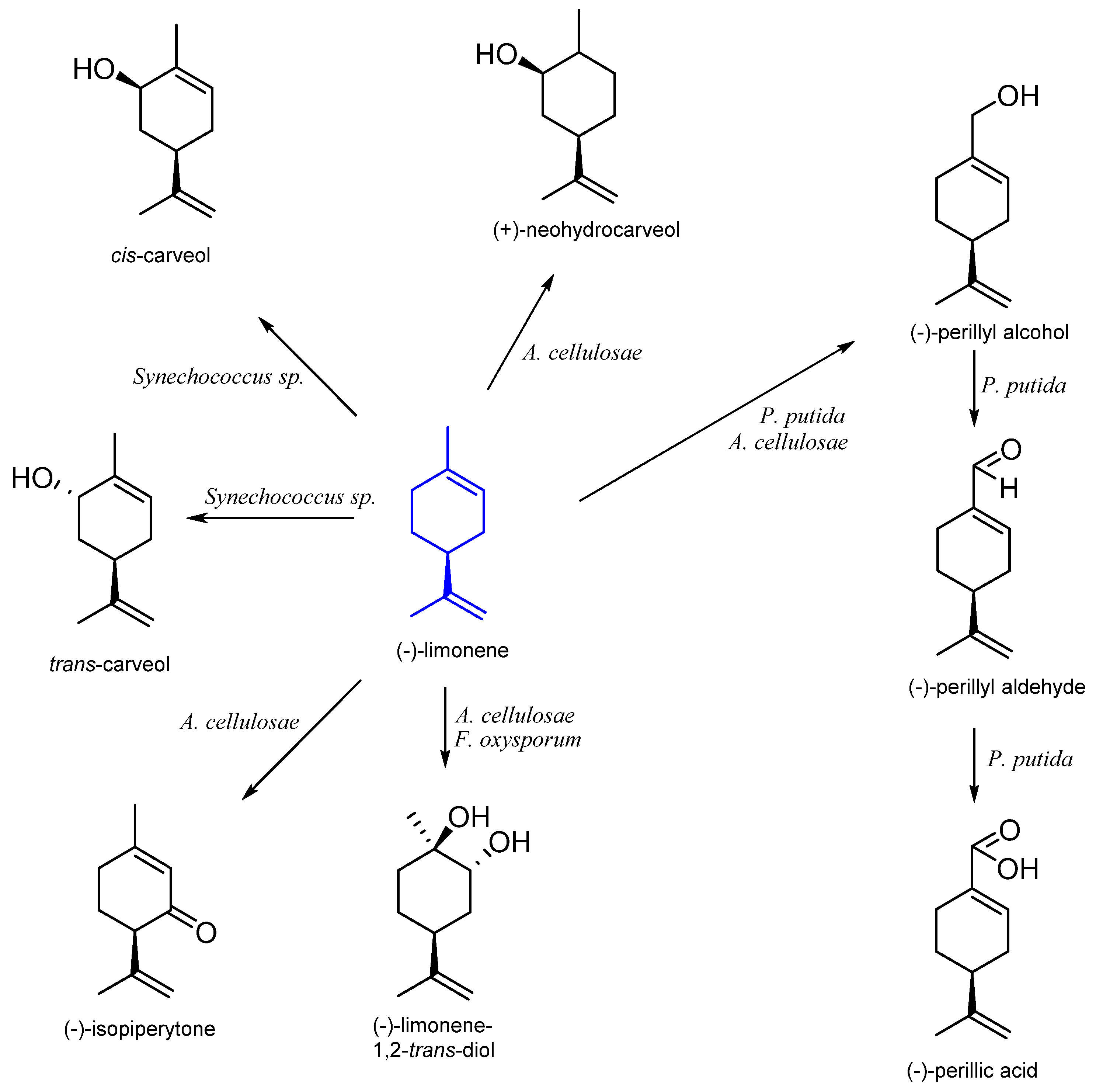
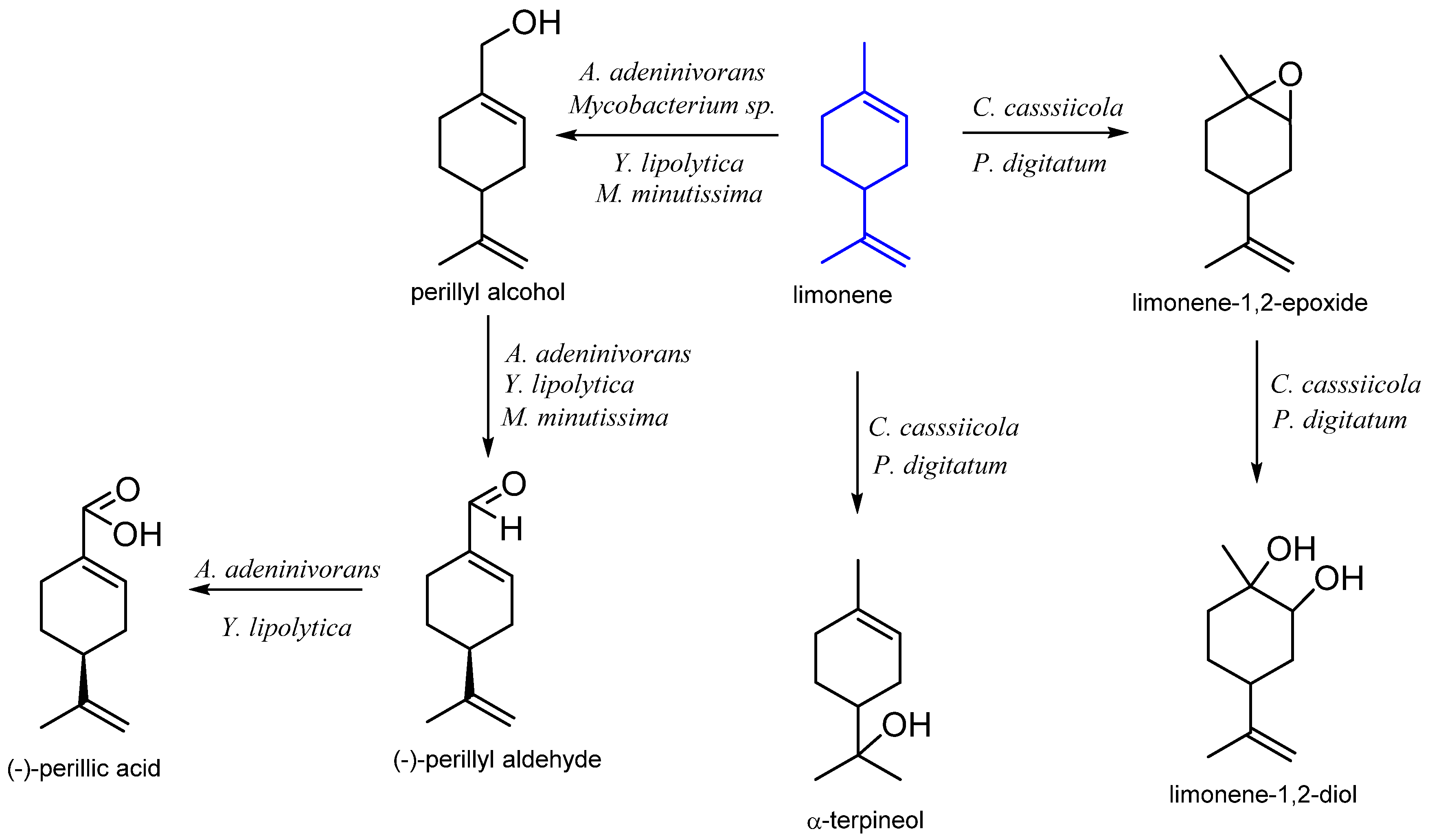
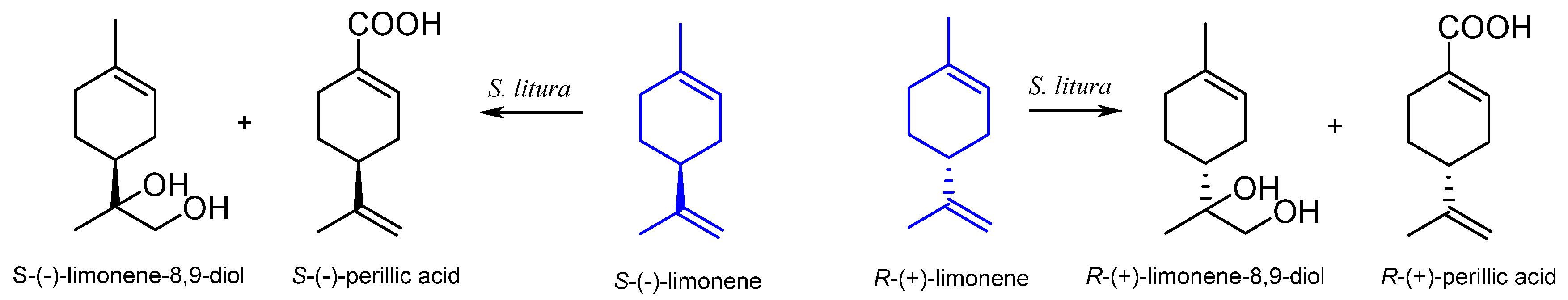




| Stereoisomer | Organism | Products | Reference |
|---|---|---|---|
| (R)-(+)- | Pseudomonas putida DSM 12264 | (R)-(+)-perillic acid | [25] |
| Trichosporon sp. UOFS Y-2041 | isopiperitone (+)-limonene-1,2-trans-diol | [26] | |
| Trichosporon sp. UOFS Y-0116 | isopiperitone | ||
| Aspergillus cellulosae M-77 | (+)-limonene-1,2-trans-diol (+)-isopiperitenone (+)-perillyl alcohol (+)-cis-carveol | [27] | |
| Diaporthe sp. | limonene-1,2-diol α-terpineol carveol | [28] | |
| Fusarium oxysporum 152B | α-terpineol | [29] | |
| Sphingobium sp. | α-terpineol | [30] | |
| Colletotrichum nymphaeae CBMAI 0864 Rhodococcus erythropolis Grosmannia claviger | limonene-1,2-epoxide limonene-1,2-diol | [31] | |
| Penicillium sp. 2025 Aspergillus sp.2038 Fusarium oxysporum 152B | α-terpineol | [32] | |
| Rhodococcus opacus PWD4 | carveol | [33] | |
| Pleurotus sapidus P 226-1 | carveol carvone | [34] | |
| (S)-(−) | Pseudomonas putida DSM 12264 | (S)-(-)-perillic acid | [25] |
| Aspergillus cellulosae M-77 | (−)-limonene-1,2-trans-diol (−)-isopiperitenone (−)-perillyl alcohol (+)-neodihydrocarveol | [27] | |
| Fusarium oxysporum 152B | limonene-1,2-epoxide limonene-1,2-diol | [29] | |
| Synechococcus sp. PCC 7942 | carveol | [35] | |
| n. d. | Mycobacterium sp. HXN-1500 | perillyl alcohol | [36] |
| Yarrowia lipolytica ATCC 18942 | perillic acid | [37] | |
| Arxula adeninivorans CSIR Y-1149 Yarrowia lipolytica CBS 599 T | perillic acid | [26] | |
| Mortierella minutissima 01 | perillyl alcohol perillyl aldehyde | [38] | |
| Penicillium digitatum Corynespora casssicola | α-terpineol limonene-1,2-diol | [39] | |
| Spodoptera litura | limonene-8,9-diol perillic acid | [40] |
| Organism | Products | Reference |
|---|---|---|
| Corynespora cassiicola DSM 62475 | 3-p-menthene-l,2-diol 2-oxo-3-p-menthenol 3-p-menthene-1,2-diol | [42,43] |
| Spodoptera litura | 4-isopropyl-1,3-cyclohexadienemethanol 4-isopropyl-1,3-cyclohexadienoic acid cumic alcohol cumic acid | [44] |
| Organism | Products | Reference |
|---|---|---|
| Corynespora cassiicola DSM 62475 | 4-p-menthene-1,2-diol | [42] |
| Stemphylium botryosum | p-mentha-1,4-dien-9-ol p-cymen-9-ol | [46] |
| S. botryosum DSMZ 62928 | p-mentha-1,4-dien-9-ol | |
| Spodoptera litura | p-mentha-1,4-dien-7-oic acid p-cymen-7-oic acid | [47] |
| Organism | Products | Reference |
|---|---|---|
| Spodoptera litura | p-menth-1-ene-4,7-diol | [47] |
| Stereoisomer | Organism | Products | Reference |
|---|---|---|---|
| n. d. | Armillariella mellea | p-menthene-6,8-diol p-menthene-1,2,8-triol | [42,48] |
| (S)-(−) | Gibberella cyanea DSM 62719 | p-menthene-7,8-diol | [42] |
| n. d. | Spodoptera litura | p-menth-1-ene-7,8-diol 8-hydroxy-p-menth-1-en-7-oic acid | [49,50] |
| Stereoisomer | Organism | Products | Reference |
|---|---|---|---|
| (1R,3S,4R)-(−)- | Aspergillus niger | (−)-1-hydroxymenthol (−)-6-hydroxymenthol (−)-8-hydroxymenthol (−)-9-hydroxymenthol | [51] |
| Cephalosporium aphidicola | (−)-6-hydroxymenthol (−)-7-hydroxymenthol (−)-8-hydroxymenthol (−)-9-hydroxymenthol | [52] | |
| Rhizoctonia solani | (−)-1-hydroxymenthol (−)-6-hydroxymenthol (−)-6,8-dihydroxymenthol | [53] | |
| Spodoptera litura | (−)-7-hydroxymenthol | [54] | |
| (1S,3R,4S)-(+)- | Aspergillus niger | (+)-1-hydroxymenthol (+)-6-hydroxymenthol (+)-7-hydroxymenthol | [51] |
| Macrophomina phaseolin | (+)-1-hydroxymenthol (+)-6-hydroxymenthol (+)-8-hydroxymenthol (+)-9-hydroxymenthol (+)-1,8-dihydroxymenthol (+)-2,8-dihydroxymenthol (+)-6,8-dihydroxymenthol (+)-7,8-dihydroxymenthol (+)-8,9-dihydroxymenthol | [55] | |
| Spodoptera litura | (+)-7-hydroxymenthol | [54] | |
| n. d. | Chlorella vulgaris | dihydroterpineol isomenthol cis-p-menth-1-en-3-ol | [56] |
| Stereoisomer | Organism | Products | Reference |
|---|---|---|---|
| (1S,4R)-(+)- | Acinetobacter NCIEI 9871Acinetobacter TD63 | (4S,7R)-(+)-mentholactone | [57] |
| Spodoptera litura | 7-hydroxymenthone 7-hydroxyneomenthol p-menth-3-on-9-oic acid | [58] | |
| (1R,4S)-(−)- | Hormonema sp. UOFS Y-0067 | neomenthol | [59] |
| Chlorella minutissima Nannochloris atomus Dunaliella parva Porphyridium purpureum Isochrysis galbana | menthol | [60] | |
| Oocystis pusilla | [61] | ||
| Chlorella vulgaris MCCS 012 | [62] | ||
| Synechococcus PCC 6716 | [63] | ||
| Spodoptera litura | 7-hydroxymenthone 7-hydroxyneomenthol p-menth-3-on-9-oic acid | [58] |
| Stereoisomer | Organism | Products | Reference |
|---|---|---|---|
| (S)-(+) | Pseudomonas ovalis | dihydrocarvone | [65] |
| Staphylococcus epidermidis | |||
| Nocardia alba | |||
| Pseudomonas oleovorans | |||
| Trichodema pseudokoningii | carveol neoiso-dihydrocarveol | ||
| Fusarium sulphureum | isodihydrocarvone isodihydrocarveol neoiso-dihydrocarveol | [66] | |
| F. solani var. coeruleum | |||
| Diplogelasinospora grovesii IMI 171018 | carveol | [67] | |
| Mucor circinelloides | dihydrocarvone dihydrocarveol | [68] | |
| Spodoptera litura | (4S)-(+)-10-hydroxycarvone (4S)-(+)-7-hydroxycarvone 8,9-dihydroxy-8,9-dihydrocarvone | [58] | |
| (R)-(−) | Mucor circinelloides | dihydrocarvone dihydrocarveol p-menthane-2,8,9-triol | [68] |
| Lasiodiplodia theobromae BRF118 | (1R,2S,4R,8S)-p-menthane-2,8,9-triol | ||
| Trichoderma harzianum BRF117 | neodihydrocarveol | ||
| Diplogelasinospora grovesii IMI 171018 Gongronella butleri CBS 157.25 Schizosaccharomyces octosporus NCYC 427 | dihydrocarvone dihydrocarveol | [69] | |
| Trichosporum cutaneum CCT 1903 | dihydrocarveol epoxydihydrocarveol 6-isopropenyl-3-methyl-2-oxo-oxepanone isopropenyl-6-oxoheptanoic acid | [70] | |
| Acinetobacter sp. NCIB 9871 Acinetobacter sp. TD63 | 6-isopropenyl-3-methyl-2-oxo-oxepanone | [57] | |
| Spodoptera litura | 7-hydroxycarvone 10-hydroxycarvone 10-hydroxycarveol 8,9-dihydroxy-8,9-dihydrocarvone | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarczyk, M.; Mączka, W.; Żołnierczyk, A.K.; Wińska, K. Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects. Molecules 2020, 25, 4840. https://doi.org/10.3390/molecules25204840
Grabarczyk M, Mączka W, Żołnierczyk AK, Wińska K. Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects. Molecules. 2020; 25(20):4840. https://doi.org/10.3390/molecules25204840
Chicago/Turabian StyleGrabarczyk, Małgorzata, Wanda Mączka, Anna K. Żołnierczyk, and Katarzyna Wińska. 2020. "Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects" Molecules 25, no. 20: 4840. https://doi.org/10.3390/molecules25204840
APA StyleGrabarczyk, M., Mączka, W., Żołnierczyk, A. K., & Wińska, K. (2020). Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects. Molecules, 25(20), 4840. https://doi.org/10.3390/molecules25204840






