Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development
Abstract
1. Introduction
1.1. Motivation
1.2. Basic Cannabis Chemotaxonomy
1.3. Brief Introduction to the Endocannabinoid System
1.4. Cannabinergic Compounds
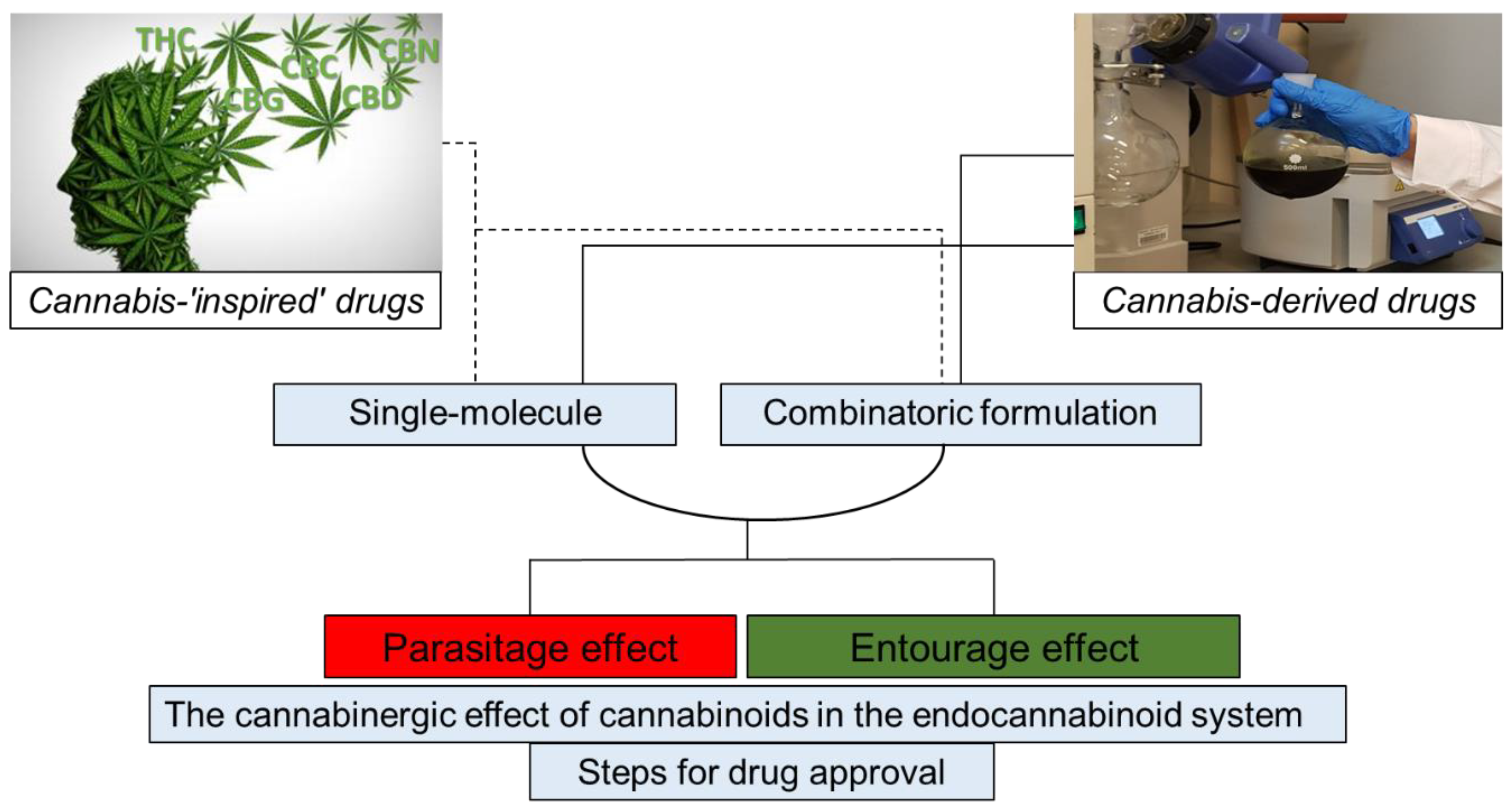
2. Chronology of Cannabis-Based Drug Development
2.1. Drugs of One Molecule
2.1.1. Cannabis-“Inspired” Drugs
2.1.2. Cannabis-Derived Drugs
2.2. Drugs of Combinatoric Formulations
3. Taking Advantage of the “Entourage Effect”
4. Other “Entourage” Considerations
4.1. The “Parasitage Effect”
4.2. Interaction between Endocannabinoids and Cannabinoids (Synthetic or Plant-Derived)
4.3. Degradation and Loss of Activation
5. Drug–Drug Interactions
6. Registration Drugs: One Molecule or Combinatoric Formulations
7. Conclusions and Future Prospects
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Small, E.; Beckstead, H.D. Cannabinoid Phenotypes in Cannabis sativa. Nat. Cell Biol. 1973, 245, 147–148. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB 1 and CB 2 Receptor Pharmacology. In Studies in Surface Science and Catalysis; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 80, pp. 169–206. [Google Scholar]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; de Medina, V.S. Pharmacological data of cannabidiol-and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Goutopoulos, A.; Makriyannis, A. From cannabis to cannabinergics: New therapeutic opportunities. Pharmacol. Ther. 2002, 95, 103–117. [Google Scholar] [CrossRef]
- Lu, D.; Meng, Z.; Thakur, G.A.; Fan, P.; Steed, J.; Tartal, C.L.; Hurst, D.P.; Reggio, P.H.; Deschamps, J.R.; Parrish, D.A.; et al. Adamantyl Cannabinoids: A Novel Class of Cannabinergic Ligands. J. Med. Chem. 2005, 48, 4576–4585. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Gomes, F.V.; Fonseca, M.D.; Mechoulam, R.; Breuer, A.; Cunha, T.M.; Guimarães, F.S. Antinociceptive effects of HUF-101, a fluorinated cannabidiol derivative. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 79, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Nikas, S.P.; Sharma, R.; Jiang, S.; Paronis, C.A.; Leonard, M.Z.; Zhang, B.; Honrao, C.; Mallipeddi, S.; Raghav, J.G.; et al. Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues. J. Med. Chem. 2016, 59, 6903–6919. [Google Scholar] [CrossRef]
- Aggarwal, S.K. Cannabinergic Pain Medicine. Clin. J. Pain 2013, 29, 162–171. [Google Scholar] [CrossRef]
- Lu, D.; Vemuri, V.K.; Duclos, R.I.; Makriyannis, A. The Cannabinergic System as a Target for Anti-inflammatory Therapies. Curr. Top. Med. Chem. 2006, 6, 1401–1426. [Google Scholar] [CrossRef]
- Felberbaum, M. FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms (accessed on 25 June 2018).
- Sekar, K.; Pack, A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Res 2019, 8, 234. [Google Scholar] [CrossRef]
- O’Keefe, L. FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare (accessed on 31 July 2020).
- Mannucci, C.; Navarra, M.; Calapai, F.; Spagnolo, E.V.; Busardò, F.P.; Cas, R.D.; Ippolito, F.M.; Calapai, G. Neurological aspects of medical use of cannabidiol. CNS Neurol. Disord. Drug Targets 2017, 16, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Marinol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018651s021lbl.pdf (accessed on 15 September 2004).
- EPIDIOLEX® (cannabidiol) oral solution, CX pending DEA scheduling action. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (accessed on 10 June 2018).
- SYNDROS (dronabinol) oral solution, CII. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205525s003lbl.pdf (accessed on 15 May 2017).
- CESAMET™ (nabilone). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf (accessed on 20 May 2006).
- Carvalho, J. Phase 1 Trial of EB Cannabinol Cream Completes Treatment in Healthy Volunteers. Available online: https://epidermolysisbullosanews.com/2020/04/09/phase-1-trial-of-eb-cannabinol-cream-completes-treatment-in-healthy-volunteers (accessed on 9 April 2020).
- Sativex Oromucosal Spray. Available online: https://www.medicines.org.uk/emc/product/602/smpc (accessed on 27 May 2020).
- Keating, G.M. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (Sativex®): A review in multiple sclerosis-related spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Trainor, D.; Evans, L.; Bird, R. Severe motor and vocal tics controlled with Sativex®. Australas Psychiatry 2016, 24, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Litscher, G.; Gao, S.H.; Zhou, S.F.; Yu, Z.L.; Chen, H.Q.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ko, K.M. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evid. Based Complement Alternat. Med. 2014, 2014, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S. Whole organisms or pure compounds? Entourage effect versus drug specificity. In Plant Medicines, Healing and Psychedelic Science; Springer Nature: Cham, Switzerland, 2018; pp. 133–149. [Google Scholar]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Gilbert, B.; Alves, L. Synergy in plant medicines. Curr. Med. Chem. 2003, 10, 13–20. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S. From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: The ongoing story of cannabis. Nat. Prod. Rep. 1999, 16, 131–143. [Google Scholar] [CrossRef]
- Koltai, H.; Namdar, D. Cannabis Phytomolecule ’Entourage’: From Domestication to Medical Use. Trends Plant Sci. 2020, 25, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mazuz, M.; Tiroler, A.; Moyal, L.; Hodak, E.; Nadarajan, S.; Vinayaka, A.C.; Gorovitz-Haris, B.; Lubin, I.; Drori, A.; Drori, G.; et al. Synergistic cytotoxic activity of cannabinoids from Cannabis sativa against cutaneous T-cell lymphoma (CTCL) in-vitro and ex-vivo. Oncotarget 2020, 11, 1141–1156. [Google Scholar] [CrossRef]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef] [PubMed]
- Nallathambi, R.; Mazuz, M.; Ion, A.; Selvaraj, G.; Weininger, S.; Fridlender, M.; Ahmad, N.; Sagee, O.; Kumari, P.; Nemichenizer, D.; et al. Anti-inflammatory activity in colon models is derived from δ9-tetrahydrocannabinolic acid that interacts with additional compounds in cannabis extracts. Cannabis Cannabinoid Res. 2017, 2, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Poulin, P.; Namdar, D. Promoting cannabis products to pharmaceutical drugs. Eur. J. Pharm. Sci. 2019, 132, 118–120. [Google Scholar] [CrossRef]
- Szarka, L. Geophysical aspects of man-made electromagnetic noise in the earth—A review. Surv. Geophys. 1988, 9, 287–318. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Corroon, J.; Felice, J.F. The endocannabinoid system and its modulation by cannabidiol (CBD). Altern. Ther. Health Med. 2019, 25, 6–14. [Google Scholar] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Valenti, M.; Vaccani, A.; Gasperi, V.; Perletti, G.; Marras, E.; Fezza, F.; Maccarrone, M.; Parolaro, D. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008, 104, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab Med. 2018, 56, 94–96. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Velez, L.I.; O’Connell, E.; Rice, J.; Benitez, F.; LoVecchio, F. Adverse reactions to cannabis and cannabinoids. Emerg. Med. Rep. 2018, 39, 20. [Google Scholar]
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Lovegrove, M.C.; Wolpert, B.J.; Timbo, B.B.; Mozersky, R.P.; Budnitz, D.S. Emergency department visits for adverse events related to dietary supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef]
- Bushra, A.; Aslam, N.; Khan, A.Y. Food-Drug Interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N. Inspired by Nature: Modern Medicines Derived from Cannabis. Front. Nat. Prod. Chem. 2018, 4, 119–155. [Google Scholar]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, T.; Bodkin, J.; Ho, J.M.-W. Drug interactions with cannabinoids. Can. Med Assoc. J. 2020, 192, E206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug–Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef]
- Alsherbiny, M.; Li, C.G. Medicinal Cannabis—Potential Drug Interactions. Medicines 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.R.; Weigle, A.T.; Das, A. Cross-talk of cannabinoid and endocannabinoid metabolism is mediated via human cardiac CYP2J2. J. Inorg. Biochem. 2018, 184, 88–99. [Google Scholar] [CrossRef]
- Bettiol, A.; Lombardi, N.; Crescioli, G.; Maggini, V.; Gallo, E.; Mugelli, A.; Firenzuoli, F.; Baronti, R.; Vannacci, A. Galenic Preparations of Therapeutic Cannabis sativa Differ in Cannabinoids Concentration: A Quantitative Analysis of Variability and Possible Clinical Implications. Front. Pharmacol. 2019, 9. [Google Scholar] [CrossRef]
- Guidance for Industry and FDA Staff: Current Good Manufacturing Practice Requirements for Combination Products. Final guidance, January 2017, U.S. Department of Health and Human Services, Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/current-good-manufacturing-practice-requirements-combination-products (accessed on 18 January 2017).
- FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). Available online: https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd (accessed on 10 January 2020).
- The drug review and approval process in Canada. An e-guide. Available online: https://spharm-inc.com/the-drug-review-and-approval-process-in-canada-an-eguide/ (accessed on 10 January 2020).
| Drug Name | Indication | Basic Formulation | Clinical Studies | Proved Efficacy | Key Limiting Toxicity | Recommended Dosage | Drug–Drug Interactions Reported | Approval | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Drugs of one molecule | |||||||||
| MARINOL® (GW Pharmaceuticals, Cambridge, UK) | Appetite stimulation; antiemetic associated with cancer chemotherapy | Dronabinol (synthetic THC) | Yes | Yes | 100 mg/day or 30 mg/kg | Appetite stimulation: 2.5 mg twice daily; antiemetic: 5 mg 3–4 times daily | FDA | [16] | |
| EPIDIOLEX® (GW Pharmaceuticals) | Lennox–Gastaut syndrome and Dravet syndrome in patients | Plant derived CBD | Yes | Yes | 20 mg/kg/day | 5–20 mg/kg/day | CYP1A2, CYP2B6 substrates, uridine 5’ diphospho-glucuronosyltransferase 1A9 (UGT1A9) and UGT2B7. CYP2C8 and CYP2C9 substrates | FDA | [17] |
| SYNDROS® (Benuvia Therapeutics Inc, Chandler, AZ, USA) | Anorexia; nausea and vomiting associated with cancer chemotherapy | Dronabinol (synthetic THC) | Yes | Yes | 25 mg/day | 4.2 mg/day | Neuropsychiatric adverse reactions; hemodynamic instability | FDA | [18] |
| CESAMET® (Valeant Pharma Int, Laval, QC, Canada) | Nausea and vomiting induced by cancer chemotherapy | Nabilone (synthetic THC) | Yes | 2 mg/day | Diazepam 5 mg; sodium secobarbital 100 mg; alcohol (absolute) 45 mL; codeine 65 mg | FDA | [19] | ||
| INM-755 cream (Inmed pharma, Vancouver, BC, Canada) | Skin diseases and wounds; epidermolysis bullosa | Bacteria Escherichia coli fermentation derived from one rare CBN (Cannabinol) | Yes Phase 1–2 ongoing | No | Two doses of INM-755 cream are currently being tested | [20] | |||
| Drugs of combinatoric formulations | |||||||||
| SATIVEX® Oromucosal spray (GW Pharmaceuticals) | Pain relief | Plant-derived CBD:THC and terpenes | Yes | Yes | 90 mg/day | 5–60 mg/day | Reversible inhibitor of CYP3A4, 1A2, 2B6, 2C9, and 2C19 | EU, Canada | [21] |
| Stage | Activities |
|---|---|
| 1. Initial drug research | Discovering and identifying various chemical and biological substances or other products on the way toward developing a drug; testing for activity, efficacy, toxicity, and ultimately gathering preliminary information on its effectiveness and safety. If the results are promising, researchers proceed to the next stage of development. |
| 2. Preclinical studies | Administration of the drug to selected species of animals (in vivo) or cells (in vitro). The drug must be shown to cause no serious harm (toxicity) at the doses required to have an effect either in a single compound or in DDIs. If results from these initial studies are promising and further tests show acceptable safety levels and clear or potential efficacy, then the next step would be to submit a clinical trial application. |
| 3. Clinical trials | The results of clinical trials conducted with humans are key components of the review process by the regulatory agency. The purpose of a trial is to gather clinical information about a drug’s effectiveness and safety, determine best dosing/usage in humans, evaluate any adverse drug reactions and DDIs, and compare results to already existing treatments for the same disease or condition or to placebo when no treatment already exists for the aimed pathology (when ethically possible). The information gathered from these trials is then included in the dossiers to be reviewed by the relevant agencies. |
| 4. Drug approval process | If results of all the preclinical studies and the clinical trials show that a drug’s potential therapeutic benefit outweighs its risks (side effects, toxicity, etc.), and the chemistry and manufacturing dossier is complete, then the sponsor may decide to file a new drug submission (NDS) with the appropriate regulatory agency in order to be granted authorization to sell the drug in the country. |
| 5. After approval | The regulatory agency requires a sponsor to ensure that the use of its drug is done under the terms of its market authorization. In addition, life cycle management activities (post-approval submissions, for new indications, new dosage forms, new strengths, manufacturing changes, etc.) are required to ensure the maintenance of the product license with its related improvements. In summary, sponsors need to ensure its continued compliance with the food and drug regulations while their products are on the market. On the other hand, the regulatory agency monitors drug information and adverse drug reaction reporting, conducts market surveillance, investigates complaints, and manages recalls if necessary, amongst other things. |
| 6. Additional regulations | There are also more processes and regulations to follow and consider before, during, or after the review process, and before the drug is officially marketed, distributed, and sold in a country. Topics included licensing, warehousing, wholesale distribution rules, and the Drug Establishment License (DEL), as well as regulations around distribution to consumers, regulations around the marketing and advertising activities, provincial requirements, and health insurance funding rules, among others. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namdar, D.; Anis, O.; Poulin, P.; Koltai, H. Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules 2020, 25, 4821. https://doi.org/10.3390/molecules25204821
Namdar D, Anis O, Poulin P, Koltai H. Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules. 2020; 25(20):4821. https://doi.org/10.3390/molecules25204821
Chicago/Turabian StyleNamdar, Dvora, Omer Anis, Patrick Poulin, and Hinanit Koltai. 2020. "Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development" Molecules 25, no. 20: 4821. https://doi.org/10.3390/molecules25204821
APA StyleNamdar, D., Anis, O., Poulin, P., & Koltai, H. (2020). Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules, 25(20), 4821. https://doi.org/10.3390/molecules25204821








