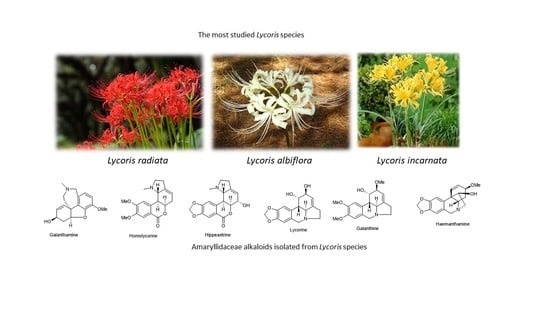
Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae)
Abstract
1. Introduction
2. Genus Lycoris: Occurrence, Ethnobotany
3. Phytochemistry of the Genus Lycoris
| L. albiflora | L. aurea | L. caldwelii | L. chinensis | L. guangxiensis | L. haywardii | L. incarnata | L. longituba | L. radiata | L. radiata var. pumila | L. sanguinea | L. sprengeri | L. squamigera | L. traubii | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belladine-type | ||||||||||||||
| 2R-Hydroxy-N,O-dimethylnorbelladine (1) | [34] | |||||||||||||
| Crinine-type | ||||||||||||||
| Amabiline (2) | [28] | |||||||||||||
| Ambelline (3) | [32] | [32] | ||||||||||||
| Crinine (4) | [35] | |||||||||||||
| Crinamabine (5) | [32] | [32] | ||||||||||||
| Crinamidine (6) | [32] | [32] | [32] | |||||||||||
| Galanthamine-type | ||||||||||||||
| 1,2,11,12-Tetradehydrogalanthamine * (7) | [32] | |||||||||||||
| 11β-Hydroxygalanthamine (8) | [30] | [28] | ||||||||||||
| N-Norgalanthamine/N-Demethylgalanthamine (9) | [31,32] | [32,35] | [30] | [32] | [34] | |||||||||
| N-Allylnorgalanthamine (10) | [35] | [28] | ||||||||||||
| Galanthamine N-oxide (11) | [36] | [37,38] | ||||||||||||
| N-(Chloromethyl)galanthamine (12) | [31] | [30] | ||||||||||||
| Galanthamine (13) | [39,40,41] | [32,41,42] | [41,43] | [32,35] | [41] | [36,41,42] | [30,41] | [13,28,32,37,38,41,42] | [41] | [41,44] | [34,41] | [45] | ||
| O-Demethyllycoramine-N-oxide (14) | [37] | |||||||||||||
| O-Demethyllycoramine (15) | [36] | [30,41] | [37,38,46] | [34] | ||||||||||
| N-(Chloromethyl)lycoramine (16) | [31] | [30] | ||||||||||||
| Lycoramine-N-oxide (17) | [39] | [32] | [32,37,38] | |||||||||||
| Lycoramine (18) | [39,41] | [32,41] | [41,43] | [32,35] | [41] | [36,41] | [41] | [32,37,38,41] | [41] | [41,44] | [34,41] | [45] | ||
| Norlycoramine (19) | [41] | [41] | [41] | [41] | [41] | [41] | [41] | |||||||
| Narwedine (20) | [41] | [32] | [32,35] | [41] | [41] | |||||||||
| Sanguinine (21) | [41] | [41] | [36] | [30,41] | [37,46] | [34] | ||||||||
| Galanthindole-type | ||||||||||||||
| Lycosinine B (22) | [44] | |||||||||||||
| Haemanthamine-type | ||||||||||||||
| 3α-Hydroxy-6β-acetylbulbispermine (23) | [47] | |||||||||||||
| 3α-Methoxy-6β-acetylbulbispermine (24) | [47] | |||||||||||||
| 3α,6β-Diacetylbulbispermine (25) | [47] | |||||||||||||
| 6β-Acetyl-8-hydroxy-9-methoxycrinamine (26) | [48] | |||||||||||||
| 6-Hydroxycrinamine (27) | [13] | |||||||||||||
| 6β-Acetoxycrinamine (28) | [13,48] | |||||||||||||
| Haemanthamine (29) | [39] | [32,41] | [41] | [41] | [32,41] | [41,44] | [34] | |||||||
| Haemanthidine (30) | [39] | [42] | [30] | [38] | [44] | [34,41] | ||||||||
| 8-O-Demethylmaritidine (31) | [32,46] | |||||||||||||
| 11-Hydroxyvittatine-N-oxide (32) | [32] | |||||||||||||
| 11-Hydroxyvittatine (33) | [46] | [34] | ||||||||||||
| Vittatine (34) | [31,32] | [32] | [32,38] | |||||||||||
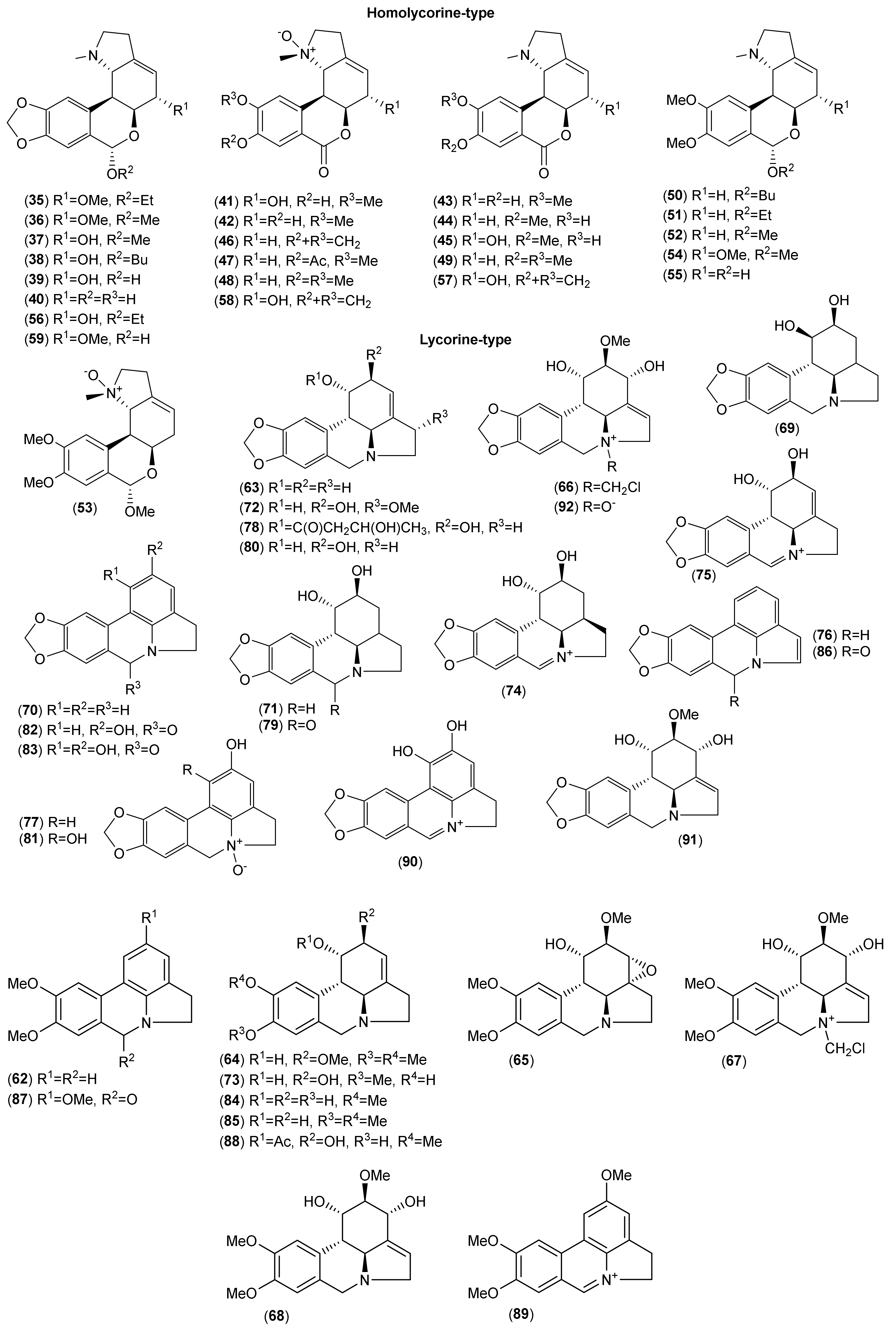
| Homolycorine-type | ||||||||||||||
| 2α-Methoxy-6-O-ethyloduline (35) | [14,37] | |||||||||||||
| 2α-Methoxy-6-O-methyloduline (36) | [49] | [32] | [14] | |||||||||||
| 2α-Hydroxy-6-O-methyloduline (37) | [39] | [31,32,49] | [13,32,37] | |||||||||||
| 2α-Hydroxy-6-O-n-butyloduline (38) | [31] | |||||||||||||
| 2α-Hydroxyoduline (39) | [49] | |||||||||||||
| Oduline (40) | [32,49] | [32] | [14,32] | |||||||||||
| 2α-Hydroxy-8-O-demethylhomolycorine-N-oxide (41) | [48] | |||||||||||||
| 8-O-Demethylhomolycorine-N-oxide (42) | [50] | [48] | ||||||||||||
| 8-O-Demethylhomolycorine (43) | [41] | [14,38,41] | [41] | |||||||||||
| 9-O-Demethylhomolycorine (44) | [39] | [14,37] | ||||||||||||
| 9-O-Demethyl-2α-hydroxyhomolycorine (45) | [37] | |||||||||||||
| 8,9-Methylenedioxyhomolycorine-N-oxide (46) | [32,47] | |||||||||||||
| 8-O-Acetylhomolycorine-N-oxide (47) | [13] | |||||||||||||
| Homolycorine-N-oxide (48) | [39] | [38,47] | ||||||||||||
| Homolycorine (49) | [39,40] | [31,49] | [41] | [13,14,38,41,42] | [41] | [44] | ||||||||
| O-n-Butyllycorenine (50) | [31] | |||||||||||||
| O-Ethyllycorenine (51) | [14,37] | |||||||||||||
| O-Methyllycorenine (52) | [31] | [14,37,38,41] | [44] | |||||||||||
| O-Methyllycorenine-N-oxide (53) | [38] | |||||||||||||
| 2α-Methoxy-6-O-methyllycorenine (54) | [44] | |||||||||||||
| Lycorenine (55) | [40] | [14,42] | [44] | |||||||||||
| Radiatine (56) | [37] | |||||||||||||
| Hippeastrine (57) | [39] | [31,32,49] | [13,14,32,37,38,41] | |||||||||||
| Hippeastrine-N-oxide (58) | [39] | [38] | ||||||||||||
| Unsevine (59) | [32] | |||||||||||||
| Hostasinine-type | ||||||||||||||
| Hostasinine A (60) | [39] | |||||||||||||
| Ismine-type | ||||||||||||||
| Ismine (61) | [34] | |||||||||||||
| Lycorine-type | ||||||||||||||
| Assoanine (62) | [41] | [41] | ||||||||||||
| Caranine (63) | [41] | [41] | [41] | [41] | [41] | [41] | [41] | [41] | ||||||
| Galanthine (64) | [41] | [41] | [41] | [41] | [36,41] | [41] | [46] | [41,44] | [41] | |||||
| Incartine (65) | [41] | [41] | [41] | [41] | [36,41] | [30] | [41] | [41] | ||||||
| N-(Chloromethyl)ungiminorine (66) | [37] | |||||||||||||
| N-(Chloromethyl)narcissidine (67) | [30] | [44] | ||||||||||||
| Narcissidine (68) | [44] | |||||||||||||
| (−)-epi-Zephyranthine (69) | [37] | |||||||||||||
| Anhydrolycorine (70) | [41] | [41] | [41] | [41] | [41] | [41] | [41] | [41] | [41] | |||||
| Dihydrolycorine (71) | [32] | [32] | [13,37] | |||||||||||
| 11-Methoxylycorine (72) | [37] | |||||||||||||
| Pseudolycorine (73) | [35] | [37,51] | [34] | |||||||||||
| 5,6-Dehydrodihydrolycorine (74) | [41] | [13] | ||||||||||||
| 5,6-Dehydrolycorine (75) | [32,47] | |||||||||||||
| 11,12-Didehydroanhydrolycorine (76) | [41] | [41] | [41] | [41] | [41] | [41] | [41] | [41] | [41] | |||||
| 2-Hydroxyanhydrolycorine-N-oxide (77) | [52] | |||||||||||||
| 1-O-(3′-Hydroxybutanoyl)lycorine (78) | [45] | |||||||||||||
| 6-Oxodihydrolycorine (79) | [13] | |||||||||||||
| Lycorine (80) | [39,40,41] | [31,32,41,42] | [41,43] | [32,35] | [41] | [36,41,42] | [30,41] | [13,28,32,38,41,42] | [41] | [41,44] | [34,41] | [45] | ||
| 1,2-Dihydroxy-anhydrolycorine-N-oxide (81) | [50] | |||||||||||||
| 2-Hydroxy-6-oxoanhydrolycorine (82) | [50] | |||||||||||||
| 1,2-Dihydroxy-6-oxoanhydrolycorine (83) | [50] | |||||||||||||
| Norpluviine (84) | [41] | [41] | ||||||||||||
| Pluviine (85) | [31,32] | [32] | [41] | [41] | [32,37] | [41] | [44] | [41] | ||||||
| Hippadine (86) | [32] | [30] | [37] | [44] | ||||||||||
| Lycosprenine (87) | [44] | |||||||||||||
| Sternbergine (88) | [45] | |||||||||||||
| Tortuosine (89) | [44] | |||||||||||||
| 1-Hydroxyungeremine (90) | [48] | |||||||||||||
| Ungiminorine (91) | [36] | [45] | ||||||||||||
| Ungiminorine-N-oxide (92) | [36] | |||||||||||||
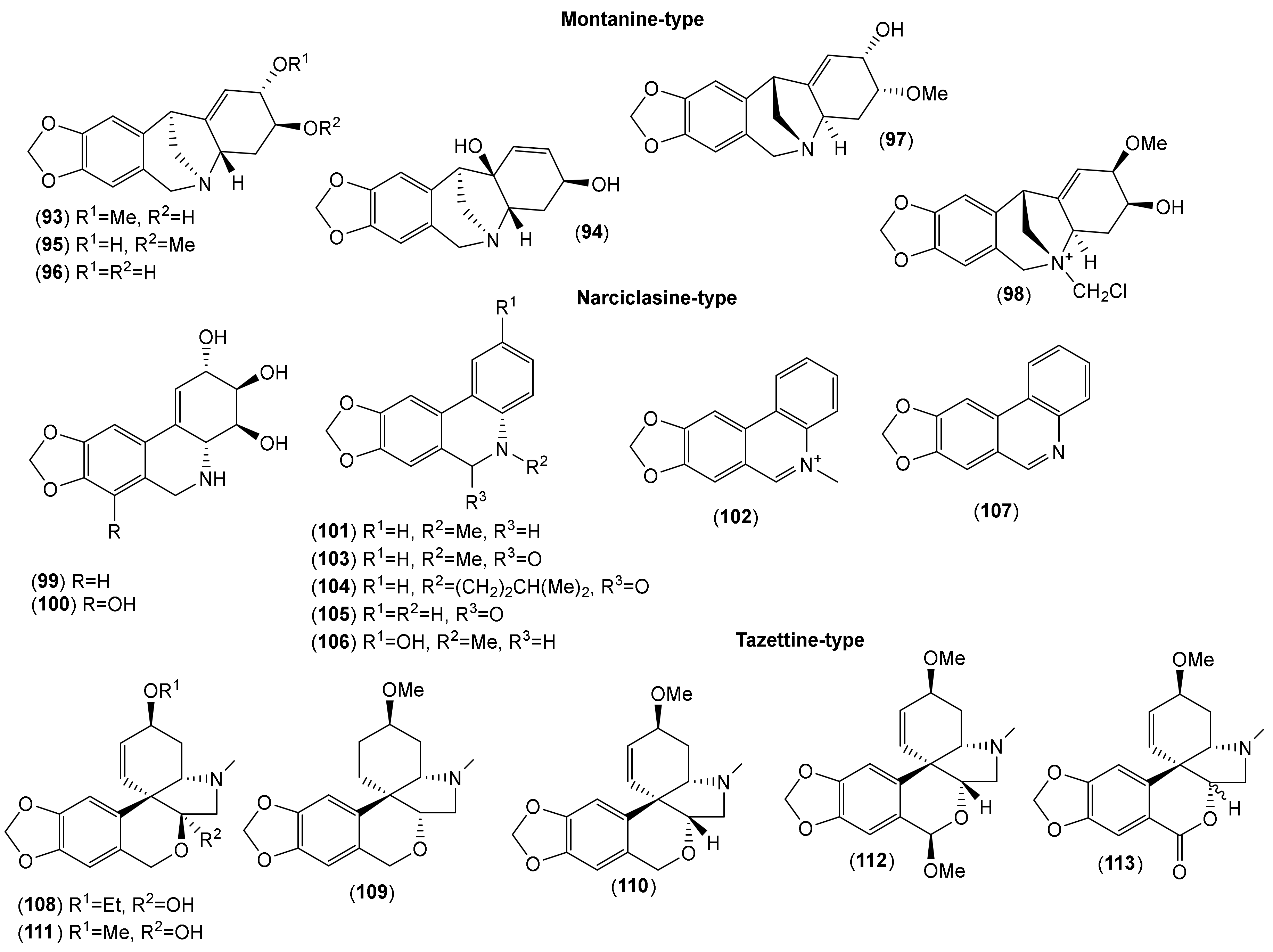
| Montanine-type | ||||||||||||||
| Montanine (93) | [41] | [41] | [41] | [41] | [34,41] | |||||||||
| Pancratinine C/Squamigine (94) | [41] | [37] | [34] | |||||||||||
| (−)-3-O-Menthylpancracine (95) | [37] | |||||||||||||
| Pancracine (96) | [37] | |||||||||||||
| Montabuphine (97) | [44] | |||||||||||||
| Lycolongirine C (98) | [30] | |||||||||||||
| Narciclasine-type | ||||||||||||||
| 7-Deoxynarciclasine/Lycoricidine (99) | [39] | [13] | [53] | [34] | [45] | |||||||||
| Narciclasine/Lycoricidinol (100) | [39] | [16,37] | [53] | [34] | [45] | |||||||||
| 5,6-Dihydrobicolorine (101) | [13] | [44] | ||||||||||||
| Bicolorine (102) | [46] | |||||||||||||
| N-Methylcrinasiadine (103) | [30] | |||||||||||||
| N-Isopentylcrinasiadine (104) | [44] | |||||||||||||
| Crinasiadine (105) | [44] | |||||||||||||
| 5,6-Dihydro-5-methyl-2-hydroxyphenanthridine (106) | [50] | [47] | ||||||||||||
| Trisphaeridine (107) | [30,41] | [46] | [41,44] | |||||||||||
| Tazettine-type | ||||||||||||||
| 3-O-Ethyltazettinol (108) | [54] | |||||||||||||
| Deoxydihydrotazettine (109) | [28] | |||||||||||||
| Deoxypretazettine (110) | [30,41] | [28,41] | ||||||||||||
| Tazettine (111) | [41] | [41] | [41] | [41] | [30,41] | [38,41] | [41] | [41,44] | [34,41] | |||||
| 6-O-Methylpretazettine (112) | [34] | |||||||||||||
| 3-Epimacronine (113) | [28] | |||||||||||||
| 3-Hydroxylatifaliumin C * (114) | [32] | [32] | ||||||||||||
| Dihydrolatifaliumin C * (115) | [32] | [32] | [32] | |||||||||||
| Latifaliumin C * (116) | [32] | |||||||||||||
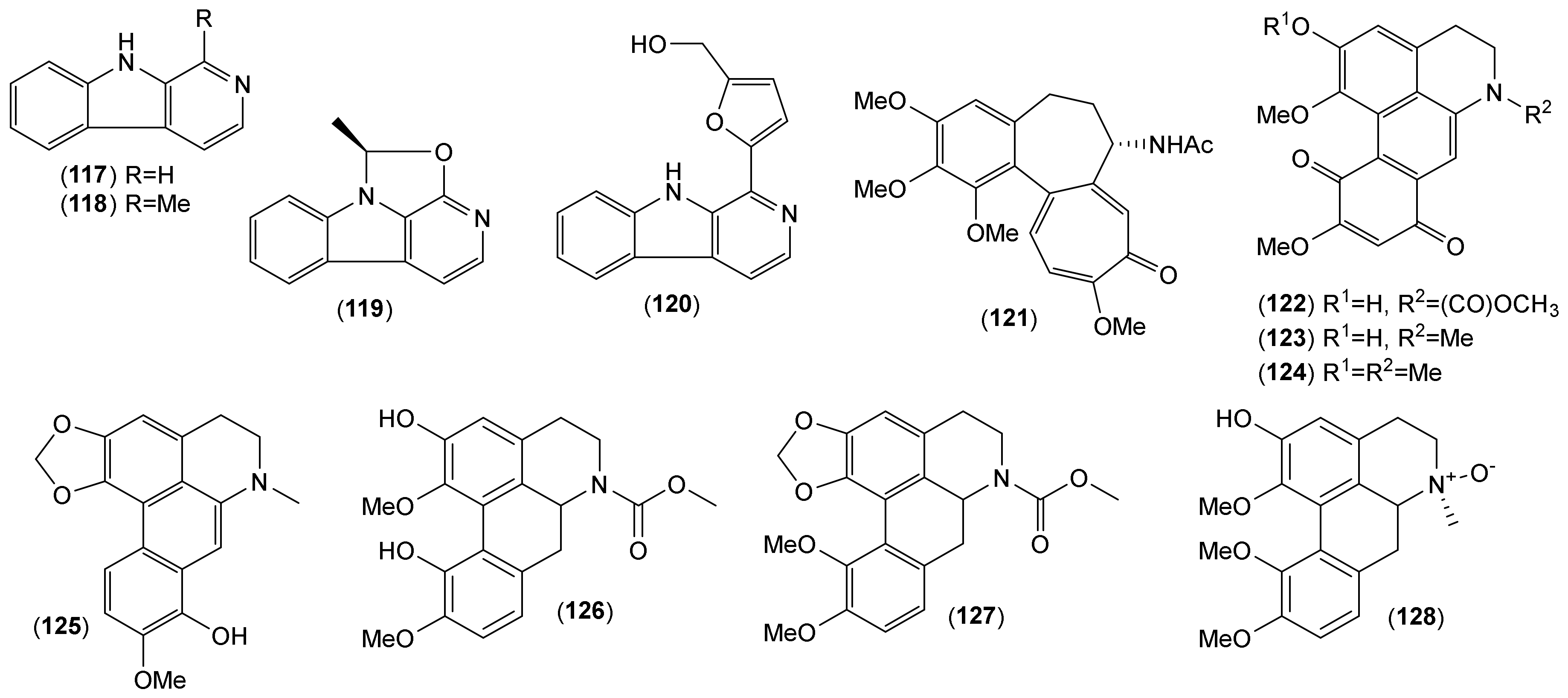
| Other structural types | ||||||||||||||
| Norharmane (117) | [30] | |||||||||||||
| Harmane (118) | [30] | |||||||||||||
| Lycolongirine A (119) | [30] | |||||||||||||
| Perlolyrine (120) | [30] | |||||||||||||
| Colchicine (121) | [28] | |||||||||||||
| N-Methoxycarbonyl-2-demethylisocorydione (122) | [48] | |||||||||||||
| 2-Demethylisocorydione (123) | [50] | |||||||||||||
| Isocorydione (124) | [50] | |||||||||||||
| 8-Demethyldehydrocrebanine (125) | [50] | |||||||||||||
| N-Methoxycarbonyllindcarpine (126) | [52] | |||||||||||||
| N-Methoxycarbonylnandigerine (127) | [52] | |||||||||||||
| 10-O-Methylhernovine-N-oxide (128) | [52] |
4. Studied Biological Activities of Extracts and Alkaloids Isolated from Lycoris Species
4.1. Antitumor Activity
4.2. Biological Activity Connected with Alzheimer’s Disease
4.3. Antimalarial Activity
4.4. Further Studied Biological Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef] [PubMed]
- Fennell, C.; van Staden, J. Crinum species in traditional and modern medicine. J. Ethnopharmacol. 2001, 78, 15–26. [Google Scholar] [CrossRef]
- Havelek, R.; Muthna, D.; Tomsik, P.; Kralovec, K.; Seifrtova, M.; Cahlikova, L.; Hostalkova, A.; Safratova, M.; Perwein, M.; Cermakova, E.; et al. Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem. Biol. Interact. 2017, 275, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Vaněčková, N.; Hošt‘álková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpánková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Marco, L.; do Carmo Carreiras, M. Galanthamine, a natural product for the treatment of Alzheimer’s disease. Recent Pat. CNS Drug Discov. 2006, 1, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.; Ko, S. New taxa of the genus Lycoris. Korean J. Plant Taxon. 1993, 23, 233–241. [Google Scholar] [CrossRef]
- Tae, K.; Ko, S. A taxonomic study on the genus Lycoris (Amaryllidaceae). Korean J. Plant Taxon. 1996, 26, 19–35. [Google Scholar] [CrossRef]
- Ping-Sheng, H.S.U.; Kurita, S.; Zhi-Zhou, Y.U.; Jin-Zhen, L.I.N. Synopsis of the genus Lycoris (Amaryllidaceae). Sida 1994, 16, 301–331. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/search?q=Lycoris (accessed on 10 September 2020).
- Chen, G.-L.; Tian, Y.-Q.; Wu, J.-L.; Li, N.; Guo, M.-Q. Antiproliferative activities of Amaryllidaceae alkaloids from Lycoris radiata targeting DNA topoisomerase I. Sci. Rep. 2016, 6, 38284. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, Y.-Y.; Su, J.; Li, Y.; Cai, X.-H.; Luo, X.-D. Amaryllidaceae alkaloids from Lycoris Radiata. Helv. Chim. Acta 2011, 94, 178–183. [Google Scholar] [CrossRef]
- Huang, S.-D.; Zhang, Y.; He, H.-P.; Li, S.-F.; Tang, G.-H.; Chen, D.-Z.; Cao, M.-M.; Di, Y.-T.; Hao, X.-J. A new Amaryllidaceae alkaloid from the bulbs of Lycoris radiata. Chin. J. Nat. Med. 2013, 11, 406–410. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.Q.; Yin, Z.Q.; Wang, Y.; Ye, W.C. Two new Amaryllidaceae alkaloids from the bulbs of Lycoris Radiata. Chem. Pharm. Bull. 2009, 57, 610–611. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Xu, X.-L.; Yang, L.-J.; Jiang, J.-G. Identification of narciclasine from Lycoris radiata (L’Her.) Herb. and its inhibitory effect on LPS-induced inflammatory responses in macrophages. Food Chem. Toxicol. 2019, 125, 605–613. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2011, 28, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China, 2nd ed.; Reference Publications: Algonac, MI, USA, 1985; p. 398. [Google Scholar]
- Chase, M.; Reveal, J.; Fay, M. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2009, 161, 132–136. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2016, 15, 317–337. [Google Scholar] [CrossRef]
- Armengol, J.B.I.; Berkov, S.; Claveria, L.T.; Pigni, N.B.; de Andrade, J.P.; Martínez, V.; Mahrer, C.C.; Meya, F.V. Chemical and biological aspects of Amaryllidaceae alkaloids. In Recent Advances in Pharmaceutical Sciences; Muñoz-Torrero, D., Ed.; Transworld Research Network: Kerala, India, 2011; pp. 65–100. [Google Scholar]
- Desgagne-Penix, I. Biosynthesis of the Amaryllidaceae alkaloids. Plant Sci. Today 2014, 1, 114–120. [Google Scholar]
- Berkov, S.; Martínez-Francés, V.; Bastida, J.; Codina, C.; Ríos, S. Evolution of alkaloid biosynthesis in the genus Narcissus. Phytochemistry 2014, 99, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Vaněčková, N.; Safratova, M.; Breiterová, K.; Blunden, G.; Hulcova, D.; Opletal, L. The genus Nerine Herb. (Amaryllidaceae): Ethnobotany, phytochemistry, and biological activity. Molecules 2019, 24, 4238. [Google Scholar] [CrossRef]
- Desgagné-Penix, I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2020, 5, 239–270. [Google Scholar] [CrossRef]
- Ang, S.; Liu, X.M.; Huang, X.J.; Zhang, D.M.; Zhang, W.; Wang, L.; Ye, W.C. Four new Amaryllidaceae alkaloids from Lycoris radiata and their cytotoxicity. Planta Med. 2015, 81, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xie, N.; Zhong, C.; Su, A.; Hui, X.; Zhang, X.; Jin, Z.; Li, Z.; Feng, J.; He, J. Aphicidal activities of Amaryllidaceae alkaloids from bulbs of Lycoris radiata against Aphis citricola. Ind. Crop. Prod. 2018, 123, 372–378. [Google Scholar] [CrossRef]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chapter Two-Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 83, pp. 113–185. [Google Scholar]
- Zhu, Y.-Y.; Li, X.; Yu, H.-Y.; Xiong, Y.-F.; Zhang, P.; Pi, H.-F.; Ruan, H.-L. Alkaloids from the bulbs of Lycoris longituba and their neuroprotective and acetylcholinesterase inhibitory activities. Arch. Pharm. Res. 2014, 38, 604–613. [Google Scholar] [CrossRef]
- Jin, A.; Li, X.; Zhu, Y.Y.; Yu, H.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl2 and H2O2-induced SH-SY5Y cell injuries. Arch. Pharm. Res. 2014, 37, 315–323. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, C.; Guo, M. Comparative analysis of Amaryllidaceae alkaloids from three Lycoris species. Molecules 2015, 20, 21854–21869. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Liang, X.; Huang, H.; Dai, W.; Shen, Y.; Yan, S.; Zhang, W. Analysis of Amaryllidaceae alkaloids from Crinum by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 2009, 23, 2903–2916. [Google Scholar] [CrossRef]
- Takayama, H.; Kinoshita, E.; Kitajima, M.; Kogure, N. Two new alkaloids from bulbs of Lycoris squamigera. Heterocycles 2009, 77, 1389–1396. [Google Scholar] [CrossRef]
- Li, H.; Ma, G.; Xu, Y.; Hong, S. Alkaloids of Lycoris Guangxiensis. Planta Med. 1987, 53, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Kihara, M.; Xu, L.; Konishi, K.; Kida, K.; Nagao, Y.; Kobayashi, S.; Shingu, T. Isolation and structure elucidation of a novel alkaloid, incartine, a supposed biosynthetic intermediate, from flowers of Lycoris incarnata. Chem. Pharm. Bull. 1994, 42, 289–292. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.Y.; Wang, Z.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia 2013, 88, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kihara, M.; Konishi, K.; Xu, L.; Kobayashi, S. Alkaloidal constituents of the flowers of Lycoris radiata HERB: Amaryllidaceae. Chem. Pharm. Bull. 1991, 39, 1849–1853. [Google Scholar] [CrossRef]
- Jitsuno, M.; Yokosuka, A.; Hashimoto, K.; Amano, O.; Sakagami, H.; Mimaki, Y. Chemical constituents of Lycoris albiflora and their cytotoxic activities. Nat. Prod. Commun. 2011, 6, 1934578X1100600208. [Google Scholar] [CrossRef]
- Boit, H.G.; Döpke, W.; Stender, W. Alkaloide aus Hippeastrum rutilum, Lycoris albiflora, Zephyranthes andersoniana und Sternbergia fischeriana. Naturwissenschaften 1958, 45, 390. [Google Scholar] [CrossRef]
- Guo, Y.; Pigni, N.; Zheng, Y.; de Andrade, J.; Torras-Claveria, L.; Borges, W.; Francesc, V.; Codina, C.; Bastida, J. Analysis of bioactive Amaryllidaceae alkaloid profiles in Lycoris species by GC-MS. Nat. Prod. Commun. 2014, 9, 1081–1086. [Google Scholar] [CrossRef]
- Boit, H.-G.; Ehmke, H. XVI. Mitteil. über Amaryllidaceen-Alkaloide. Alkaloide von Nerine corusca, N. flexuosa, Pancratium illyricum, Lycoris aurea und L. Incarnata. Chem. Ber. 1957, 90, 369–373. [Google Scholar] [CrossRef]
- Mu, H.M.; Wang, R.; Li, X.D.; Jiang, Y.M.; Peng, F.; Xia, B. Alkaloid accumulation in different parts and ages of Lycoris Chinensis. Z. Nat. C 2010, 65, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-M.; Zhu, Y.-Y.; Li, H.-R.; Yu, H.-Y.; Zhang, P.; Pi, H.-F.; Ruan, H.-L. Two new alkaloids from the bulbs of Lycoris sprengeri. J. Asian Nat. Prod. Res. 2014, 16, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Toriizuka, Y.; Kinoshita, E.; Kogure, N.; Kitajima, M.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Ōmura, S.; Takayama, H. New lycorine-type alkaloid from Lycoris traubii and evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives. Bioorganic Med. Chem. 2008, 16, 10182–10189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Z.-Q.; Cai, Y.; Zhang, X.-Q.; Yao, X.-S.; Ye, W.-C. Amaryllidaceae alkaloids from the bulbs of Lycoris Radiata. BioChem. Syst. Ecol. 2010, 38, 444–446. [Google Scholar] [CrossRef]
- Hao, B.; Shen, S.F.; Zhao, Q.J. Cytotoxic and antimalarial Amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Huang, X.-Y.; Cui, M.-R.; Zhang, X.-D.; Chen, Z.; Yang, B.-S.; Zhao, X.-K. Amaryllidaceae alkaloids from the bulbs of Lycoris radiata with cytotoxic and anti-inflammatory activities. Fitoterapia 2015, 101, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Ao, M.; Zhang, P.; Yu, L. Extracts of Lycoris aurea induce apoptosis in murine sarcoma S180 cells. Molecules 2012, 17, 3723–3735. [Google Scholar] [CrossRef]
- Song, J.-H.; Zhang, L.; Song, Y. Alkaloids from Lycoris aurea and their cytotoxicities against the head and neck squamous cell carcinoma. Fitoterapia 2014, 95, 121–126. [Google Scholar] [CrossRef]
- Deng, B.; Ye, L.; Yin, H.; Liu, Y.; Hu, S.; Li, B. Determination of pseudolycorine in the bulb of Lycoris radiata by capillary electrophoresis combined with online electrochemiluminescence using ultrasonic-assisted extraction. J. Chromatogr. B 2011, 879, 927–932. [Google Scholar] [CrossRef]
- Cao, P.; Pan, D.S.; Han, S.; Yu, C.Y.; Zhao, Q.J.; Song, Y.; Liang, Y. Alkaloids from Lycoris caldwellii and their particular cytotoxicities against the astrocytoma and glioma cell lines. Arch. Pharm. Res. 2013, 36, 927–932. [Google Scholar] [CrossRef]
- Yun, Y.S.; Tajima, M.; Takahashi, S.; Takahashi, Y.; Umemura, M.; Nakano, H.; Park, H.S.; Inoue, H. Two alkaloids from bulbs of Lycoris sanguinea MAXIM. suppress PEPCK expression by inhibiting the phosphorylation of CREB. Phytother. Res. 2016, 30, 1689–1695. [Google Scholar] [CrossRef]
- Pi, H.F.; Zhang, P.; Ruan, H.L.; Zhang, Y.H.; Sun, H.D.; Wu, J.Z. A new alkaloid from Lycoris aurea. Chin. Chem. Lett. 2009, 20, 1319–1320. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1934578X1400900834. [Google Scholar] [CrossRef]
- Habartová, K.; Cahlíková, L.; Řezáčová, M.; Havelek, R. The biological activity of alkaloids from the Amaryllidaceae: From cholinesterases inhibition to anticancer activity. Nat. Prod. Commun. 2016, 11, 1934578X1601101038. [Google Scholar] [CrossRef]
- Nair, J.J.; Rárová, L.; Strnad, M.; Bastida, J.; van Staden, J. Mechanistic insights to the cytotoxicity of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2015, 10, 1934578X1501000138. [Google Scholar] [CrossRef]
- Nair, J.J.; Bastida, J.; Viladomat, F.; van Staden, J. Cytotoxic agents of the crinane series of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2013, 8, 1934578X1300800501. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Bastida, J.; Pandey, S.; Griffin, C. Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells. Phytochemistry 2009, 70, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, N.M.; Lamoral-Theys, D.; Mathieu, V.; Andolfi, A.; Frolova, L.V.; Pelly, S.C.; van Otterlo, W.A.; Magedov, I.V.; Kiss, R.; Evidente, A.; et al. In search of a cytostatic agent derived from the alkaloid lycorine: Synthesis and growth inhibitory properties of lycorine derivatives. Bioorganic Med. Chem. 2011, 19, 7252–7261. [Google Scholar] [CrossRef]
- Griffin, C.; Sharda, N.; Sood, D.; Nair, J.; McNulty, J.; Pandey, S. Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: Evaluation of the activity of two new compounds. Cancer Cell Int. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Kawano, I.; Rezáčová, M.; Blunden, G.; Hulcova, D.; Havelek, R. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 2020, 26, 1519–1524. [Google Scholar] [CrossRef]
- Lefranc, F.; Sauvage, S.; Goietsenoven, G.; Mégalizzi, V.; Lamoral-Theys, D.; Debeir, O.; Spiegl-Kreinecker, S.; Berger, W.; Mathieu, V.; Decaestecker, C.; et al. Narciclasine, a plant growth modulator, activates Rho and stress fibers in glioblastoma cells. Mol. Cancer 2009, 8, 1739–1750. [Google Scholar] [CrossRef]
- Pettit, G.R.; Pettit, G.R.; Backhaus, R.A.; Boyd, M.R.; Meerow, A.W. Antineoplastic agents, 256. Cell growth inhibitory isocarbostyrils from Hymenocallis. J. Nat. Prod. 1993, 56, 1682–1687. [Google Scholar] [CrossRef]
- Van Goietsenoven, G.; Hutton, J.; Becker, J.-P.; Lallemand, B.; Robert, F.; Lefranc, F.; Pirker, C.; Vandenbussche, G.; Van Antwerpen, P.; Evidente, A.; et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010, 24, 4575–4584. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Matta, H.; Choi, S.; Chaudhary, P. Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma. Sci. Rep. 2020, 10, 5712. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Ingrassia, L.; Rouzeau, S.; Ribaucour, F.; Thomas, S.; Roland, I.; Darro, F.; Lefranc, F.; Kiss, R. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts 1. Neoplasia 2007, 9, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Mathieu, V.; Lefranc, F.; Kornienko, A.; Evidente, A.; Kiss, R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013, 33, 439–455. [Google Scholar] [CrossRef]
- Stark, A.; Schwenk, R.; Wack, G.; Zuchtriegel, G.; Hatemler, M.; Bräutigam, J.; Schmidtko, A.; Reichel, C.; Bischoff, I.; Fürst, R. Narciclasine exerts anti-inflammatory actions by blocking leukocyte–endothelial cell interactions and down-regulation of the endothelial TNF receptor 1. FASEB J. 2019, 33, 8771–8781. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Yu, W.; Hao, W.; Hong-zhuan, C. AChE Inhibition-based multi-target-directed ligands, a novel pharmacological approach for the symptomatic and disease-modifying therapy of Alzheimer’s Disease. Curr. Neuropharmacol. 2016, 14, 364–375. [Google Scholar]
- De Vita, D.; Pandolfi, F.; Ornano, L.; Feroci, M.; Chiarotto, I.; Sileno, I.; Pepi, F.; Costi, R.; Di Santo, R.; Scipione, L. New N,N-dimethylcarbamate inhibitors of acetylcholinesterase: Design, synthesis and biological evaluation. J. Enzym. Inhib. Med. Chem. 2016, 31, 106–113. [Google Scholar] [CrossRef][Green Version]
- Al Mamun, A.; Maříková, J.; Hulcová, D.; Janoušek, J.; Šafratová, M.; Nováková, L.; Kučera, T.; Hrabinová, M.; Kuneš, J.; Korábečný, J.; et al. Amaryllidaceae alkaloids of belladine type from Narcissus pseudonarcissus cv. Carlton as new selective inhibitors of butyrylcholinesterase. Biomolecules 2020, 10, 800. [Google Scholar] [CrossRef]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Nováková, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Moreno, R.; Tallini, L.R.; Salazar, C.; Osorio, E.H.; Montero, E.; Bastida, J.; Oleas, N.H.; Acosta León, K. Chemical profiling and cholinesterase inhibitory activity of five Phaedranassa Herb. (Amaryllidaceae) species from Ecuador. Molecules 2020, 25, 2092. [Google Scholar] [CrossRef] [PubMed]
- T Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase inhibition activity, alkaloid profiling and molecular docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.K.; Chaudhary, S. Artemisinin-derived antimalarial endoperoxides from bench-side to bed-side: Chronological advancements and future challenges. Med. Res. Rev. 2020, 40, 1220–1275. [Google Scholar] [CrossRef]
- Dedryver, C.-A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, X.; Zheng, B. Effects of sublethal doses of anticholinesterase agents on toxicity of insecticides and their induction to acetylcholinesterase (AChE) activity in Helicoverpa armigera. Acta Entomol. Sin. 2003, 46, 691–696. [Google Scholar]


| Alkaloid (No.) | Cell Line | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HL-60 | A549 | MCF-7 | BEN-MEN-1 | CCF-STTG1 | CHG-5 | SHG-44 | U251 | SMMC-7721 | W480 | ||
| 3α-Hydroxy-6β-acetylbulbispermine (23) | 7.1 ± 0.9 | >100 | 29.4 ± 4.1 | 29.4 ± 5.3 | 28.3 ± 2.7 | 15.8 ± 1.7 | 66.8 ± 9.4 | 53.5 ± 12.4 | [47] | ||
| 3α-Methoxy-6β-acetylbulbispermine (24) | 8.6 ± 1.4 | >100 | 29.7 ± 5.4 | 29.6 ± 6.3 | 29.1 ± 3.8 | 16.7 ± 2.6 | 68.2 ± 12.3 | 50.1 ± 7.8 | [47] | ||
| 3α,6β-Diacetylbulbispermine (25) | 7.3 ± 1.1 | >100 | 27.1 ± 5.1 | 30.1 ± 4.4 | 27.1 ± 3.2 | 17.4 ± 2.1 | 63.2 ± 11.8 | 51.1 ± 10.9 | [47] | ||
| 6β-Acetyl-8-hydroxy-9-methoxycrinamine (26) | 8.6 ± 1.4 | >100 | 29.4 ± 4.1 | 29.6 ± 5.3 | 27.1 ± 3.2 | 17.4 ± 2.1 | 68.2 ± 12.3 | 53.5 ± 12.4 | [48] | ||
| 6β-Acetoxycrinamine (28) | 8.1 | 24.3 | 15.0 | [13] | |||||||
| 8,9-Methylenedioxyhomolycorine-N-oxide (46) | >100 | >100 | 83.2 ± 13.7 | >100 | >100 | >100 | 86.2 ± 17.4 | >100 | [47] | ||
| 8-O-Acetylhomolycorine-N-oxide (47) | >40 | >40 | >40 | ||||||||
| Homolycorine-N-oxide (48) | >100 | >100 | >100 | 93.0 ± 21.1 | >100 | >100 | 85.0 ± 16.2 | >100 | [47] | ||
| 5,6-Dehydrodihydrolycori-ne (79) | >40 | >40 | >40 | [13] | |||||||
| 5,6-Dehydrolycorine (75) | 10.8 ± 1.6 | >100 | 10.3 ± 0.9 | 10.2 ± 1.6 | 9.4 ± 1.3 | 11.8 ± 0.7 | 10.5 ± 0.9 | 11.6 ± 1.1 | [47] | ||
| 2-Hydroxy-anhydrolycorine-N-oxide (77) | >100 | >100 | >100 | >100 | 93.7 | [52] | |||||
| 1-Hydroxyungeremine (90) | 10.8 ± 1.6 | >100 | 10.3 ± 0.9 | 10.2 ± 1.6 | 9.4 ± 1.3 | 11.8 ± 0.9 | 10.5 ± 0.9 | 11.6 ± 1.1 | [48] | ||
| 7-Deoxynarciclasine/Lyco-ricidine (99) | 0.15 | [39] | |||||||||
| Narciclasine/Lycoricidi-nol (100) | 0.018 | [39] | |||||||||
| 5,6,-Dihydro-5-methyl-2-hydroxyphenanthridine (106) | 81.3 ± 15.7 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | [47] | ||
| Alkaloid (No.) | HT-29 | HepG-2 | SK-OV-3 | SCL-1 | CAL-27 | UMSCC-1 | Detroit-562 | SCC-PKU | TCA-83 | HSC-2 | Ref. |
| 8-O-Demethylhomolycorine-N-oxide (42) | 11.6 | 13.2 | 12.3 | 12.3 | 12.9 | 13.2 | 16.7 | [50] | |||
| Hippeastrine (57) | 12.6 ± 0.92 | 37.62 ± 0.63 | [12] | ||||||||
| 2-Hydroxy-anhydrolycorine-N-oxide (77) | 67.7 | 76.2 | [52] | ||||||||
| Alkaloid (No.) | HT-29 | HepG-2 | SK-OV-3 | SCL-1 | CAL-27 | UMSCC-1 | Detroit-562 | SCC-PKU | TCA-83 | HSC-2 | Ref. |
| 1,2-Dihydroxy-anhydrolycorine-N-oxide (81) | >100 | >100 | >100 | >100 | 94.3 | >100 | >100 | >100 | [50] | ||
| 2-Hydroxy-6-oxoanhydrolycorine (82) | >100 | >100 | >100 | >100 | >100 | 95.5 | 91.2 | [50] | |||
| 1,2-Dihydroxy-6-oxoanhydrolycorine (83) | >100 | >100 | >100 | >100 | >100 | 88.3 | 91.8 | [50] | |||
| 7-Deoxynarciclasine/Lyco-ricidine (99) | 1.7 ± 0.2 | [39] | |||||||||
| Narciclasine/Lycoricidi-nol (100) | 1.373 | 0.08 | 0.05 | [16,39] | |||||||
| 5,6,-Dihydro-5-methyl-2-hydroxyphenanthridine (106) | >100 | >100 | >100 | >100 | >100 | 87.6 | >100 | [50] | |||
| Alkaloid (No.) | % AChE Inhibition (100 µM) | IC50 (µM) |
|---|---|---|
| 11β-Hydroxygalanthamine (8) | 96 ± 0 | 3.04 ± 0.61 |
| N-Norgalanthamine (9) | 92 ± 1 | 2.76 ± 0.65 |
| N-(Chloromethyl)galanthamine (12) | 94 ± 1 | 5.55 ± 0.63 |
| Galanthamine (13) | 95 ± 1 | 2.43 ± 0.66 |
| O-Demethyllycoramine (15) | 86 ± 1 | 8.13 ± 1.49 |
| N-(Chloromethyl)lycoramine (16) | 79 ± 1 | 25.76 ± 1.09 |
| Sanguinine (21) | 93 ± 0 | 5.30 ± 0.76 |
| Haemanthidine (30) | 39 ± 1 | 208.10 ± 1.58 |
| Incartine (65) | 41 ± 1 | 148.70 ± 1.46 |
| N-(Chloromethyl)narcissidine (67) | 36 ± 3 | 190.70 ± 2.00 |
| Lycorine (80) | 32 ± 2 | 224.80 ± 3.01 |
| Hippadine (86) | 42 ± 2 | 117.60 ± 1.79 |
| Lycolongirine C (98) | 45 ± 2 | 194.80 ± 2.31 |
| N-Methylcrinasiadine (103) | 85 ± 1 | 4.23 ± 1.13 |
| Trisphaeridine (107) | 33 ± 1 | 190.70 ± 2.00 |
| Deoxypretazettine (110) | 89 ± 0.38 | 8.44 ± 0.83 |
| Alkaloid (No.) | D-6 IC50 (µM) | W-2 IC50 (µM) |
|---|---|---|
| 3α-Hydroxy-6β-acetylbulbispermine (23) | 17.9 | 19.3 |
| 3α-Methoxy-6β-acetylbulbispermine (24) | 21.3 | 23.4 |
| 3α,6β-Diacetylbulbispermine (25) | 18.9 | 20.1 |
| 8,9-Methylenedioxyhomolycorine-N-oxide (46) | >100 | >100 |
| Homolycorine-N-oxide (48) | >100 | >100 |
| 5,6-Dehydrolycorine (75) | 2.3 | 1.9 |
| 5,6,-Dihydro-5-methyl-2-hydroxyphenanthridine (106) | >100 | >100 |
| Chloroquine * | 9.8 a | 6.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahlíková, L.; Breiterová, K.; Opletal, L. Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae). Molecules 2020, 25, 4797. https://doi.org/10.3390/molecules25204797
Cahlíková L, Breiterová K, Opletal L. Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae). Molecules. 2020; 25(20):4797. https://doi.org/10.3390/molecules25204797
Chicago/Turabian StyleCahlíková, Lucie, Kateřina Breiterová, and Lubomír Opletal. 2020. "Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae)" Molecules 25, no. 20: 4797. https://doi.org/10.3390/molecules25204797
APA StyleCahlíková, L., Breiterová, K., & Opletal, L. (2020). Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae). Molecules, 25(20), 4797. https://doi.org/10.3390/molecules25204797