Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches
Abstract
1. Introduction
2. Results and Discussion
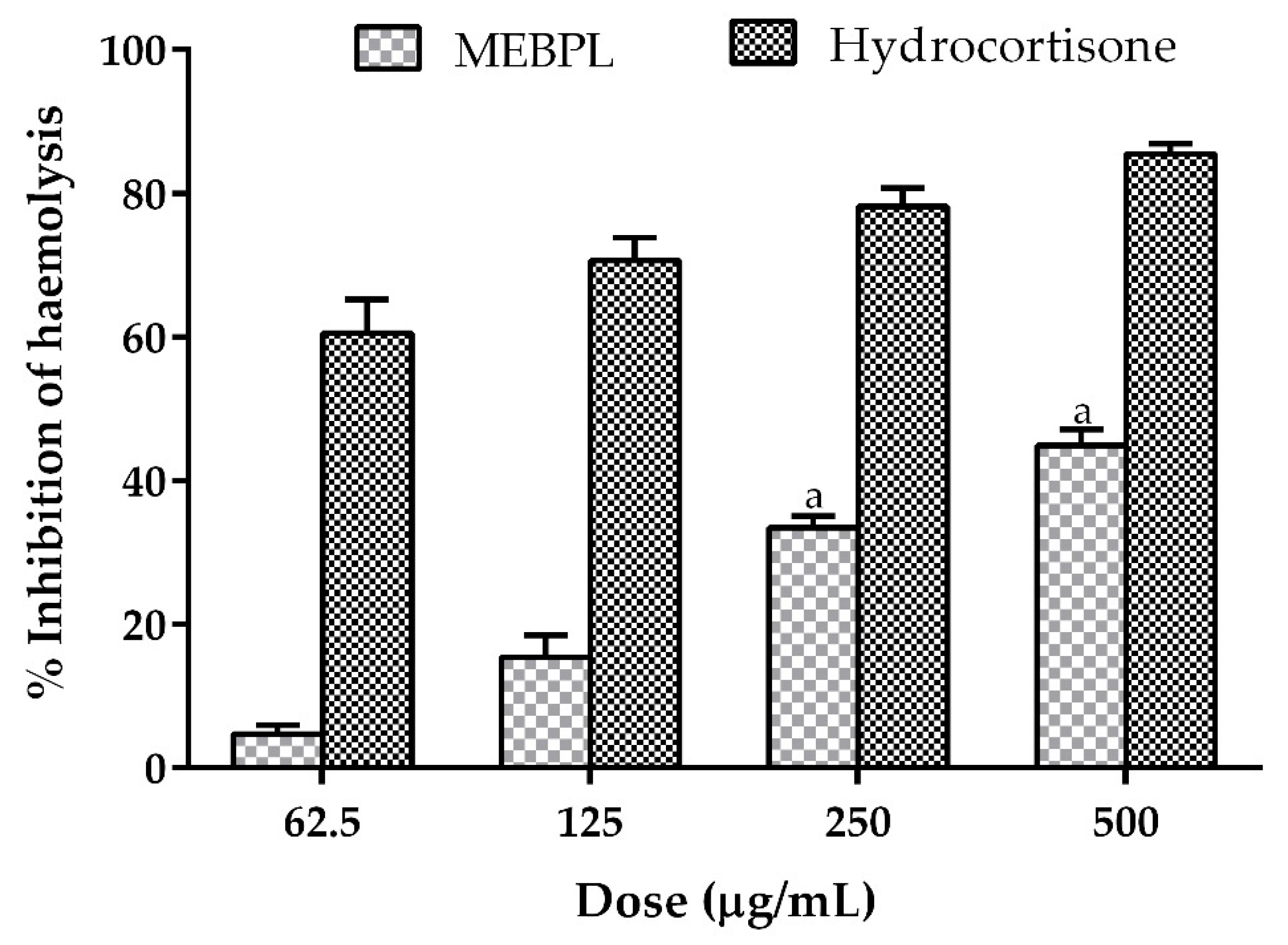
2.1. Effect of MEBPL on Anti-Inflammatory Activity
2.2. Effect of MEBPL on Analgesic Activity
2.3. Effect of MEBPL on Anxiolytic Activity
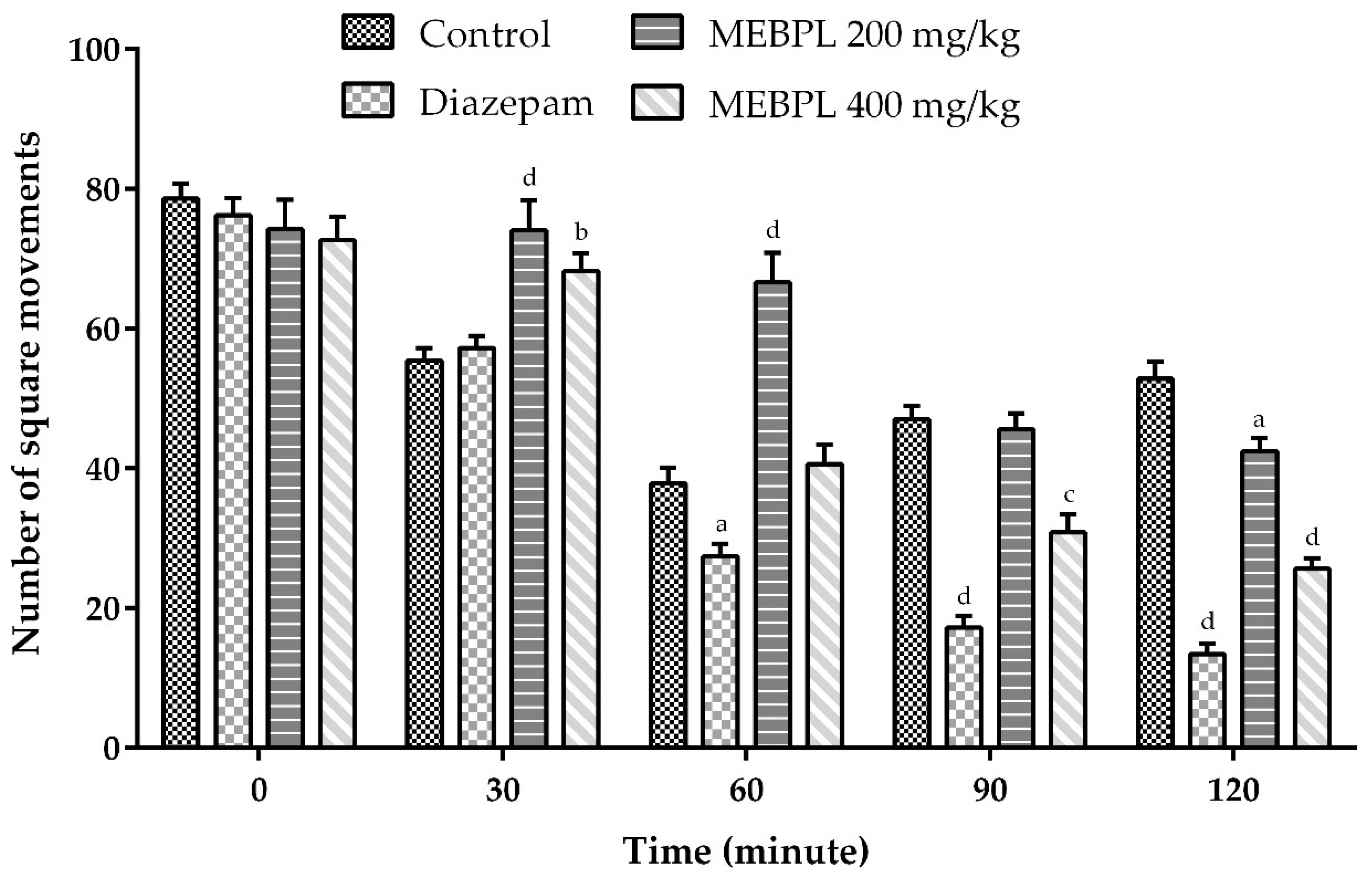
2.4. Effect of MEBPL on Locomotor Activity
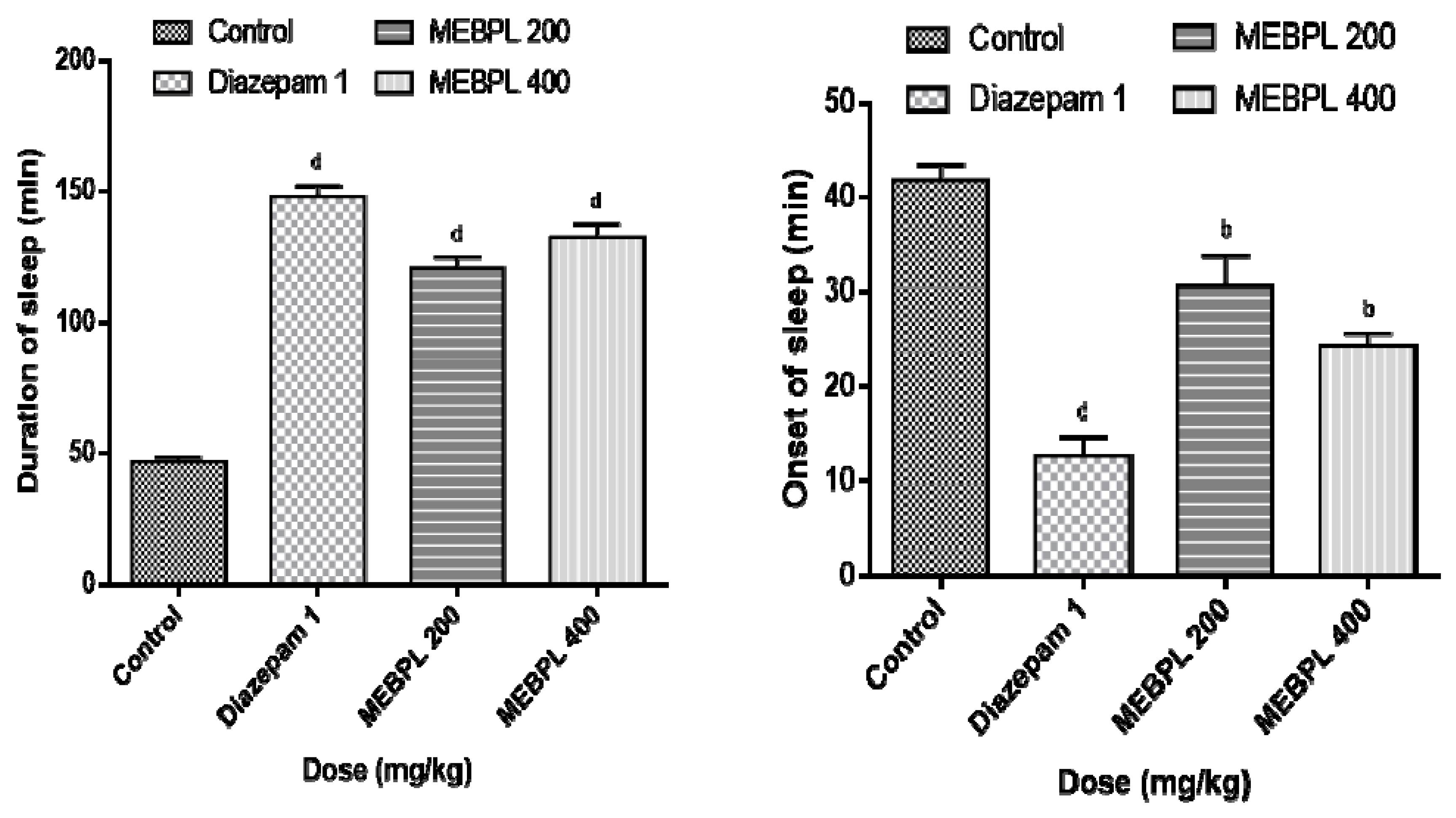
2.5. Effect of MEBPL on Sedative Activity
2.6. Effect of MEBPL on Anti-Diarrheal Activity
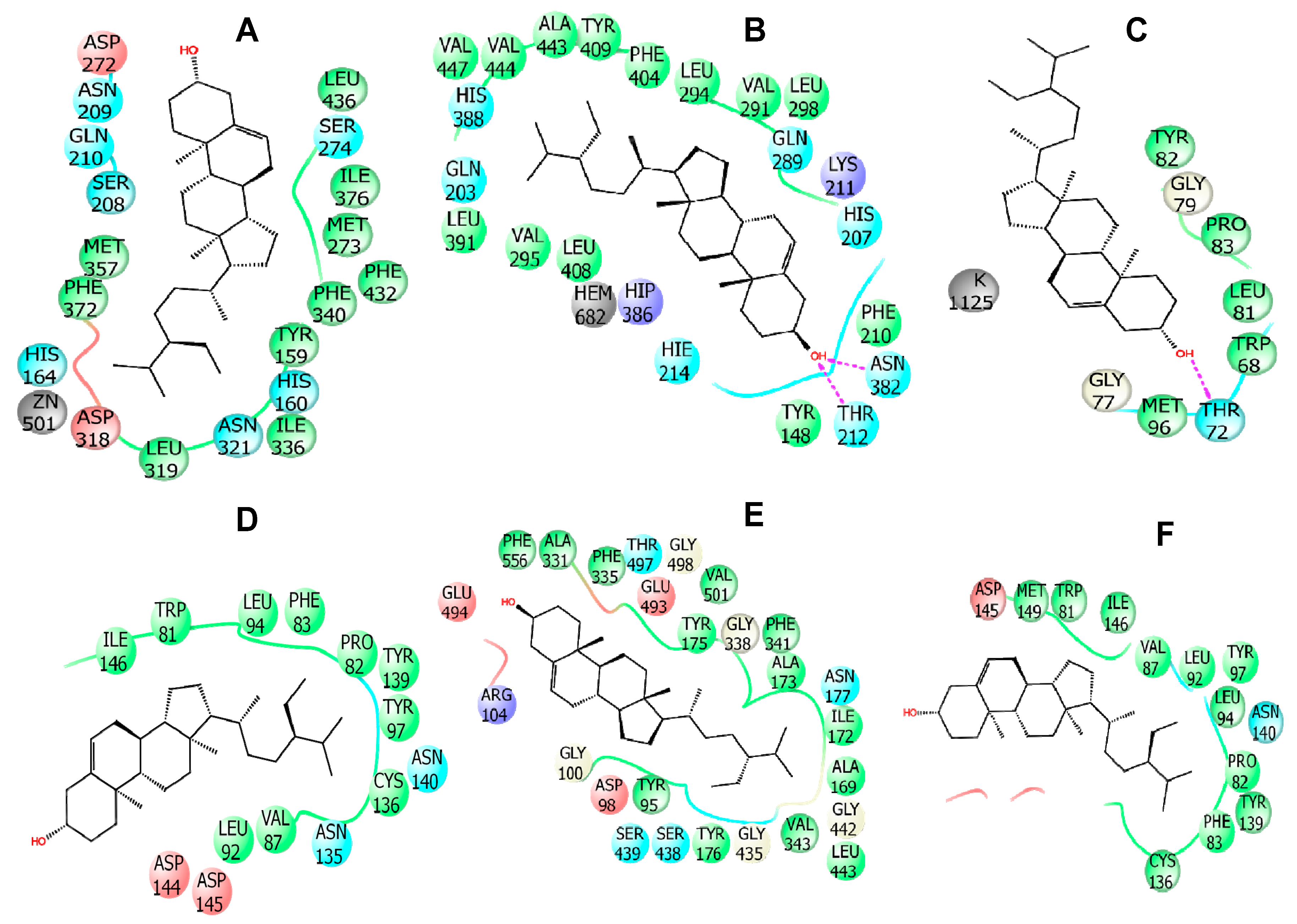
2.7. In Silico Studies
3. Materials and Methods
3.1. Plant Collection
3.2. Preparation of Plant Extract
3.3. Chemicals
3.4. Experimental Animals
3.5. Acute Toxicity Study
3.6. Phytochemical Screening
3.7. In Vitro Anti-Inflammatory Activity
3.7.1. Membrane Stabilization
3.7.2. Inhibition of Protein Denaturation
3.8. In Vivo Analgesic Activity
3.8.1. Writhing Inhibition Test by Inducing Acetic Acid
3.8.2. Paw Licking Test by Inducing Formalin
3.9. Anxiolytic Activity by Hole Board Experiment
3.10. Locomotor Activity by Open Field Experiment
3.11. Sedative Activity by Thiopental-Sodium Induced Sleeping Time
3.12. Anti-Diarrheal Activity
3.12.1. Castor-Oil Induced Diarrhoea Test
3.12.2. Gastro-Intestinal Motility Test
3.13. In Silico Studies
3.13.1. Selection of Compounds for In Silico Studies
3.13.2. Molecular Docking Analysis
3.13.3. PASS Prediction Study
3.13.4. ADME Analysis
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cock, I.E.; Winnett, V.; Sirdaarta, J.; Matthews, B. The potential of selected Australian medicinal plants with anti-Proteus activity for the treatment and prevention of rheumatoid arthritis. Pharmacogn. Mag. 2015, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Amat, N.; Amat, R.; Abdureyim, S.; Hoxur, P.; Osman, Z.; Mamut, D.; Kijjoa, A. Aqueous extract of Dioscorea opposita thunb. normalizes the hypertension in 2K1C hypertensive rats. BMC Complement. Altern. Med. 2014, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.; Martel, M.; Jamison, R.; Weiss, R.; Edwards, R.; McHugh, R. (530) Distress intolerance and prescription opioid misuse among patients with chronic pain. J. Pain 2016, 17, 806–814. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, N.U.; Kamal, A.M.; Barlow, J.W.; Faruque, M.O.; Yang, X.; Uddin, S.B. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J. Ethnopharmacol. 2019, 236, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Chy, N.U.; Kamal, A.M.; Chowdhury, R.; Islam, S.; Hossain, A.; Tareq, A.M.; Bhuiyan, I.H.; Uddin, N.; Tahamina, A.; et al. Unveiling pharmacological responses and potential targets insights of identified bioactive constituents of Cuscuta reflexa Roxb. leaves through in vivo and in silico approaches. Pharmaceuticals 2020, 13, 50. [Google Scholar] [CrossRef]
- Han, C.; Pae, C.-U. Pain and depression: A neurobiological perspective of their relationship. Psychiatry Investig. 2015, 12, 1. [Google Scholar] [CrossRef]
- Hasanat, A.; Kabir, M.S.H.; Ansari, A.; Chowdhury, T.A.; Hossain, M.M.; Islam, M.N.; Ahmed, S.; Chy, N.U.; Adnan; Kamal, A.M. Ficus cunia Buch.-Ham. ex Roxb.(leaves): An experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J. Basic Clin. Physiol. Pharmacol. 2019, 30. [Google Scholar] [CrossRef]
- Pollmann, A.S.; Murphy, A.L.; Bergman, J.C.; Gardner, D.M. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: A scoping review. BMC Pharmacol. Toxicol. 2015, 16, 19. [Google Scholar] [CrossRef]
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef]
- Pierce, N.F.; Carpenter, C.C.; Elliott, H.L.; Greenough, W.B. Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology 1971, 60, 22–32. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, N.U.; Kamal, A.M.; Azad, O.K.; Paul, A.; Uddin, S.B.; Barlow, J.W.; Faruque, M.O.; Park, C.H.; Cho, D.H. Investigation of the biological activities and characterization of bioactive constituents of Ophiorrhiza rugosa var. prostrata (D. Don) & Mondal leaves through in vivo, in vitro, and in silico approaches. Molecules 2019, 24, 1367. [Google Scholar]
- Khine, K.K. Phytochemical screening and some bioactivities of Byttneria pilosa Roxb. (Sat-le-pyat) Leaves and Stems. In 2nd Myanmar Korea Conference Research Journal; Dagon University: Yangon, Myanmar, 2019; Volume 2, Available online: https://www.dagonuniversity.edu.mm/research/ (accessed on 30 July 2020).
- Ibrahim, M.Y.; Ansari, P.; Riasat-ul-Islam, A.K.M.; Sultana, M.; Zhumur, N.A.; Mohammed, S. Evaluation of thrombolytic and cytotoxic activities of an ornamental medicinal plant: Byttneria pilosa. Am. J. Biomed. Res. 2015, 3, 35–39. [Google Scholar]
- Zaman, R.; Parvez, M.; Sekendar, A.M.; Islam, M. Possible anti-obesity activity of methanol extract of Byttneria pilosa Roxb. leaves. Middle-East. J. Sci. Res. 2015, 23, 1585–1589. [Google Scholar]
- Rahman, A.; Sultana, R.; Bin Emran, T.; Islam, M.S.; Chakma, J.S.; Rashid, H.; Hasan, C.M.M.; Rahman, M.A. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement. Altern. Med. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rashid, U.; Khan, M.R.; Jan, S.; Bokhari, J.; Shah, N.A. Assessment of phytochemicals, antimicrobial and cytotoxic activities of extract and fractions from Fagonia olivieri (Zygophyllaceae). BMC Complement. Altern. Med. 2013, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Herbal Neurotoxicity: An Introduction to Its Occurrence and Causes in Toxicology of Herbal Products; Springer: Berlin/Heidelberg, Germany, 2017; pp. 345–362. [Google Scholar]
- Boussouf, L.; Boutennoune, H.; Kebieche, M.; Adjeroud-Abdellatif, N.; Al-Qaoud, K.; Madani, K. Anti-inflammatory, analgesic and antioxidant effects of phenolic compound from Algerian Mentha rotundifolia L. leaves on experimental animals. S. Afr. J. Bot. 2017, 113, 77–83. [Google Scholar] [CrossRef]
- Guo, R.; Chang, X.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Liu, R.H. Phenolic compounds, antioxidant activity, antiproliferative activity and bioaccessibility of Sea buckthorn (Hippophaë rhamnoides L.) berries as affected byin vitrodigestion. Food Funct. 2017, 8, 4229–4240. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Serafini, M.R.; Quintans-Júnior, L.J. Terpenes and derivatives as a new perspective for pain treatment: A patent review. Expert Opin. Ther. Patents 2014, 24, 243–265. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar] [CrossRef]
- Mizushima, Y. Inhibition of protein denaturation by antirheumatic or antiphlogistic agents. Arch. Int. Pharmacodyn.Ther. 1964, 149, 1–7. [Google Scholar] [PubMed]
- Gillette, J.R.; Heinzelman, R.V.; Szmuszkovicz, J.; Leemann, H.G.; Stich, K.; Thomas, M.; Martin-Smith, M.; Khatoon, T.; Kunz, W. Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès des recherches pharmaceutiques; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1963; pp. 279–346. [Google Scholar]
- Hasan, S.R.; Akter, R.; Jamila, M.; Mazumder, M.; Alam, M.; Faruque, A.; Rana, S.; Hossain, M.; Rahman, S. Analgesic Activity of the Different Fractions of the Aerial Parts of Commelina benghalensis Linn. Int. J. Pharmacol. 2010, 6, 63–67. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Ghani, Z.D.F.A.; Nor, R.N.S.R.M.; Gopalan, H.K.; Sulaiman, M.R.; Jais, A.M.M.; Somchit, M.N.; Kader, A.A.; Ripin, J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.; Aziz, M.A.; Tareq, A.M.; Sayeed, M.A.; Alam, N.; Alam, N.U.; Uddin, M.A.; Lyzu, C.; Emran, T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 2020, 23, 100772. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Majumder, R.; Mamun, A.; Alam, E.K.; Jami, S.I.; Alam, B. Preliminary pharmacological activity of the methanolic extract of Premna integrifolia barks in rats. Avicenna J. Phytomed. 2014, 4, 215–224. [Google Scholar]
- Musa, Y.M.; Haruna, A.K.; Ilyas, M.; Yaro, A.H.; Ahmadu, A.A.; Usman, H. Phytochemical, Analgesic and Anti-Inflammatory Effects Of The Ethylacetate Extract Of The Leaves Of Pseudocedrella kotschyii. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Shanthi, P.; Sachdanandam, P. Immunomodulatory and Anti-inflammatory Effects of Semecarpus anacardium LINN. Nut Milk Extract in Experimental Inflammatory Conditions. Biol. Pharm. Bull. 2006, 29, 693–700. [Google Scholar] [CrossRef]
- Olsen, R.W.; Tobin, A.J. Molecular biology of GABA A receptors. FASEB J. 1990, 4, 1469–1480. [Google Scholar] [CrossRef]
- Ezhou, Y.; Danbolt, N.C. GABA and Glutamate Transporters in Brain. Front. Endocrinol. 2013, 4, 165. [Google Scholar]
- Chowdhury, M.R.; Chowdhury, K.H.; Hanif, N.B.; Sayeed, M.A.; Mouah, J.; Mahmud, I.; Kamal, A.T.M.M.; Chy, M.N.U.; Adnan, M. An integrated exploration of pharmacological potencies of Bischofia javanica (Blume) leaves through experimental and computational modeling. Heliyon 2020, 6, e04895. [Google Scholar] [CrossRef]
- Ezenwali, M.; Njoku, O.; Okoli, C. Studies on the anti-diarrheal properties of seed extract of Monodora tenuifolia. Int. J. App. Res. Nat. Prod. 2010, 2, 20–26. [Google Scholar]
- Raju, G.S.; Moghal, M.R.; Dewan, S.M.R.; Amin, M.N.; Billah, M. Characterization of phytoconstituents and evaluation of total phenolic content, anthelmintic, and antimicrobial activities of Solanum violaceum Ortega. Avicenna J. Phytomed. 2013, 3, 313–320. [Google Scholar] [PubMed]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Mohamad, T.A.S.T.; Mossadeq, W.M.S.; Moin, S.; Yusof, M.; Mokhtar, A.F.; Zakaria, Z.A.; Israf, D.A.; Lajis, N. Antinociceptive Activity of the Essential Oil of Zingiber zerumbet. Planta Medica 2010, 72, 107–112. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Omale, J.; Okafor, P.N. Comparative antioxidant capacity, membrane stabilization, polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata. Afr. J. Biotechnol. 2008, 7, 3129–3133. [Google Scholar]
- Ansari, P.; Uddin, J.; Rahman, M.; Abdullah-Al-Mamun, M.; Islam, R.; Ali, H.; Reza, A.A. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 51–58. [Google Scholar] [CrossRef]
- Arrigoni-Blank, M.D.F.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.; Andrade, M.R.; Marchioro, M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar] [CrossRef]
- Okokon, J.E.; Nwafor, P.A. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak. J. Pharm. Sci. 2010, 23, 385–392. [Google Scholar]
- Öztürk, Y.; Aydin, S.; Beis, R.; Baser, K.H.C.; Berberoğlu, H. Effects of Hypericum perforatum L. and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomedicine 1996, 3, 139–146. [Google Scholar] [CrossRef]
- Gupta, B.D.; Dandiya, P.C.; Gupta, M.L. A psychopharmacological analysis of behavior in rats. Jpn. J. Pharmacol. 1971, 21, 293–298. [Google Scholar] [CrossRef]
- Ferrini, R.; Miragoli, G.; Taccardi, B. Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittelforschung 1974, 24, 2029–2032. [Google Scholar] [PubMed]
- Shoba, F.; Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol. 2001, 76, 73–76. [Google Scholar] [CrossRef]
- Mascolo, N.; A Izzo, A.; Autore, G.; Barbato, F.; Capasso, F. Nitric oxide and castor oil-induced diarrhea. J. Pharmacol. Exp. Ther. 1994, 268, 291–295. [Google Scholar] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.N.U.; Rakib, A.; Emran, T.B.; Simal-Gandara, J. GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef]
- Harman, C.A.; Turman, M.V.; Kozak, K.R.; Marnett, L.J.; Smith, W.L.; Garavito, R.M. Structural Basis of Enantioselective Inhibition of Cyclooxygenase-1 by S-α-Substituted Indomethacin Ethanolamides. J. Biol. Chem. 2007, 282, 28096–28105. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nat. Cell Biol. 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nat. Cell Biol. 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Lenaeus, M.J.; Burdette, D.; Wagner, T.; Focia, P.J.; Gross, A. Structures of kcsa in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry 2014, 53, 5365–5373. [Google Scholar] [CrossRef] [PubMed]
- Felding, J.; Sørensen, M.D.; Poulsen, T.D.; Larsen, J.; Andersson, C.; Refer, P.; Engell, K.; Ladefoged, L.G.; Thormann, T.; Vinggaard, A.M.; et al. Discovery and Early Clinical Development of 2-{6-[2-(3,5-Dichloro-4-pyridyl)acetyl]-2,3-dimethoxyphenoxy}-N-propylacetamide (LEO 29102), a Soft-Drug Inhibitor of Phosphodiesterase 4 for Topical Treatment of Atopic Dermatitis. J. Med. Chem. 2014, 57, 5893–5903. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Picaud, S.; Fedorov, O.; Keller, M.; Wrobel, M.; Morgenstern, O.; Bracher, F.; Knapp, S. Benzodiazepines and benzotriazepines as protein interaction inhibitors targeting bromodomains of the BET family. Bioorg. Med. Chem. 2012, 20, 1878–1886. [Google Scholar] [CrossRef]
- Kudryashov, D.S.; Durer, Z.A.O.; Ytterberg, A.J.; Sawaya, M.R.; Pashkov, I.; Prochazkova, K.; Yeates, T.O.; Loo, R.R.O.; Loo, J.A.; Satchell, K.J.F.; et al. Connecting actin monomers by iso-peptide bond is a toxicity mechanism of the Vibrio cholerae MARTX toxin. Proc. Natl. Acad. Sci. USA 2008, 105, 18537–18542. [Google Scholar] [CrossRef]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular docking studies of (4Z, 12Z)-cyclopentadeca-4, 12-dienone from Grewia hirsuta with some targets related to type 2 diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]


| Phytochemical Test | Results |
|---|---|
| Alkaloids | ++ |
| Carbohydrates | + |
| Glycosides | ++ |
| Flavonoids | ++ |
| Phenols | + |
| Tannins | + |
| Saponins | + |
| Terpenoids | + |
| Cholesterol | - |
| Protein | - |
| Fixed oil | + |
| Quinones | + |
| Resins | + |
| Treatment | Writhing Number | (%) Inhibition |
|---|---|---|
| Control | 37.20 ± 2.46 | - |
| Diclofenac-Na (10 mg/kg) | 12.80 ± 0.97 c | 65.59 |
| MEBPL (200 mg/kg) | 28.00 ± 1.79 a | 24.73 |
| MEBPL (400 mg/kg) | 24.60 ± 2.44 b | 33.87 |
| Treatment | Early Phase Paw Licking (s) | % Inhibition of Early Phase | Late Phase Paw Licking (s) | % Inhibition of Late Phase |
|---|---|---|---|---|
| Control | 57.80 ± 3.02 | - | 46.20 ± 1.74 | - |
| Diclofenac-Na (10 mg/kg) | 17.40 ± 1.12 d | 69.89 | 16.00 ± 1.14 d | 65.37 |
| MEBPL (200 mg/kg) | 40.40 ± 2.25 b | 30.11 | 35.20 ± 2.69 b | 23.81 |
| MEBPL (400 mg/kg) | 36.00 ± 3.85 c | 37.72 | 29.40 ± 2.84 c | 36.36 |
| Treatment | Number of Head Dipping |
|---|---|
| Control | 24.80 ± 3.07 |
| Diazepam 1 mg/kg | 58.20 ± 4.02 d |
| MEBPL 200 mg/kg | 30.60 ± 2.42 |
| MEBPL 400 mg/kg | 40.20 ± 2.82 b |
| Treatment | Total Number of Faeces | % Inhibition of Defecation | Total Number of Diarrheal Faeces | % Inhibition of Diarrhoea |
|---|---|---|---|---|
| Control | 14.70 ± 0.41 | - | 7.05 ± 0.25 | - |
| Loperamide (5 mg/kg) | 5.55 ± 0.46 d | 62.24 | 2.05 ± 0.15 c | 70.92 |
| MEBPL (200 mg/kg) | 6.55 ± 0.56 d | 55.44 | 2.30 ± 0.22 c | 67.38 |
| MEBPL (400 mg/kg) | 5.50 ± 0.34 d | 62.59 | 1.80 ± 0.33 d | 74.46 |
| Treatment | Total Length of Intestine (cm) | Distance Travel by Charcoal (cm) | % Peristalsis Index | % Inhibition |
|---|---|---|---|---|
| Control | 52.80 ± 1.59 | 44.20 ± 1.66 | 83.92 ± 3.55 | - |
| Loperamide (5 mg/kg) | 53.20 ± 1.93 | 24.20 ± 1.88 d | 45.33 ± 2.27 d | 54.51 |
| MEBPL (200 mg/kg) | 55.60 ± 3.31 | 31.80 ± 1.66 c | 57.93 ± 4.44 c | 42.80 |
| MEBPL (400 mg/kg) | 57.80 ± 2.15 | 29.40 ± 2.29 c | 51.30 ± 5.04 c | 49.13 |
| Compound | 4WCU | 2OYE | 6COX | 4UUJ | 3U5K | 5I6X | 3U5J | 3CJB |
|---|---|---|---|---|---|---|---|---|
| Beta-sitosterol | −5.01 | - | −3.865 | −2.041 | −4.273 | −6.656 | −4.494 | −2.01 |
| Receptor PDB ID | Hydrogen Bond Interactions | Hydrophobic Bond Interactions |
|---|---|---|
| 6COX | ASN 382 (2), THR 212 | HIS 207 (Pi-Alkyl), HIS 214 (Pi-Alkyl), HIS 386 (Pi-Alkyl), HIS 388 (Pi-Alkyl), PHE 404 (Pi-Alkyl), VAL 291(2) (Alkyl), VAL 444 (Alkyl), VAL 447 (Alkyl), LEU 391 (Alkyl), LEU 408 (Alkyl), ALA 443 (Alkyl) |
| 5I6X | ARG 104 | PHE 355(2) (Pi-Alkyl), PHE 341(2) (Pi-Alkyl), TYR 176(2) (Pi-Alkyl), ILE 172(3) (Alkyl), ALA 169 (Alkyl), ALA 173 (Alkyl) |
| 4WCU | - | ILE 336 (Alkyl), LEU 319 (Alkyl), MET 273(2) (Alkyl), PHE 340 (Pi-Alkyl), PHE 372 (Pi-Alkyl), PHE 432(2) (Pi-Alkyl), TYR 159(2) (Pi-Alkyl), HIS 164 (Pi-Alkyl), HIS 160 (Pi-Alkyl) |
| 4UUJ | THR 72 | TYR 82 (3) (Pi-Alkyl) |
| 3U5K | - | TYR 139(2) (Pi-Alkyl), TYR 97 (Pi-Alkyl), PHE 83(2) (Pi-Alkyl), VAL 87 (Alkyl), LEU 94 (Alkyl), PRO 82 (Alkyl), CYS 136 (Alkyl) |
| 3U5J | - | VAL 87(2) (Alkyl), LEU 92 (Alkyl), LEU 94(2) (Alkyl), PRO 82(2) (Alkyl), ILE 146(3) (Alkyl), TRP 81 (Pi-Alkyl), TYR 139 (Pi-Alkyl), TYR 97(2) (Pi-Alkyl) |
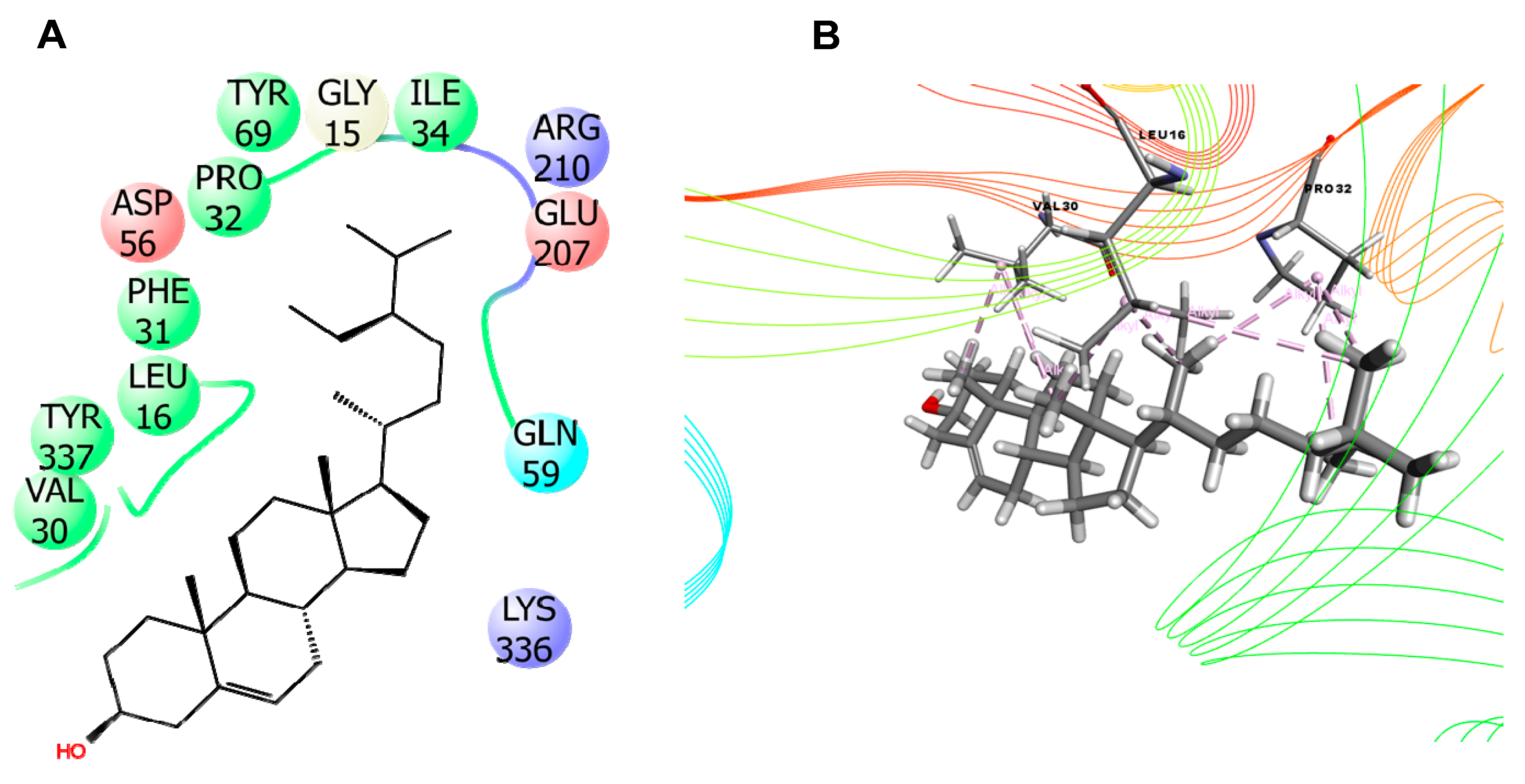
| 3CJB | - | VAL 30(3) (Alkyl), PRO 32(3) (Alkyl), LEU 16(3) (Alkyl) |
| Properties | Pa | Pi |
|---|---|---|
| Antinociceptive | 0.558 | 0.014 |
| Anti-inflammatory | 0.467 | 0.067 |
| Antisecretory | 0.427 | 0.049 |
| Neurotransmitter uptake inhibitor | 0.266 | 0.259 |
| Neurotrophic factor enhancer | 0.218 | 0.051 |
| Neuropeptide Y4 antagonist | 0.288 | 0.200 |
| ADME Analysis of Beta-Sitosterol | |
|---|---|
| Molecular weight (acceptable range: 500) | 414.7 |
| Hydrogen bond donor (acceptable range: ≤5) | 1 |
| Hydrogen bond acceptor (acceptable range: ≤10) | 1 |
| High lipophilicity (expressed as Log P, ˂5) | 9.3 |
| Rotatable bond | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyoti, M.A.; Barua, N.; Hossain, M.S.; Hoque, M.; Bristy, T.A.; Mahmud, S.; Kamruzzaman; Adnan, M.; Chy, M.N.U.; Paul, A.; et al. Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches. Molecules 2020, 25, 4737. https://doi.org/10.3390/molecules25204737
Jyoti MA, Barua N, Hossain MS, Hoque M, Bristy TA, Mahmud S, Kamruzzaman, Adnan M, Chy MNU, Paul A, et al. Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches. Molecules. 2020; 25(20):4737. https://doi.org/10.3390/molecules25204737
Chicago/Turabian StyleJyoti, Mifta Ahmed, Niloy Barua, Mohammad Shafaet Hossain, Muminul Hoque, Tahmina Akter Bristy, Shabnur Mahmud, Kamruzzaman, Md. Adnan, Md. Nazim Uddin Chy, Arkajyoti Paul, and et al. 2020. "Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches" Molecules 25, no. 20: 4737. https://doi.org/10.3390/molecules25204737
APA StyleJyoti, M. A., Barua, N., Hossain, M. S., Hoque, M., Bristy, T. A., Mahmud, S., Kamruzzaman, Adnan, M., Chy, M. N. U., Paul, A., Hossain, M. E., Emran, T. B., & Simal-Gandara, J. (2020). Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches. Molecules, 25(20), 4737. https://doi.org/10.3390/molecules25204737












