Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells
Abstract
1. Introduction
2. Results
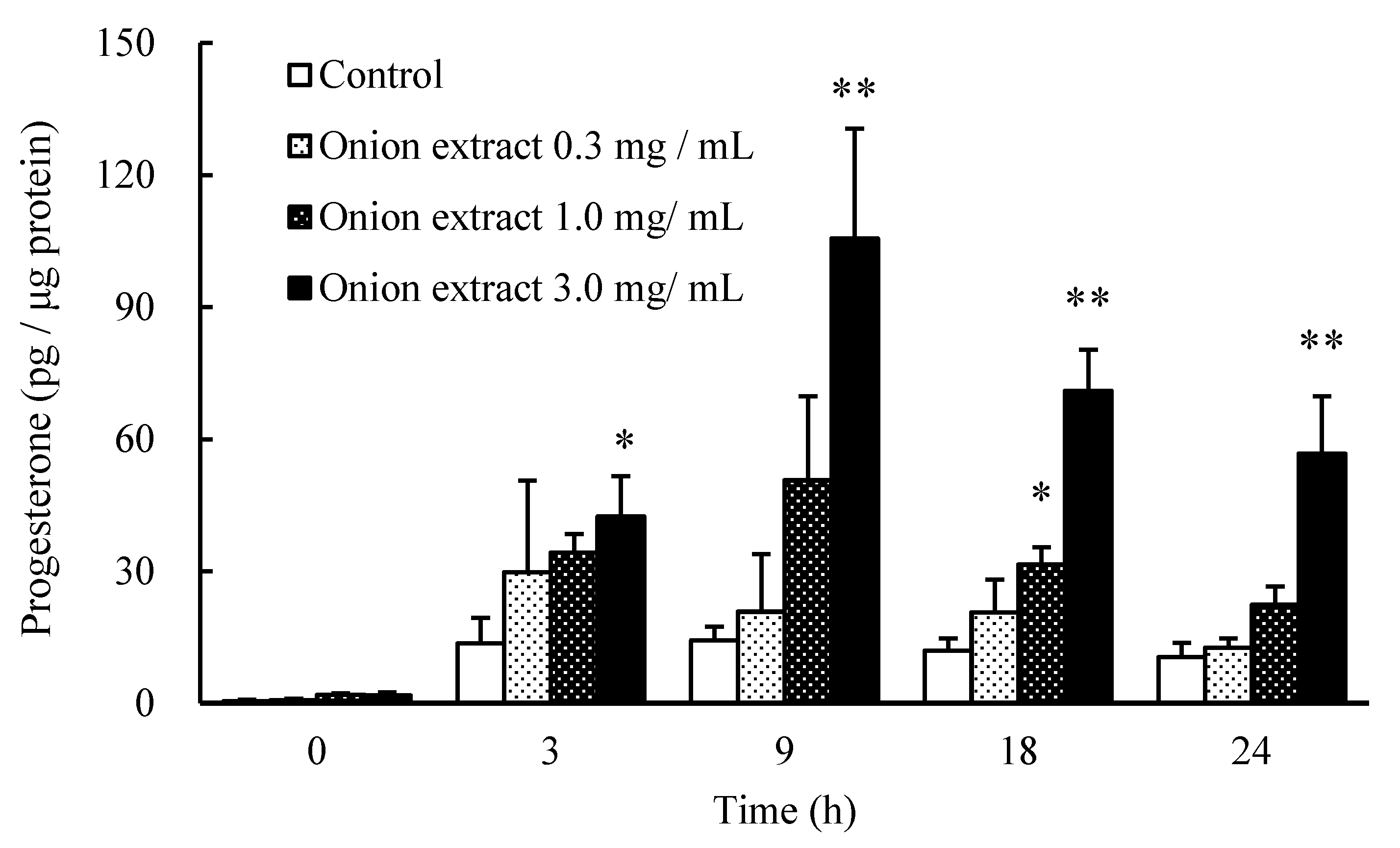
2.1. Onion Extract Stimulates Progesterone Production
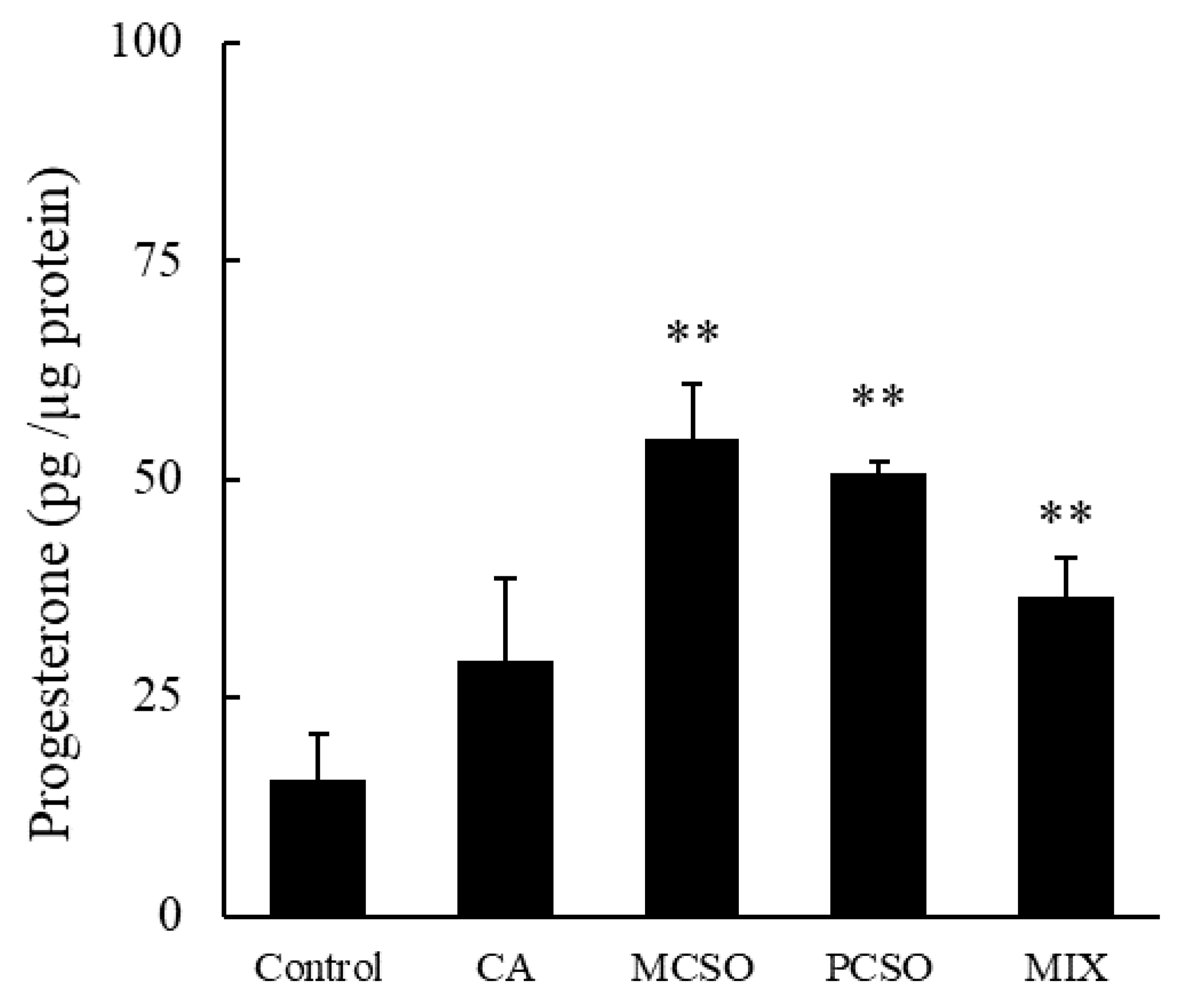
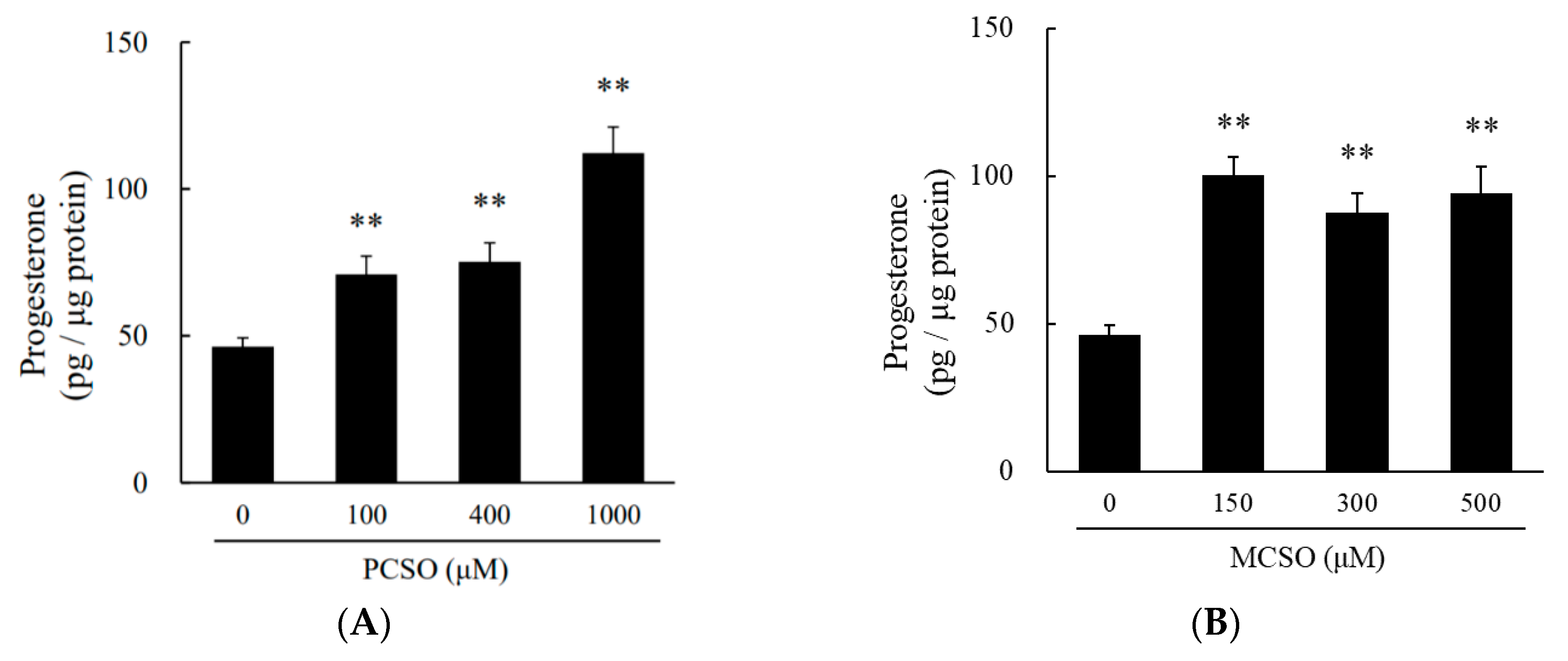
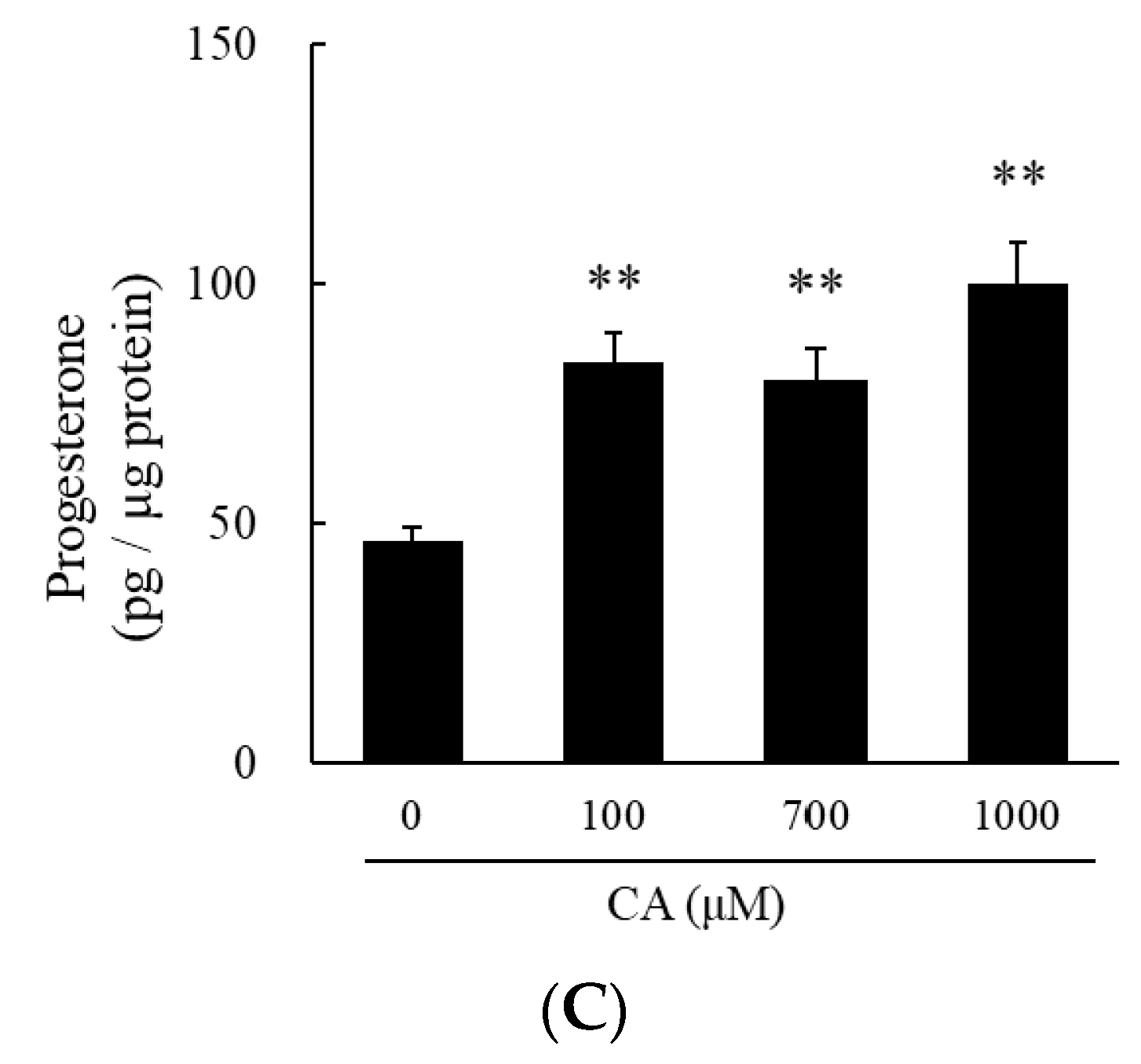
2.2. Cysteine Sulfoxides Stimulate Progesterone Production
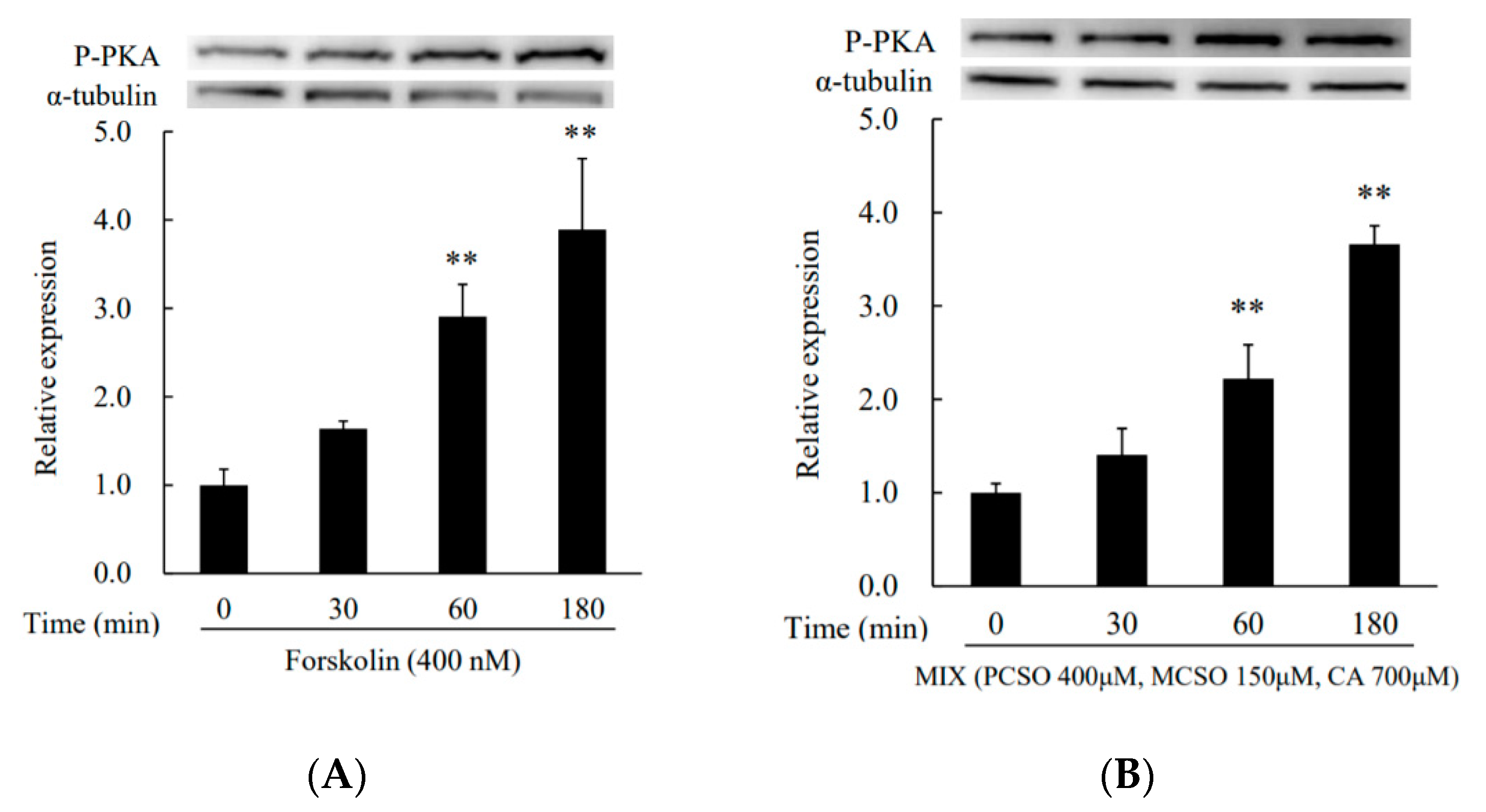
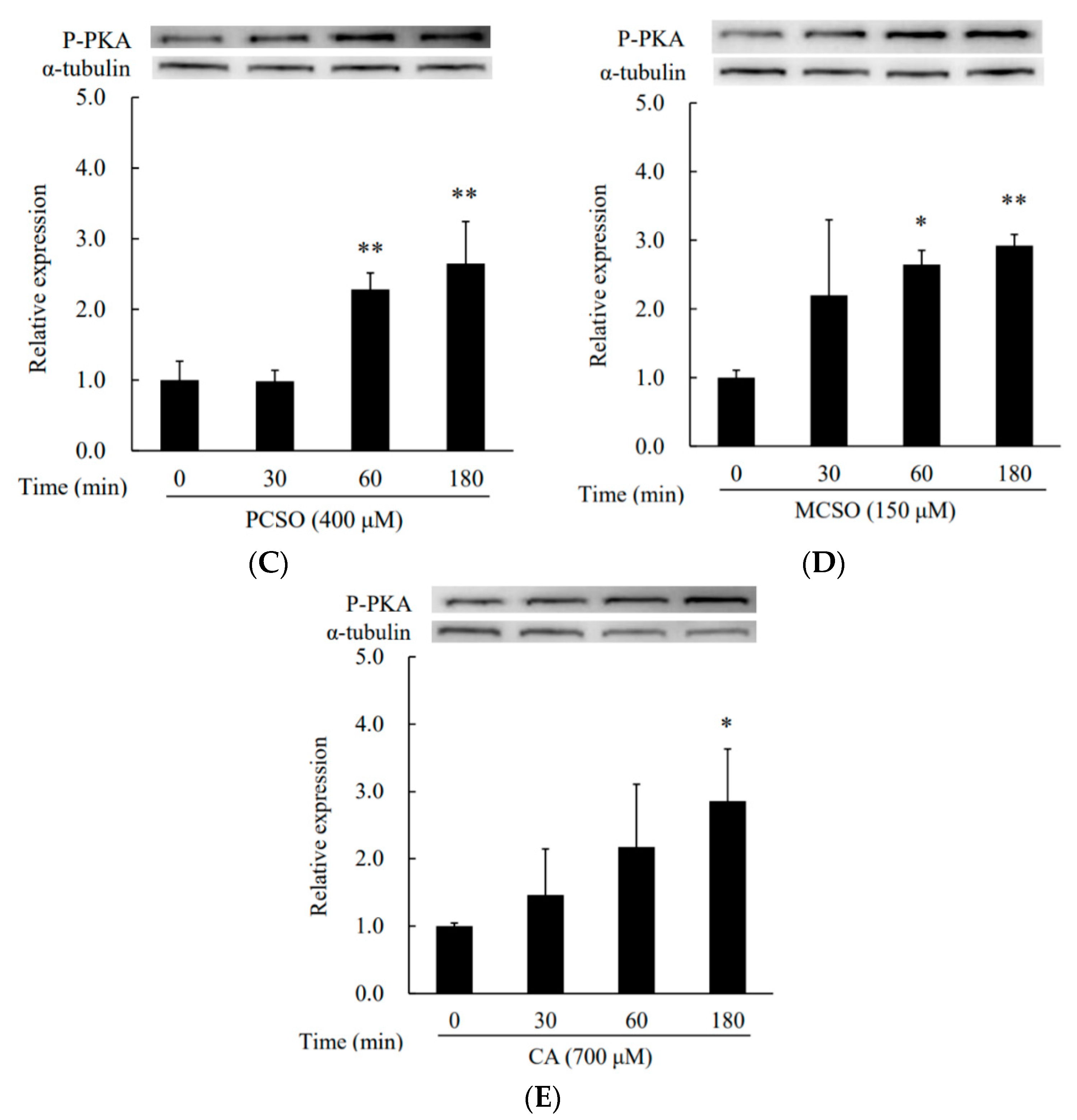
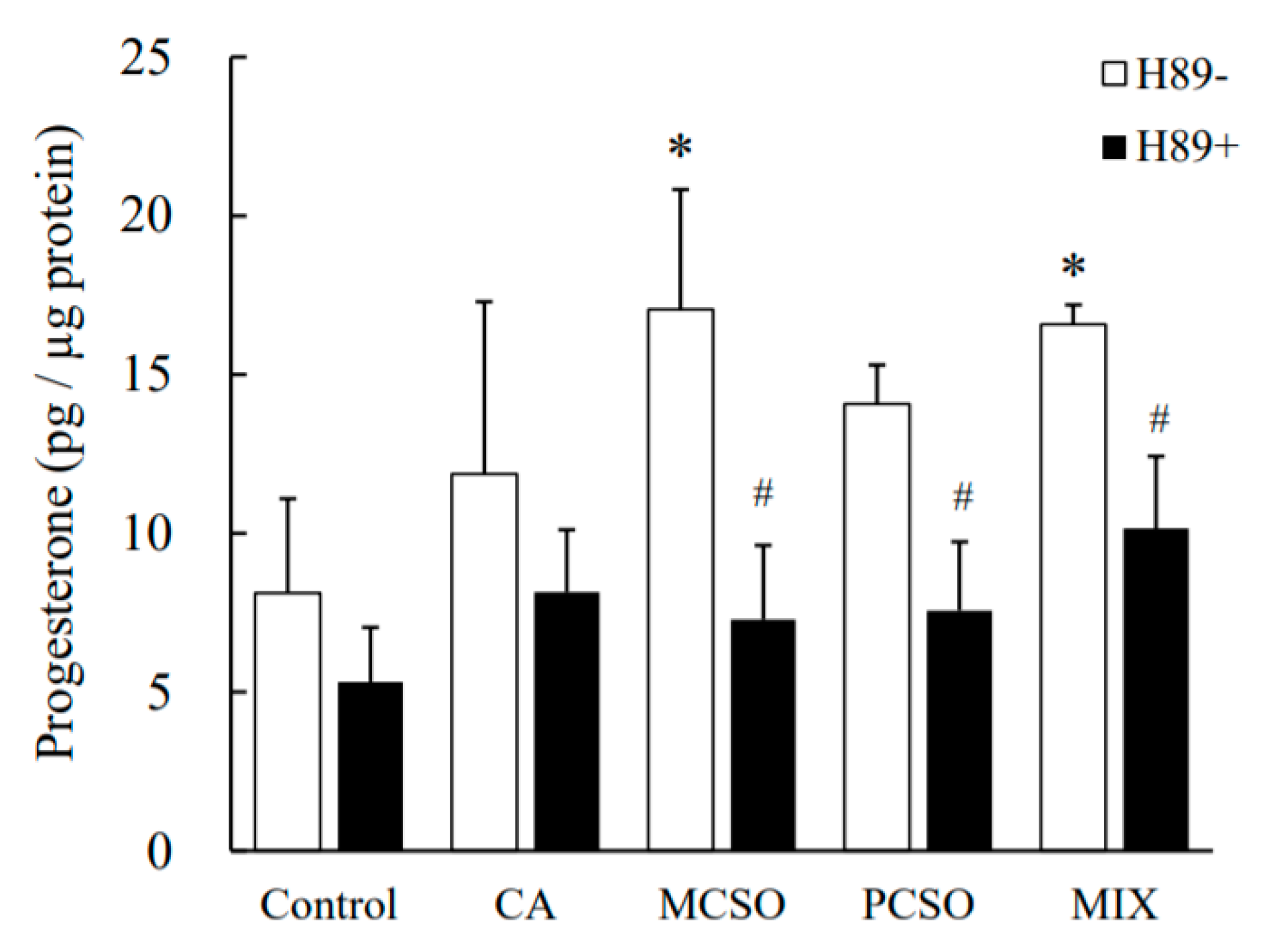
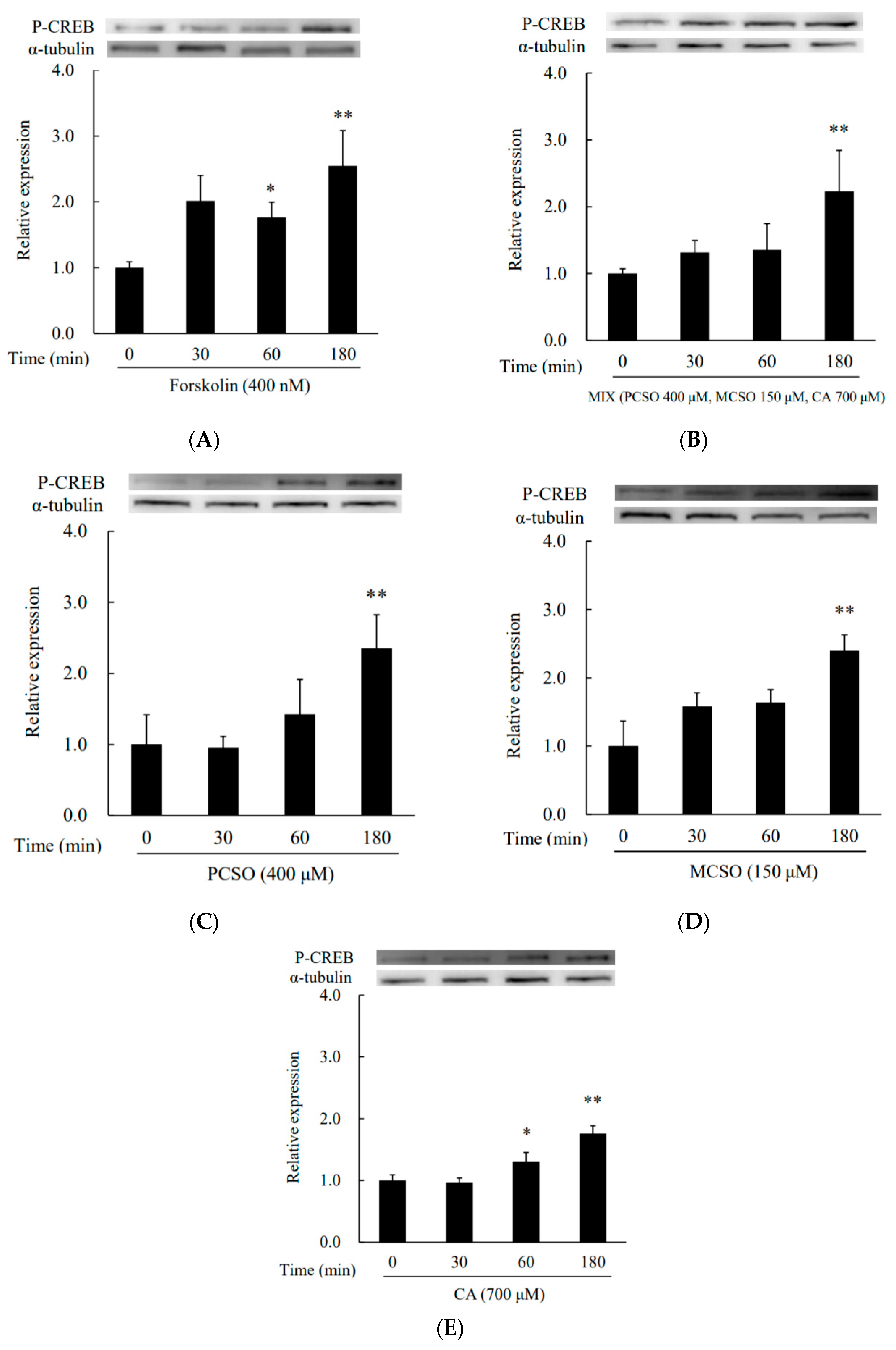
2.3. Cysteine Sulfoxides Regulate the Activation of PKA and cAMP Response Element-Binding Protein (CREB)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. High Performance Liquid Chromatography (HPLC) Analysis
4.3. Cell Culture
4.4. Measurement of Progesterone Levels in Culture Medium
4.5. Cell Growth Assays
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kristin, M.V.; Kathleen, A.C.; Janet, A.D. Maternal salivary testosterone in pregnancy and fetal neuromaturation. Dev. Psychobiol. 2017, 59, 822–831. [Google Scholar]
- Lee, B.S.; William, H.W. The Regulation of Spermatogenesis by Androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar]
- Antonio, L.; Wu, F.C.; O’Neill, T.W.; Pye, S.R.; Ahern, T.B.; Laurent, M.R.; Huhtaniemi, I.T.; Lean, M.E.; Keevil, B.G.; Rastrelli, G.; et al. Low Free Testosterone Is Associated with Hypogonadal Signs and Symptoms in Men with Normal Total Testosterone. J. Clin. Endocrinol. Metab. 2016, 101, 2647–2657. [Google Scholar] [CrossRef]
- Okamura, K.; Ando, F.; Shimokata, H. Serum total and free testosterone level of Japanese men: A population-based study. Int. J. Urol. 2005, 12, 810–814. [Google Scholar]
- Iwamoto, T.; Yanase, T.; Horie, H.; Namiki, M.; Okuyama, A. Late-onset hypogonadism (LOH) and androgens: Validity of the measurement of free testosterone levels in the diagnostic criteria in Japan. Int. J. Urol. 2009, 16, 168–174. [Google Scholar] [CrossRef]
- Desai, N.; Sabanegh, E., Jr.; Kim, T.; Agarwal, A. Free Radical Theory of Aging: Implications in Male Infertility. Urology 2010, 75, 14–19. [Google Scholar]
- Andre, B.A.; Gretchen, R.E.; Varant, K.; O’Donnell, A.B.; Thomas, T.G.; Rachel, E.; Williams, R.E.; McKinlay, J.B. Prevalence of Symptomatic Androgen Deficiency in Men. J. Clin. Endocrinol. Metab. 2007, 92, 4241–4247. [Google Scholar]
- Beatrice, A.M.; Dutta, D.; Kumar, M.; Kumbenahalli, S.S.; Sinha, A.; Ray, S.; Chowdhury, S. Testosterone levels and type 2 diabetes in men: Current knowledge and clinical implications. Diabetes Metab. Syndr. Obes. 2014, 20, 481–486. [Google Scholar]
- Akishita, M.; Hashimoto, M.; Ohike, Y.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis 2010, 210, 232–236. [Google Scholar] [CrossRef]
- Tsai, E.C.; Boyko, E.J.; Leonetti, D.L.; Fujimoto, W.Y. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, R.M.; Perry, P.J. Depression in Aging Men: The Role of Testosterone. Drugs Aging 2004, 21, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ge, R.S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell. Endocrinol. 2009, 10, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, B.; Borut, A.; Cohen, S. Production of testosterone from progesterone by rat testicular microsomes without release of the intermediates 17 alpha-hydroxyprogesterone and androstenedione. Eur. J. Biochem. 1987, 166, 425–429. [Google Scholar] [CrossRef]
- Payne, A.H. Steroidogenic enzymes in Leydig cells. In The Leydig Cell in Health and Disease; Payne, A.H., Hardy, M.P., Eds.; Human Press: Totowa, NJ, USA, 2007; pp. 157–171. [Google Scholar]
- Ito, A.; Shirakawa, H.; Takumi, N.; Minegishi, Y.; Ohashi, A.; Howlader, Z.H.; Ohashi, Y.; Sato, T.; Goto, T.; Komai, M. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Health Dis. 2011, 13, 158. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef]
- Nishimura, H.; Higuchi, O.; Tateshita, K. Antioxidative activity of sulfur-containing compounds in Allium species for human LDL oxidation in vitro. Biofactors 2004, 21, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Parkin, K.L. Antioxidant Functions of Selected Allium Thiosulfinates and S-Alk(en)yl-L-Cysteine Sulfoxides. J. Agric Food Chem. 2002, 50, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.; Kim, G.H.; Choi, K. The Antidiabetic Effect of Onion and Garlic in Experimental Diabetic Rats: Meta-Analysis. J. Med. Food. 2009, 12, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.A.; Khamenehi, H.J.; Hamadeh, M. Evaluation of androgenic activity of allium cepa on spermatogenesis in the rat. Folia Morphol. 2009, 68, 45–51. [Google Scholar]
- Nakayama, Y.; Tanaka, K.; Hiramoto, S.; Yahata, N.; Inagawa, Y.; Nukui, K.; Hori, S. Alleviation of the aging males’ symptoms by the intake of onion-extracts containing concentrated cysteine sulfoxides for 4 weeks-Randomized, double-blind, placebo-controlled, parallel-group comparative study. Jpn. Pharmacol. Ther. 2017, 45, 595–608. [Google Scholar]
- Freeman, D.A. Constitutive steroidogenesis in the R2C Leydig tumor cell line is maintained by the adenosine 3’,5’-cyclic monophosphate-independent production of a cycloheximide-sensitive factor that enhances mitochondrial pregnenolone biosynthesis. Endocrinology 1987, 120, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Kato, N.; Shao, R.X.; Hoshida, Y.; Ijichi, H.; Koike, Y.; Taniguchi, H.; Moriyama, M.; Shiratori, Y.; Kawabe, T.; et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology 2004, 40, 243–251. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, Y.; Ho, H.-J.; Yamagishi, M.; Ikemoto, H.; Komai, M.; Shirakawa, H. Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules 2020, 25, 4694. https://doi.org/10.3390/molecules25204694
Nakayama Y, Ho H-J, Yamagishi M, Ikemoto H, Komai M, Shirakawa H. Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules. 2020; 25(20):4694. https://doi.org/10.3390/molecules25204694
Chicago/Turabian StyleNakayama, Yuya, Hsin-Jung Ho, Miki Yamagishi, Hiroyuki Ikemoto, Michio Komai, and Hitoshi Shirakawa. 2020. "Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells" Molecules 25, no. 20: 4694. https://doi.org/10.3390/molecules25204694
APA StyleNakayama, Y., Ho, H.-J., Yamagishi, M., Ikemoto, H., Komai, M., & Shirakawa, H. (2020). Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules, 25(20), 4694. https://doi.org/10.3390/molecules25204694





