Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment
Abstract
1. Introduction
2. Results
2.1. Visible Spectroscopy
2.2. FTIR Spectroscopy
2.3. Theoretical Calculations
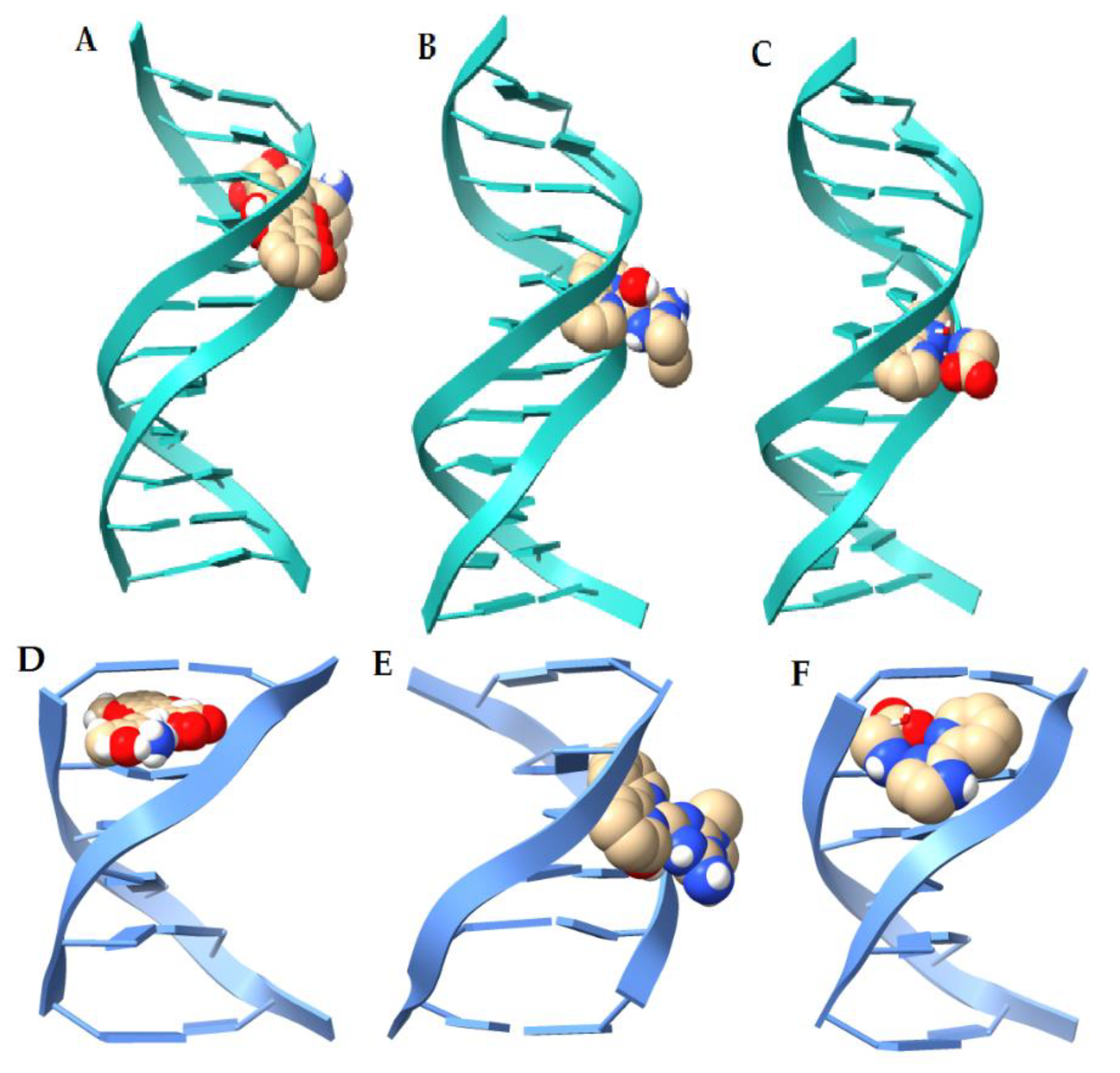
2.4. Molecular Docking (DNA)
| Ligand | Binding Energy (kcal/mol) 1BNA [52] | Binding Energy (kcal/mol) 151D [53] | Interaction |
|---|---|---|---|
| Doxorubicine | −11.09 | −11.54 | H bond, π-anion |
| 1’ [Cu(Metf)(bipy)(H2O)]2+ | −9.69 | −7.05 | H bond, salt-bridge |
| 2. [Cu(Impy)(Gly)(H2O)]+ | −8.82 | −6.73 | H bond, π-anion |
| 3. [Cu(phen)(Lys)(H2O)]2+ [37] | −11.03 | −9.98 | H Bond, π-anion, salt-bridge |
| 4. [Cu(bipy)(Orn)(H2O)]2+ [37] | −11.12 | −9.68 | H bond, salt-bridge, π-anion |
| 5. [Cu(phen)(Gly)(H2O)]+ [38] | −9.5 | −8.52 | H bond, |
| 6. [Cu(phen)(Orn))(H2O)]2+ [#] | −11.05 | −9.43 | H bond, salt-bridge, π-anion |
| 7. [Cu(bipy)(Lys)(H2O)]2+ [#] | −11.04 | −8.72 | H bond, salt-bridge |
| 8. [Cu(phen)2(H2O)]+ [54] | −8.79 | −8.53 | H bond, π-anion |
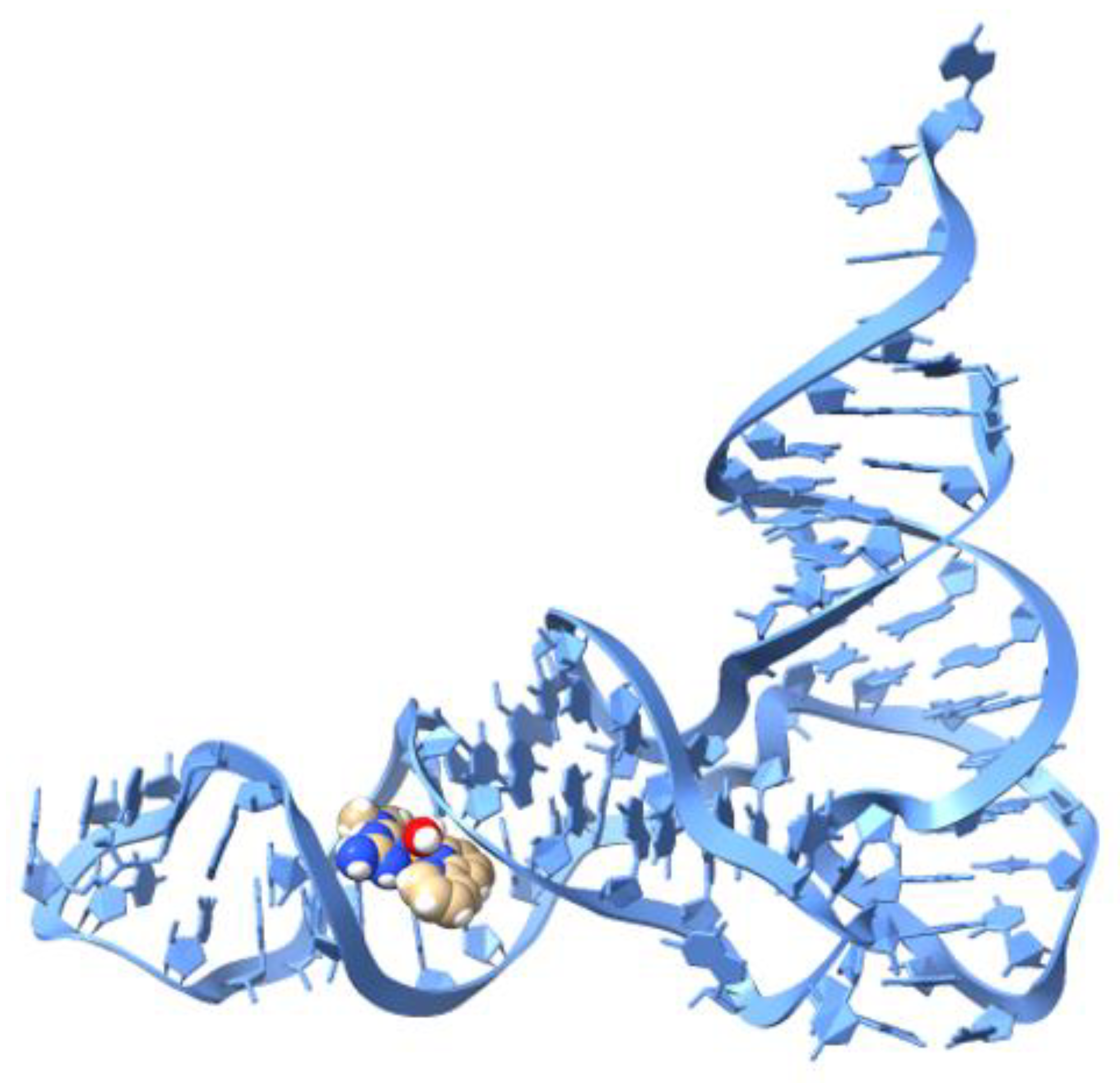
2.5. Molecular Docking (tRNA)
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Computational Methods
4.3. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- OPS/OMS, Programa de Cáncer. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=292:cancer-program&Itemid=3904&lang=es (accessed on 30 August 2020).
- Ezhilarasan, D.; Arumugham, M.N. Synthesis, characterization DNA binding and biological activity of Copper(II) complexes with mixed ligands. J. Chem. Biol. Phys. Sci. 2017, 7, 896–905. [Google Scholar]
- Spreckelmeyer, S.; Orvig, C.; Casini, A. Cellular transport mechanisms of cytotoxic metallodrugs: An overview beyond cisplatin. Molecules 2014, 19, 15584–15610. [Google Scholar] [CrossRef]
- Seiji, K.; Casini, A. Next-generation anti-cancer metallodrugs. Curr. Trends Med. Chem. 2012, 12, 219–235. [Google Scholar]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Ndagi, U.; Ndumiso, M.; Mahmoud, E.S. Metal complexes in cancer therapy–an update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum Neurotoxicity Pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Gaál, A.; Orgován, G.; Mihucz, V.G.; Pape, I.; Ingerle, D.; Streli, C.; Szoboszlai, N.J. Metal Transport Capabilities of Anticancer Copper Chelators. Trace Elem. Med. Biol. 2018, 47, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Krajčiová, D.; Melník, M.; Havránek, E.; Forgácsová, A.; Mikuš, P. Copper compounds in nuclear medicine and oncology. J. Coord. Chem. 2014, 67, 1493–1519. [Google Scholar] [CrossRef]
- Dhakshanamoorthy, S.; Krishnan, M.M.; Arumugham, M.N. Synthesis, characterisation, DNA binding/cleavage, anticancer and antimicrobial activity of ternary copper(II) complexes. Asian J. Res. Chem. 2017, 10, 312–318. [Google Scholar] [CrossRef]
- Paterson, B.M.; Donnelly, P.S. Copper complexes of bis(thiosemicarbazones): From chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gómez, M.E. Copper compounds in cancer chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Manzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Tardito, S.; Marchiò, L. Copper compounds in anti-cancer strategies. Curr. Med. Chem. 2009, 16, 1325–1348. [Google Scholar] [CrossRef]
- Shobha, C.; Thulasiram, B.; Aerva, R.R.; Nagababu, P. Recent Advances in Copper Intercalators as Anticancer Agents. J. Fluoresc. 2018, 28, 1195–1205. [Google Scholar] [CrossRef]
- Kuwabara, M.; Yoon, C.; Goyne, T.; Thederahn, T.; Sigman, D.S. Nuclease activity of 1, 10-phenanthroline-copper ion: Reaction with CGCGAATTCGCG and its complexes with netropsin and EcoRI. Biochemistry 1986, 25, 7401–7408. [Google Scholar] [CrossRef]
- Tabti, R.; Tounsi, N.; Gaiddon, C.; Bentouhami, E.; Désaubry, L. Progress in Copper Complexes as Anticancer Agents. Med. Chem. (Los Angeles) 2017, 7, 875–879. [Google Scholar] [CrossRef]
- Sigman, D.S.; Graham, D.R.; Aurora, V.D.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar]
- Kwik, W.L.; Ang, K.P.; Chen, G. Complexes of (2,2′-bipyridyl) copper(II) and (1,10-phenanthroline) copper(II) with some amino acids. J. Inorg. Nucl. Chem. 1980, 42, 303–313. [Google Scholar] [CrossRef]
- Zelenko, O.; Gallagher, Y.X.; Sigman, D.S. Chemical Nuclease Activity of 1, 10-Phenanthroline−Copper. Isotopic Probes of Mechanism. Inorg. Chem. 1998, 37, 2198–2204. [Google Scholar] [CrossRef]
- Zhang, S.; Chun, X.; Chen, Y.; Zhou, J. Synthesis, Crystal Structure and DNA Cleavage Activity of a Ternary Copper(II) Complex of Dipyrido[3,2-d:2′,3′-f]-quinoxaline and Glycine. Chin. J. Chem. 2011, 29, 65–71. [Google Scholar] [CrossRef]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anti-cancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stepniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef]
- Irving, E.; Stoker, A.W. Vanadium Compounds as PTP Inhibitors. Molecules 2017, 22, 2269. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar] [CrossRef]
- Correia, I.; Roy, S.; Matos, C.P.; Borovic, S.; Butenko, N.; Cavaco, I.; Marques, F.; Lorenzo, J.; Rodríguez, A.; Moreno, V.; et al. Vanadium(IV) and copper(II) complexes of salicylaldimines and aromatic heterocycles: Cytotoxicity, DNA binding, and DNA cleavage properties. J. Inorg. Biochem. 2015, 147, 134–146. [Google Scholar] [CrossRef]
- Novotny, L.; Kombian, S.B. Vanadium: Possible Use in Cancer Chemoprevention and Therapy. J. Cancer Res. Updates 2014, 3, 97–102. [Google Scholar] [CrossRef]
- Ling-Pan, L.; Feng-Zhi, S.; Ya-Li, F.; Li-Li, S.; Ying, L.; Yang-Jie, L.; Kai-Ti, W. Synthesis and biological evaluation of vanadium complex as novel antitumor agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar]
- Griffin, E.; Levina, A.; Lay, P.A. Vanadium(V) tris-3,5-di-tert-butylcatecholato complex: Links between speciation and antiproliferative activity in human pancreatic cancer cells. J. Inorg. Biochem. 2019, 201, 110815. [Google Scholar] [CrossRef]
- Rozzo, C.; Sanna, D.; Garribba, E.; Serra, M.; Cantara, A.; Palmieri, G.; Pisano, M. Antitumoral effect of vanadium compounds in malignant melanoma cell lines. J. Inorg. Biochem. 2017, 174, 14–24. [Google Scholar] [CrossRef]
- Pisano, M.; Arru, C.; Serra, M.; Galleri, G.; Sanna, D.; Garribba, E.; Palmieri, G.; Rozzo, C. Antiproliferative activity of vanadium compounds: Effects on the major malignant melanoma molecular pathways. Metallomics 2019, 11, 1687–1699. [Google Scholar] [CrossRef]
- León, I.L.; Ruiz, M.C.; Franca, C.A.; Parajón-Costa, B.S.; Baran, E.J. Metvan, bis(4,7-Dimethyl-1,10-phenanthroline) sulfatooxidovanadium(IV): DFT and Spectroscopic Study—Antitumor Action on Human Bone and Colorectal Cancer Cell Lines. Biol. Trace Elem. Res. 2018. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Noriega, L.; Sánchez-Gaytán, B.L.; Castro, M.E.; Meléndez-Bustamante, F.J.; González-Vergara, E. Cyclo-tetravanadate bridged copper complexes as potential double bullet pro-metallodrugs for cancer treatment. J. Inorg. Biochem. 2020, 208, 111081. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Sánchez-Gaytán, B.L.; Cerro-López, M.; Mendoza, A.; Castro, M.E.; Meléndez-Bustamante, F.J.; González-Vergara, E. Synthesis and Experimental-Computational Characterization of a Copper/Vanadium Compound with Potential Anticancer Activity. Crystals 2020, 10, 492. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Wang, G.; Cheng, Y.; Zhang, B. Methyl-substituted enhancement of antitumor activity in square-planar metal complex and analysis of DE, DG, CV, UV-vis, and luminescence. New J. Chem. 2015, 39, 4869–4875. [Google Scholar] [CrossRef]
- Sciortino, G.; Maréchal, J.D.; Fábián, I.; Lihi, N.; Garribba, E. Quantitative prediction of electronic absorption spectra of copper (II)–bioligand systems: Validation and applications. J. Inorg. Biochem. 2020, 204, 110953. [Google Scholar]
- Gunasekaran, S.; Natarajan, R.K.; Renganayaki, V.; Natarajan, S. Vibrational spectra and thermodynamic analysis of metformin. Indian J. Pure Appl. Phys. 2006, 44, 495–500. [Google Scholar]
- Sharma, R.P.; Ajnesh, S.; Venugopalan, P.; Dansby-Sparks, R.; Xue, Z.L.; Rossetti, S.; Ferretti, V. Stabilization of tetrameric metavanadate ion by tris (1, 10-phenanthroline) cobalt (III): Synthesis, spectroscopic, and X-ray structural study of [Co (phen) 3] 3 (V4O12) 2Cl·27H2O. J. Coord. Chem. 2010, 63, 3016–3027. [Google Scholar] [CrossRef][Green Version]
- Yurdakul, Ş.; Badoğlu, S. FT-IR spectra, vibrational assignments, and density functional calculations of imidazo [1, 2-a] pyridine molecule and its Zn (II) halide complexes. Struct. Chem. 2009, 20, 423–434. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Facile synthesis, characterization and optical properties of copper vanadate nanostructures for enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 4871–4878. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sanchez-Gaytan, B.L.; Castro, M.E.; Bernes, S.; Méndez-Rojas, M.A.; Meléndez-Bustamante, F.J.; González-Vergara, E. A one-dimensional supramolecular chain based on [H2V10O28]4− units decorated with 4-dimethylaminopyridinium ions: An experimental and theoretical characterization. New J. Chem. 2019, 43, 17746–17755. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199. [Google Scholar] [CrossRef]
- Jenkins, S.; Morrison, I. The chemical character of the intermolecular bonds of seven phases of ice as revealed by ab initio calculation of electron densities. Chem. Phys. Lett. 2000, 317, 97–102. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440. [Google Scholar] [CrossRef]
- Galindo-Murillo, R.; García-Ramos, J.C.; Ruiz-Azuara, L.; Cheatham, T.E.; Cortés-Guzmán, F. Intercalation processes of copper complexes in DNA. Nucleic Acids Res. 2015, 43, 5364–5376. [Google Scholar] [CrossRef]
- Robertazzi, A.; Vargiu, A.V.; Magistrato, A.; Ruggerone, P.; Carloni, P.; de Hoog, P.; Reedijk, J. Copper-1, 10-phenanthroline complexes binding to DNA: Structural predictions from molecular simulations. J. Phys. Chem. B 2009, 113, 10881–10890. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.A.; Peek, M.E.; Zhou, F.X.; Bertrand, J.A.; VanDerveer, D.; Williams, L.D. Water ring structure at DNA interfaces: Hydration and dynamics of DNA-anthracycline complexes. Biochemistry 1994, 33, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N.; Yogendra, P.S.; Yogendra, S.; Raymond, J.B. Synthesis, crystal structure, DFT computation, and bioactivity measurements of copper (II) polypyridyl complexes. Polyhedron 2016, 104, 116–126. [Google Scholar] [CrossRef]
- Santos, M.; Fidalgo, A.; Varanda, A.S.; Oliveira, C.; Santos, M.A. tRNA deregulation and its consequences in cancer. Trends Mol. Med. 2019, 25, 853–865. [Google Scholar] [CrossRef]
- Sussman, J.L.; Holbrook, S.R.; Warrant, R.W.; Church, G.M.; Kim, S.H. Crystal structure of yeast phenylalanine transfer RNA. I. Crystallographic refinement. J. Mol. Biol. 1978, 123, 607–630. [Google Scholar] [CrossRef]
- Arjmand, F.; Yousuf, I.; Zaidi, Y.; Toupet, L. Crystal structure determination, spectroscopic characterization and biological profile of a tailored ionic molecular entity, Sn (iv) iminodiacetic acid–piperazinediium conjugate: In vitro DNA/RNA binding studies, Topo I inhibition activity, cytotoxic and systemic toxicity studies. RSC Adv. 2015, 5, 16250–16264. [Google Scholar]
- Agudelo, D.; Bourassa, P.; Beauregard, M.; Bérubé, G.; Tajmir-Riahi, H.A. tRNA binding to antitumor drug doxorubicin and its analogue. PLoS ONE 2013, 8, e69248. [Google Scholar] [CrossRef]
- Parveen, S.; Fatima, K.; Zehra, S.; Arjmand, F. RNA-targeted Cu (II)-based potential antitumor drug entity: Comprehensive structural, biological {DNA/RNA binding, cleavage, cytotoxicity} and computational studies. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper complex as anti-cancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and its complexes in medicine: A biochemical approach. Mol. Biol. Int. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Rajendiran, V.; Karthik, R.; Palaniandavar, M.; Stoeckli-Evans, H.; Periasamy, V.S.; Akbarsha, M.A.; Srinag, B.S.; Krishnamurthy, H. Mixed-ligand copper(II)-phenolate complexes: Effect of coligand on enhanced DNA and protein binding, DNA cleavage, and anti-cancer activity. Inorg. Chem. 2007, 46, 8208–8221. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Zheng, Y.-J.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Synthesis and structure of new bicopper(II) complexes bridged by N-(2-aminopropyl)-N′-(2-oxidophenyl)oxamide: The effects of terminal ligands on structures, anti-cancer activities, and DNA-binding properties. Eur. J. Med. Chem. 2011, 46, 3851–3857. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Zanetti, S.; Pongi, R.; Basosi, R. An electron spin resonance study and antimicrobial activity of copper (II)-phenanthroline complexes. J. Inorg. Biochem. 1996, 63, 291–300. [Google Scholar] [CrossRef]
- Patel, R.N.; Singh, N.; Shukla, K.K.; Niclós-Gutiérrez, J.; Castinerias, A.; Vaidyanathan, V.G.; Nair, B.U. Characterization and biological activities of two copper(II) complexes with diethylenetriamine and 2,2′-bipyridine or 1,10-phenanthroline as ligands. Spectrochim. Acta Part A 2005, 62, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rufino-González, Y.; Ponce-Macotela, M.; García-Ramos, J.C.; Martínez-Gordillo, M.N.; Galindo-Murillo, R.; González-Maciel, A.; Reynoso-Robles, R.; Tovar-Tovar, A.; Flores-Alamo, M.; Toledano-Magaña, Y.; et al. Antigiardiasic activity of Cu(II) coordination compounds: Redox imbalance and membrane damage after a short exposure time. J. Inorg. Biochem. 2019, 195, 83–90. [Google Scholar] [CrossRef]
- Paixão, D.A.; Lopez, C.D.; Carneiro, Z.A.; Sousa, L.M.; de Oliveira, L.P.; Lopes, N.P.; Pivatto, M.; Chaves, J.D.S.; de Almeida, M.V.; Ellena, J. In vitro anti-Trypanosoma cruzi activity of ternary copper(II) complexes and in vivo evaluation of the most promising complex. Biomed. Pharm. 2019, 109, 157–166. [Google Scholar] [CrossRef] [PubMed]
- McGivern, T.J.P.; Afsharpour, S.; Marmion, C.J. Copper complexes as artificial DNA metallonucleases: From Sigman’s reagent to next-generation anti-cancer agent? Inorg. Chim. Acta 2018, 472, 12–39. [Google Scholar] [CrossRef]
- Alvarez, N.; Viña, D.; Leite, C.M.; Mendes, L.F.S.; Batista, A.A.; Ellena, J.; Costa-Filho, A.; Facchin, G. Synthesis and structural characterization of a series of ternary copper(II)-L-dipeptide-neocuproine complexes. Study of their cytotoxicity against cancer cells including MDA-MB-231, triple-negative breast cancer cells. J. Inorg. Biochem. 2019, 203, 110930. [Google Scholar] [CrossRef]
- Mroueh, M.; Daher, C.; Hariri, E.; Demirdjian, S.; Isber, S.; Choid, E.S.; Mirtamizdoust, B.; Hammud, H.H. Magnetic property, DFT calculation, and biological activity of bis [(μ2-chloro) chloro (1, 10-phenanthroline) copper (II)] complex. Chem. Biol. Interact. 2015, 231, 53–60. [Google Scholar] [CrossRef]
- Hayat, F.; Faryad, A.R.; Zia-ur, R.; Bélanger-Gariepy, F. Molecular, supramolecular, DNA-binding and biological studies of piperazine and piperidine based dithiocarbamates of biocompatible copper. Inorg. Chem. Commun. 2020, 121, 108190. [Google Scholar] [CrossRef]
- Gordon, A.T.; Abosede, O.O.; Ntsimango, S.; van Vuuren, S.; Hosten, E.C.; Ogunlaga, A.S. Synthesis, characterization, molecular docking and antimicrobial activity of T copper(II) complexes of metronidazole and 1,10 phenanthroline. Inorg. Chim. Acta 2020, 510, 119744. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R.; Vatan, Ö.; Zorlu, Y. A potent drug candidature of Cu(II) pyrazino[2,3 f][1,10]phenanthroline complexes with bioactive ligands: Synthesis, crystal structures, biomolecular interactions, radical scavenging and cytotoxicities. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef] [PubMed]
- Alemon-Medina, R.; Brena-Valle, M.; Muñoz-Sánchez, J.L.; Gracia-Mora, M.I.; Ruiz-Azuara, L. Induction of oxidative damage by copper-based antineoplastic drugs (Casiopeínas). Cancer Chemother. Pharm. 2007, 60, 219–228. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate Complexes from Phenylate Phenathrolines to Be Used as Anticancerigenic Agents. European Patent EP 0434444A1, 26 June 1991. [Google Scholar]
- Bravo-Gómez, M.E.; Campero-Peredo, C.; García-Conde, D.; Mosqueira-Santillán, M.J.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA-binding mode of antitumoral copper compounds (Casiopeinas®) and analysis of its biological meaning. Polyhedron 2015, 102, 530–538. [Google Scholar] [CrossRef]
- Serment-Guerrero, J.; Cano-Sanchez, P.; Reyes-Perez, E.; Velazquez-Garcia, F.; Bravo-Gomez, M.E.; Ruiz-Azuara, L. Genotoxicity of the copper antineoplastic coordination complexes casiopeinas®. Toxicol. In Vitro 2011, 25, 1376–1384. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bastian, G.; Bravo-Gómez, M.E.; Cañas, R.C.; Flores-Alamo, M.; Fuentes, I.; Mejia, C.; García-Ramos, J.C.; Serrano, A. Abstract CT408: Phase I study of one mixed chelates copper(II) compound, Casiopeína CasIIIia with antitumor activity and its mechanism of action. Cancer Res. 2014, 74. [Google Scholar] [CrossRef]
- Cobar, E.A.; Horn, P.H.; Bergman, R.G.; Head-Gordon, M. Examination of the hydrogen-bonding networks in small water clusters (n = 2–5, 13, 17) using absolutely localized molecular orbital energy decomposition analysis. Phys. Chem. Chem. Phys. 2012, 14, 15328–15339. [Google Scholar] [CrossRef]
- McLauchlan, C.C.; Murakami, H.A.; Wallace, C.A.; Crans, D.C. Coordination environment changes of the vanadium in vanadium-dependent haloperoxidase enzymes. JIB 2018, 186, 267–279. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Calvo, C. The crystal chemistry of the M+ VO3 (M+= Li, Na, K, NH4, Tl, Rb, and Cs) pyroxenes. JSSC 1977, 22, 157–170. [Google Scholar] [CrossRef]
- Pérez-Benítez, A.; Bernès, S. Redetermination of ammonium metavanadate. IUCrData 2018, 3, x181080. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Ferreira Da Silva, J.L.; Minas da Piedade, M.F.; Duarte, M.T. Decavanadates: A building-block for supramolecular assemblies. Inorg. Chim. Acta. 2003, 356, 222–242. [Google Scholar] [CrossRef]
- Amanchi, S.R.; Das, S.K. A Versatile Polyoxovanadate in Diverse Cation Matrices: A Supramolecular Perspective. Front. Chem. 2018, 6, 469. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van de Street, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Austin, A.; Peterson, G.A.; Frisch, M.; Dobek, F.; Scalmani, G.; Throssell, K. A density functional with spherical atom dispersion terms. J. Chem. Theor. Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems: Excitation energies of first row transition metals Sc-Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J.J. How does basis set superposition error change the potential surfaces for hydrogen bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, B; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Turner, M.J.; MacKiinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 4 October 2020).
- Keith, T.A. TK Gristmill Software, Version 19.02.13; AIMAll: Overland Park, KS, USA, 2019. [Google Scholar]
- Gilad, Y.; Senderowitz, H. Docking studies on DNA intercalators. J. Chem. Inf. Model. 2014, 54, 96–107. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

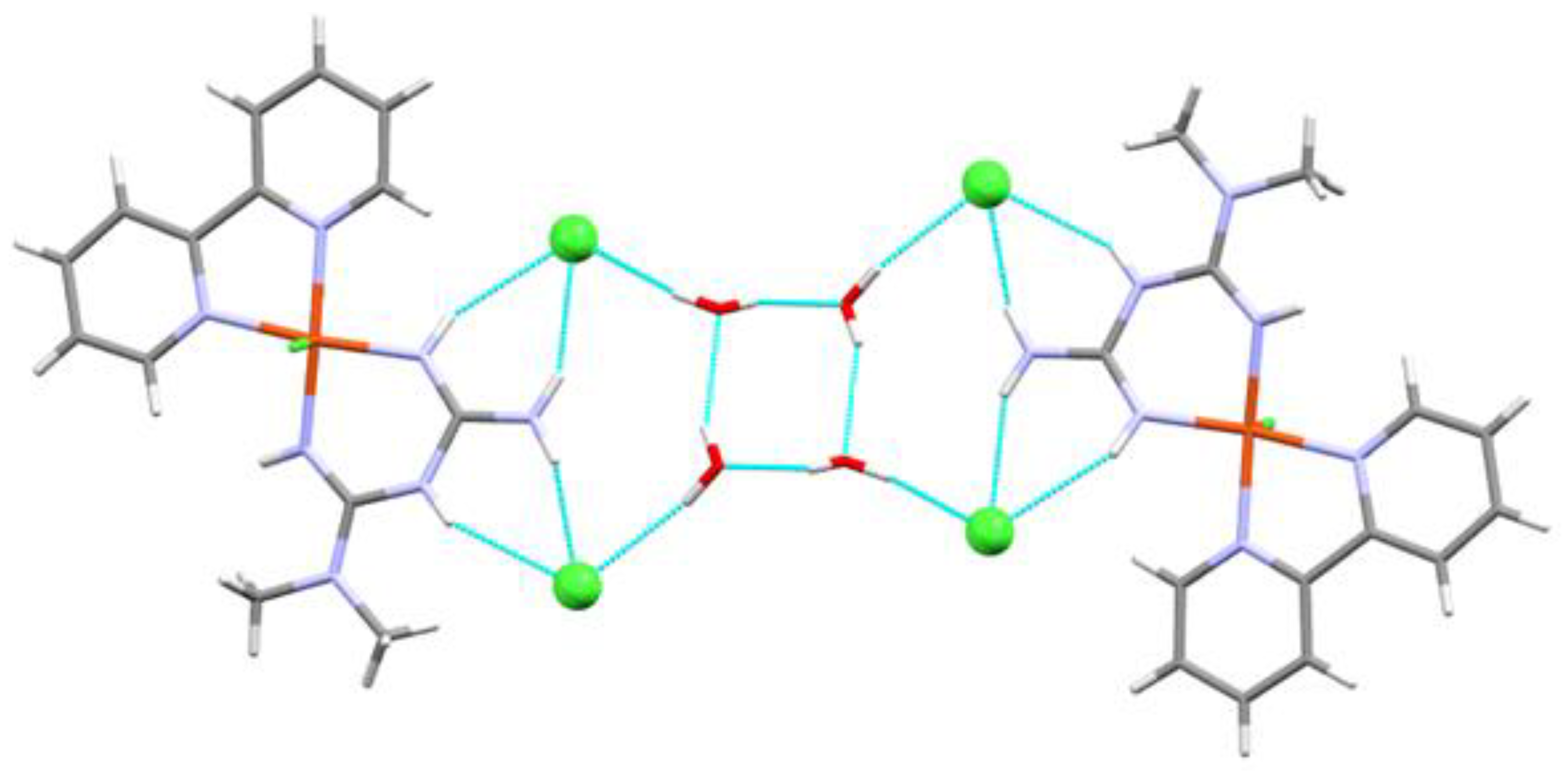

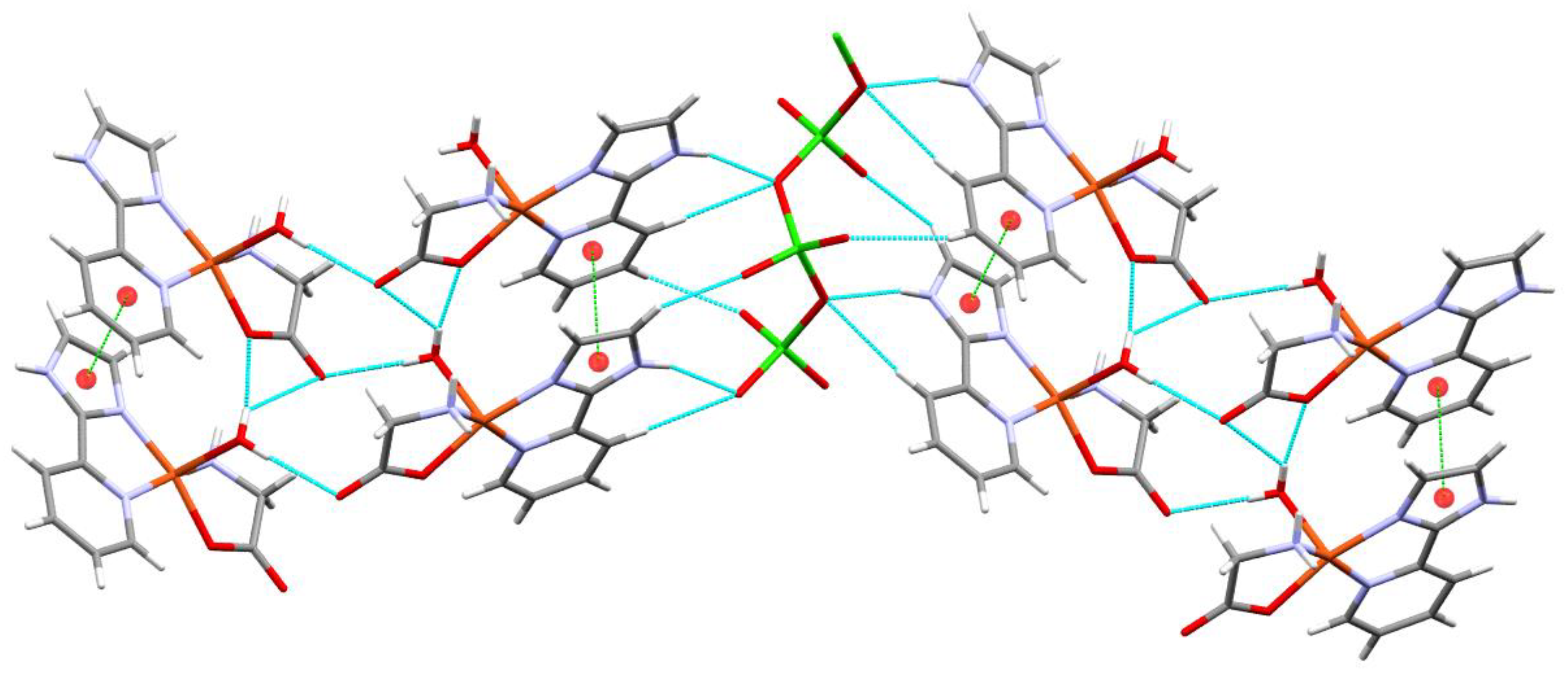
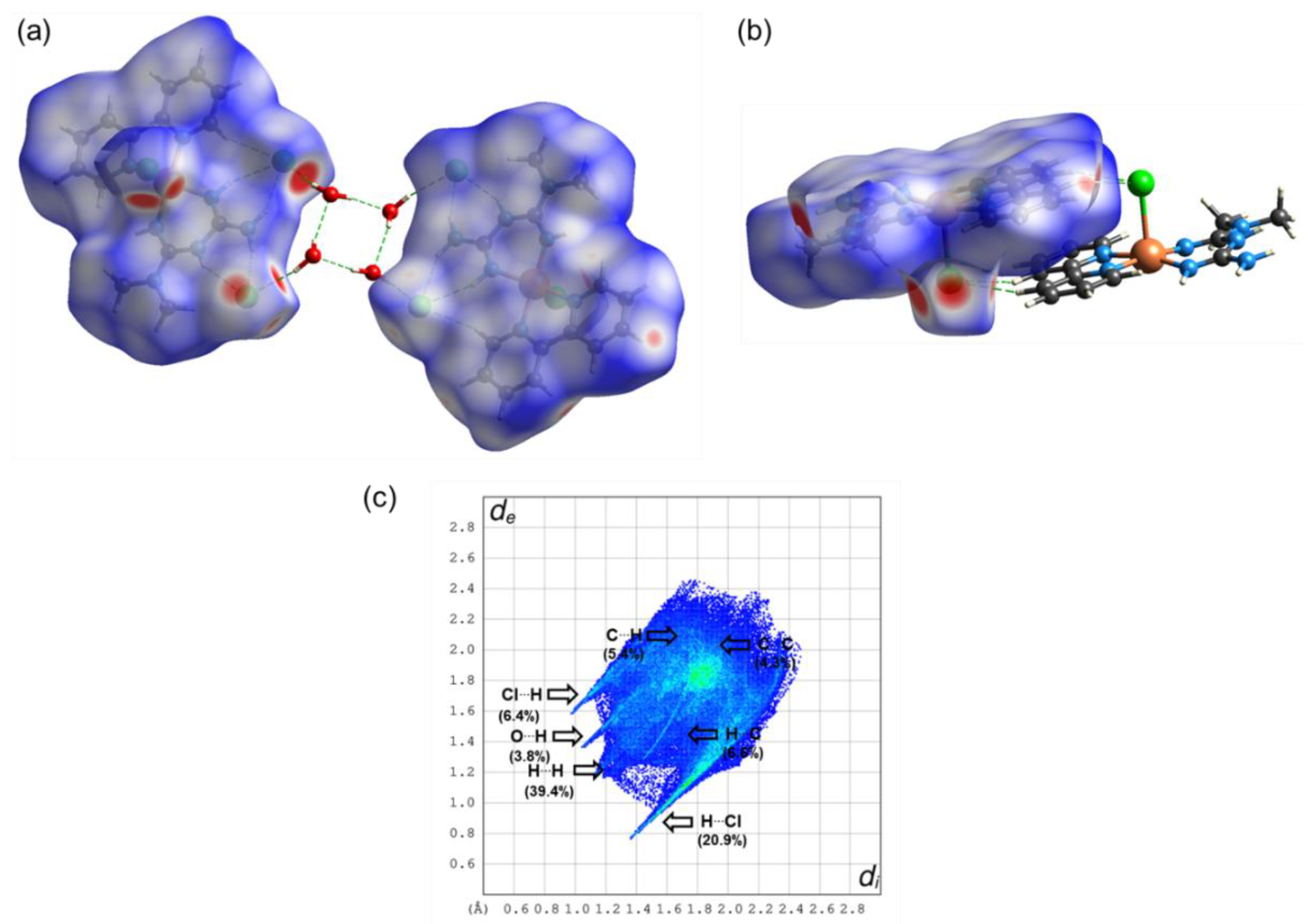
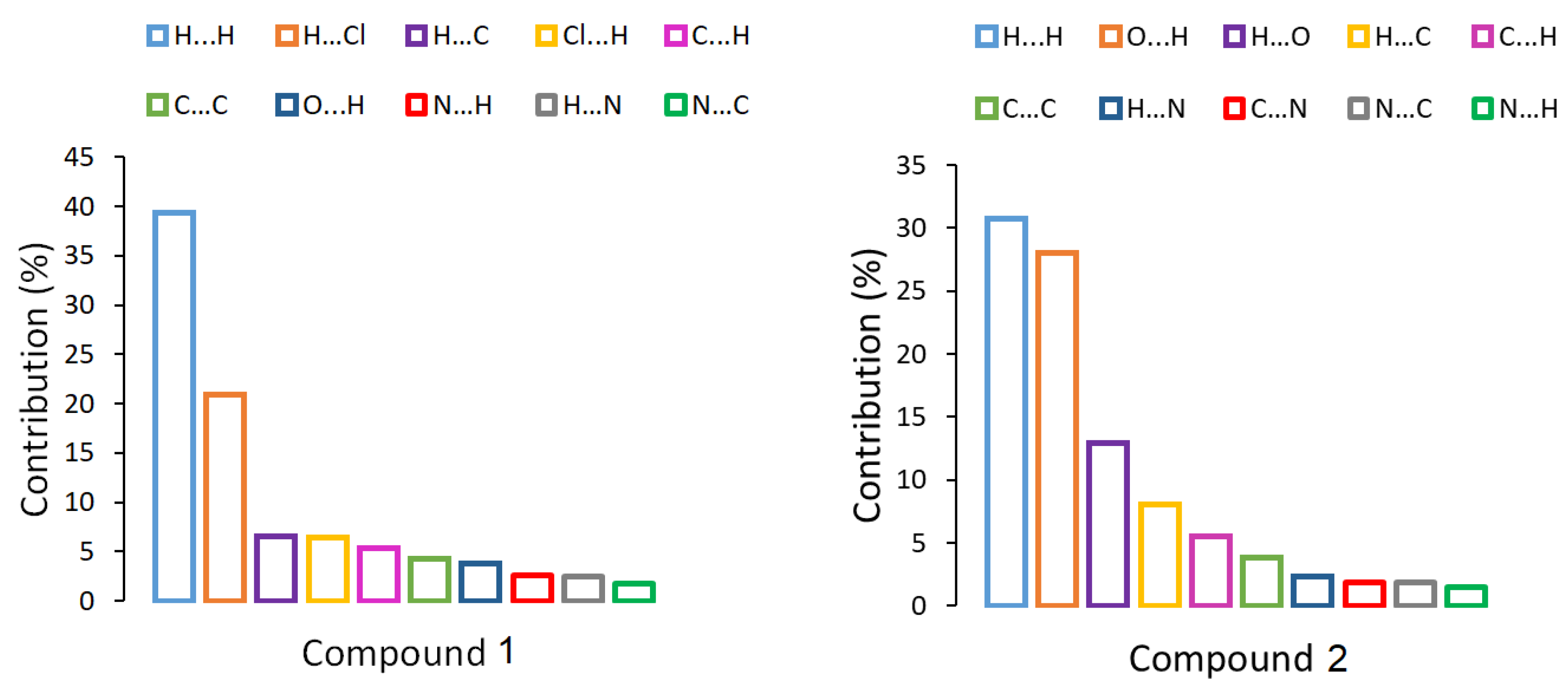
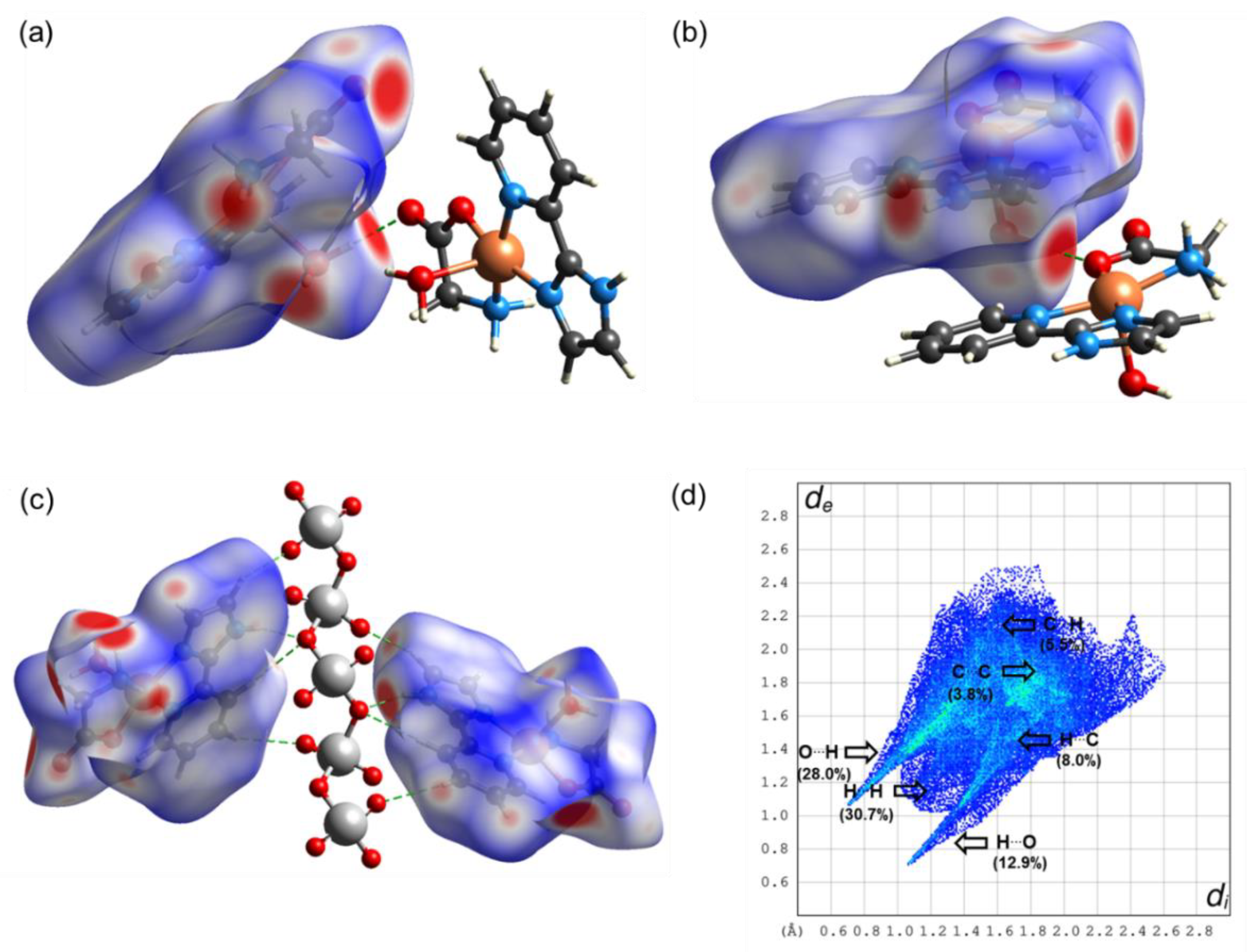
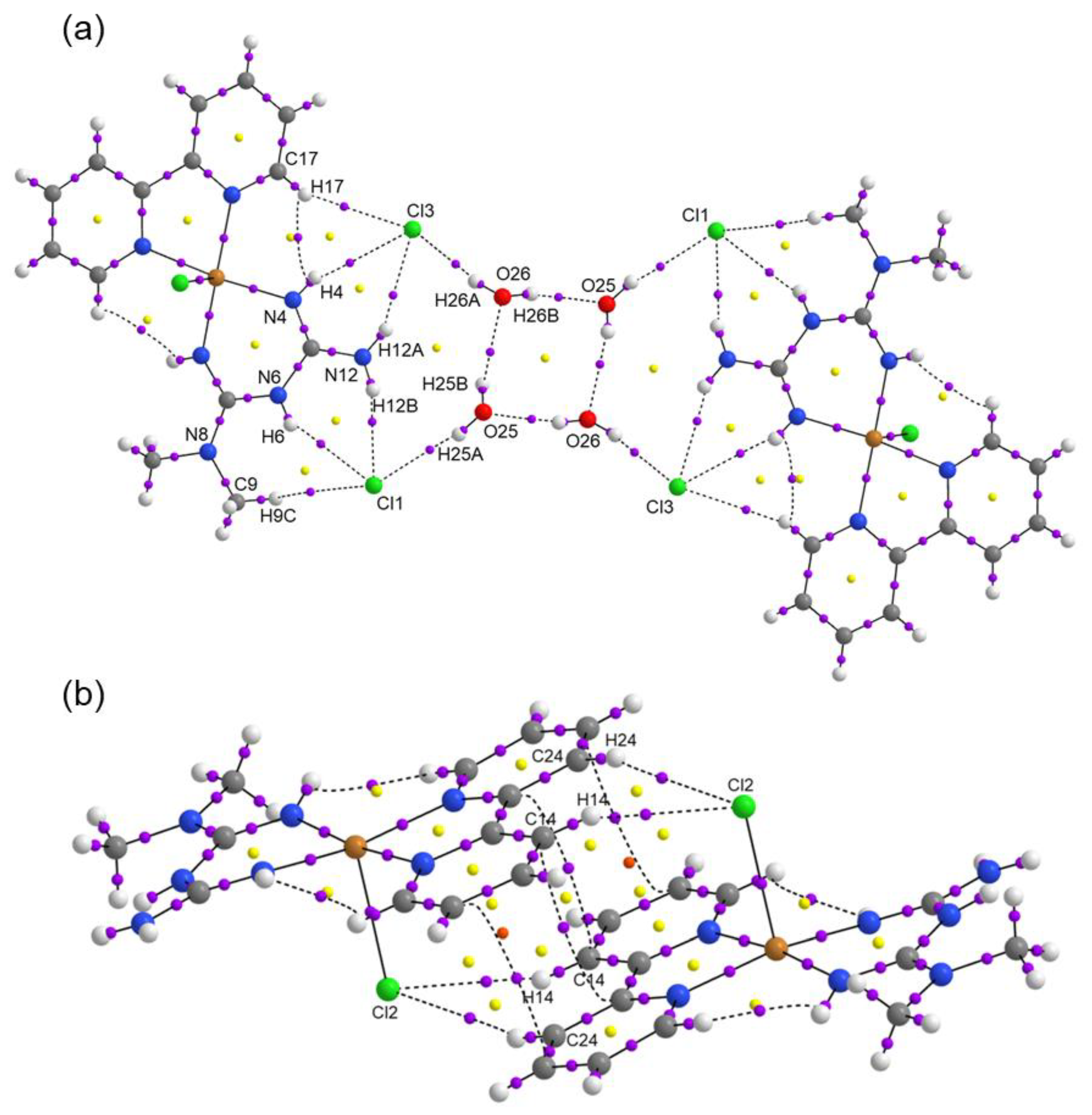
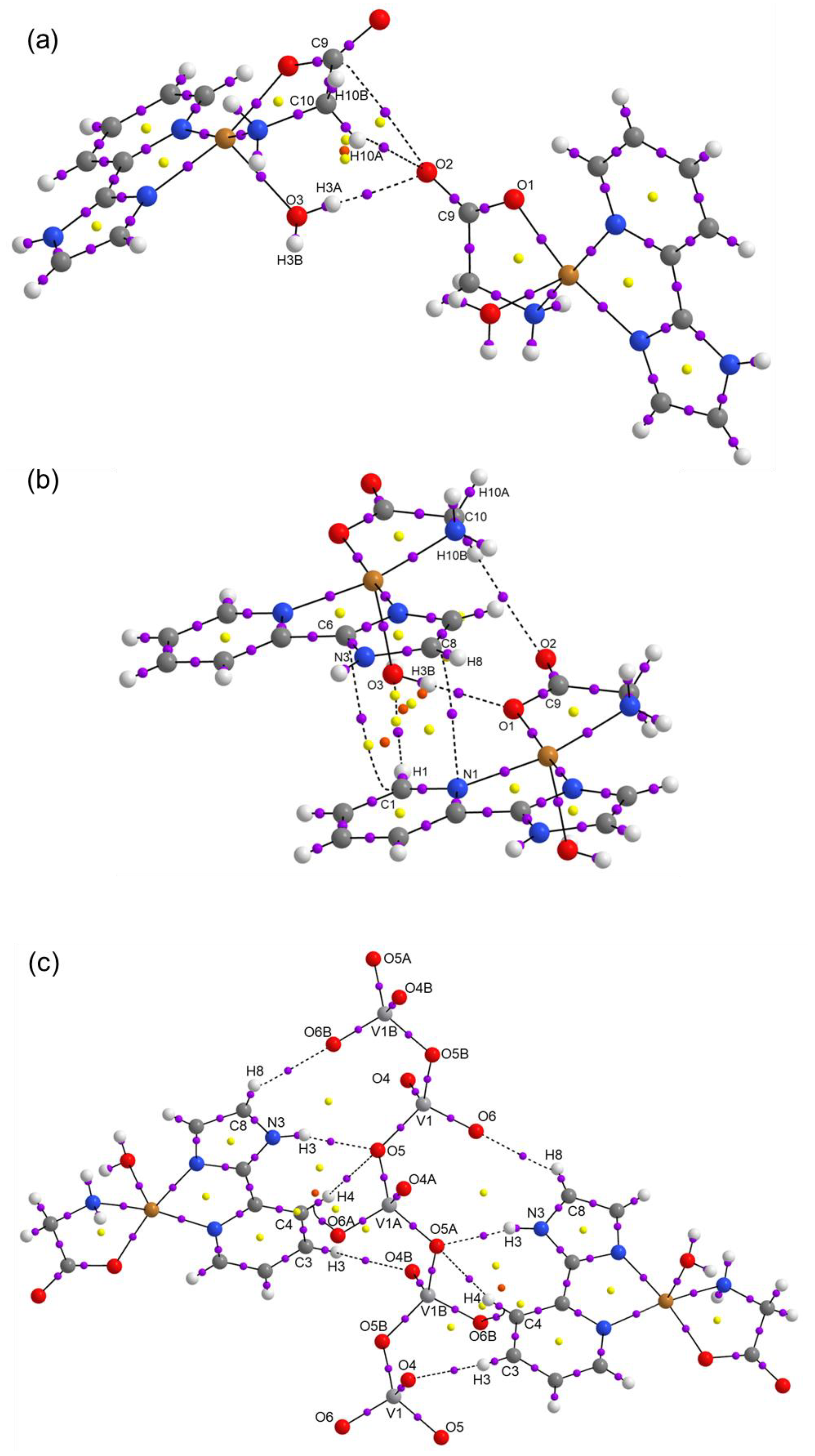
| Compound 1 | Compound 2 | |
|---|---|---|
| Empirical formula | C14H23Cl2CuN7O2 | C10H13CuN4O6V |
| Formula weight | 453.83 | 399.72 |
| Temperature/K | 293 (2) | 293 (2) |
| Crystal system | Triclinic | Monoclinic |
| Space group | P-1 | P21/c |
| a/Å | 8.4235 (3) | 13.3902 (4) |
| b/Å | 10.8688 (5) | 5.21481 (14) |
| c/Å | 11.2005 (4) | 20.6731 (5) |
| α/° | 108.249 (4) | 90 |
| β/° | 93.220 (3) | 107.316 (3) |
| γ/° | 90.608 (3) | 90 |
| Volume/Å3 | 971.91 (7) | 1378.13 (7) |
| Z | 2 | 4 |
| δcalc g/cm3 | 1.551 | 1.927 |
| μ/mm−1 | 1.421 | 2.257 |
| F(000) | 468 | 804 |
| Crystal size/mm3 | 0.67 × 0.275 × 0.12 | 0.34 × 0.211 × 0.093 |
| Radiation | Mo Kα (λ = 0.71073 Å) | Mo Kα (λ = 0.71073 Å) |
| 2Θ range for data collection/° | 5.99 to 77.408 | 5.918 to 70.408 |
| Index ranges | −13 ≤ h ≤ 13, −17 ≤ k ≤ 17, −17 ≤ l ≤ 17 | −20 ≤ h ≤ 21, −8 ≤ k ≤ 8, −33 ≤ l ≤ 33 |
| Reflections collected | 40,564 | 30,742 |
| Independent reflections | 8328 [Rint = 0.0940, Rsigma = 0.0687] | 5883 [Rint = 0.0415, Rsigma = 0.0357] |
| Data/restraints/parameters | 8328/0/247 | 5883/4/211 |
| Goodness-of-fit on F2 | 1.009 | 1.031 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0579, wR2 = 0.1322 | R1 = 0.0405, wR2 = 0.0837 |
| Final R indexes [all data] | R1 = 0.1070, wR2 = 0.1659 | R1 = 0.0659, wR2 = 0.0962 |
| Largest diff. peak/hole/e Å−3 | 0.58/−0.59 | 0.57/−0.85 |
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| O25-H25B···O26 | 0.851 | 2.169 | 2.822 | 133.47 |
| O26-H26B···O25 | 0.850 | 2.002 | 2.828 | 163.68 |
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| NH···OVO2- (N3H3···O5) | 0.861 | 2.050 | 2.877 | 160.96 |
| HOH··· OCO- (O3H3A···O2) | 0.755 | 2.019 | 2.771 | 174.53 |
| HNH···OVO2- (N4H4B···O6) | 0.788 | 2.196 | 2.914 | 151.77 |
| HOH····OCO coord (O3H3B···O1) | 0.773 | 1.995 | 2.762 | 171.17 |
| CH····OCO- (C3H3C···O2) | 0.930 | 2.569 | 3.436 | 155.30 |
| CH····OH2 (C1H1···O3) | 0.930 | 2.580 | 3.426 | 150.50 |
| CH2····OCO- (C10H10A···O2) | 0.970 | 2.587 | 3.406 | 142.16 |
| CH····OVO2- (C3H3C···O6) | 0.930 | 2.541 | 3.139 | 122.36 |
| CH····OVO2- (C4H4····O5) | 0.931 | 2.653 | 3.506 | 152.71 |
| CH····OVO2- (C8H8···O4) | 0.930 | 2.431 | 3.101 | 128.85 |
| Compound | ΔE0 (a.u.) | ΔGsol (kcal mol−1) | Eint (kcal mol−1) |
|---|---|---|---|
| 1 | 0.00 | −67.58 | −152.87 |
| 1′ | 384.03 | −137.26 | −11.33 |
| 2 | 554.52 | −62.49 | −12.50 |
| BCP | ρ(r) | ∇2ρ(r) | G (r) | V (r) | H (r) | EH…Y | Dinter |
|---|---|---|---|---|---|---|---|
| Compound 1 | |||||||
| Cl3⋯H17 | 0.0098 | 0.0031 | 0.0062 | −0.0046 | 0.0108 | 1.44 | 2.279 |
| Cl3⋯H4 | 0.0094 | 0.0325 | 0.0064 | −0.0046 | 0.0110 | 1.44 | 2.699 |
| Cl3⋯H12A | 0.0133 | 0.0469 | 0.0093 | −0.0069 | 0.0162 | 2.16 | 2.504 |
| Cl1⋯H12B | 0.0189 | 0.0661 | 0.0139 | −0.0114 | 0.0253 | 3.58 | 2.334 |
| Cl1⋯H6 | 0.0113 | 0.0379 | 0.0075 | −0.0056 | 0.0131 | 1.76 | 2.603 |
| Cl1⋯H9C | 0.0072 | 0.0217 | 0.0044 | −0.0034 | 0.0078 | 1.07 | 2.933 |
| Cl3⋯H26A | 0.0204 | 0.0686 | 0.0151 | −0.0130 | 0.0281 | 4.08 | 2.279 |
| O25⋯H26B | 0.0213 | 0.0885 | 0.0189 | −0.0158 | 0.0347 | 4.96 | 2.002 |
| Cl1⋯H25A | 0.0126 | 0.0428 | 0.0085 | −0.0062 | 0.0147 | 1.95 | 2.505 |
| O26⋯H25B | 0.0166 | 0.0644 | 0.0140 | −0.0119 | 0.0259 | 3.73 | 2.168 |
| Cl2⋯H24 | 0.0062 | 0.0177 | 0.0036 | −0.0028 | 0.0064 | 0.88 | 2.951 |
| Cl2⋯H14 | 0.0094 | 0.0290 | 0.0058 | −0.0044 | 0.0102 | 1.38 | 2.724 |
| Compound 2 | |||||||
| O2⋯H3A | 0.0056 | 0.0204 | 0.0043 | −0.0034 | 0.0077 | 1.07 | 2.019 |
| O2⋯H10A | 0.0194 | 0.0906 | 0.0187 | −0.0147 | 0.0334 | 4.61 | 2.767 |
| O6B⋯H8 | 0.0096 | 0.0362 | 0.0075 | −0.0060 | 0.0135 | 1.88 | 2.431 |
| O2⋯H10B | 0.0071 | 0.0232 | 0.0050 | −0.0042 | 0.0092 | 1.32 | 2.587 |
| O1⋯H3B | 0.0211 | 0.0966 | 0.0203 | −0.0165 | 0.0368 | 5.18 | 1.995 |
| O5⋯H3 | 0.0193 | 0.0794 | 0.0167 | −0.0136 | 0.0303 | 4.27 | 2.049 |
| O5⋯H4 | 0.0064 | 0.0211 | 0.0045 | −0.0038 | 0.0083 | 1.19 | 2.653 |
| O4B⋯H3 | 0.0083 | 0.0304 | 0.0064 | −0.0052 | 0.0116 | 1.63 | 2.541 |
| Ligand | Binding Energy (kcal/mol) 6TNA | Interaction |
|---|---|---|
| Doxorubicin | −9.82 | H bond, van der Waals, π-anion |
| [Cu(hydroxynaphthaldehyde)(H2O)] | −7.98 | H bond, van der Waals, π-anion, π-π |
| 1′ [Cu(Metf)(bipy)(H2O)]2+ | −12.76 | H bond, van der Waals, π-anion |
| 2 [Cu(Impy)(Gly)(H2O)]+ | −8.86 | H bond, van der Waals, π-anion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Méndez-Rojas, M.Á.; Melendez, F.J.; Castro, M.E.; González-Vergara, E. Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment. Molecules 2020, 25, 4679. https://doi.org/10.3390/molecules25204679
Corona-Motolinia ND, Martínez-Valencia B, Noriega L, Sánchez-Gaytán BL, Méndez-Rojas MÁ, Melendez FJ, Castro ME, González-Vergara E. Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment. Molecules. 2020; 25(20):4679. https://doi.org/10.3390/molecules25204679
Chicago/Turabian StyleCorona-Motolinia, Nidia D., Beatriz Martínez-Valencia, Lisset Noriega, Brenda L. Sánchez-Gaytán, Miguel Ángel Méndez-Rojas, Francisco J. Melendez, María Eugenia Castro, and Enrique González-Vergara. 2020. "Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment" Molecules 25, no. 20: 4679. https://doi.org/10.3390/molecules25204679
APA StyleCorona-Motolinia, N. D., Martínez-Valencia, B., Noriega, L., Sánchez-Gaytán, B. L., Méndez-Rojas, M. Á., Melendez, F. J., Castro, M. E., & González-Vergara, E. (2020). Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment. Molecules, 25(20), 4679. https://doi.org/10.3390/molecules25204679








