β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte
Abstract
1. Introduction
2. Results
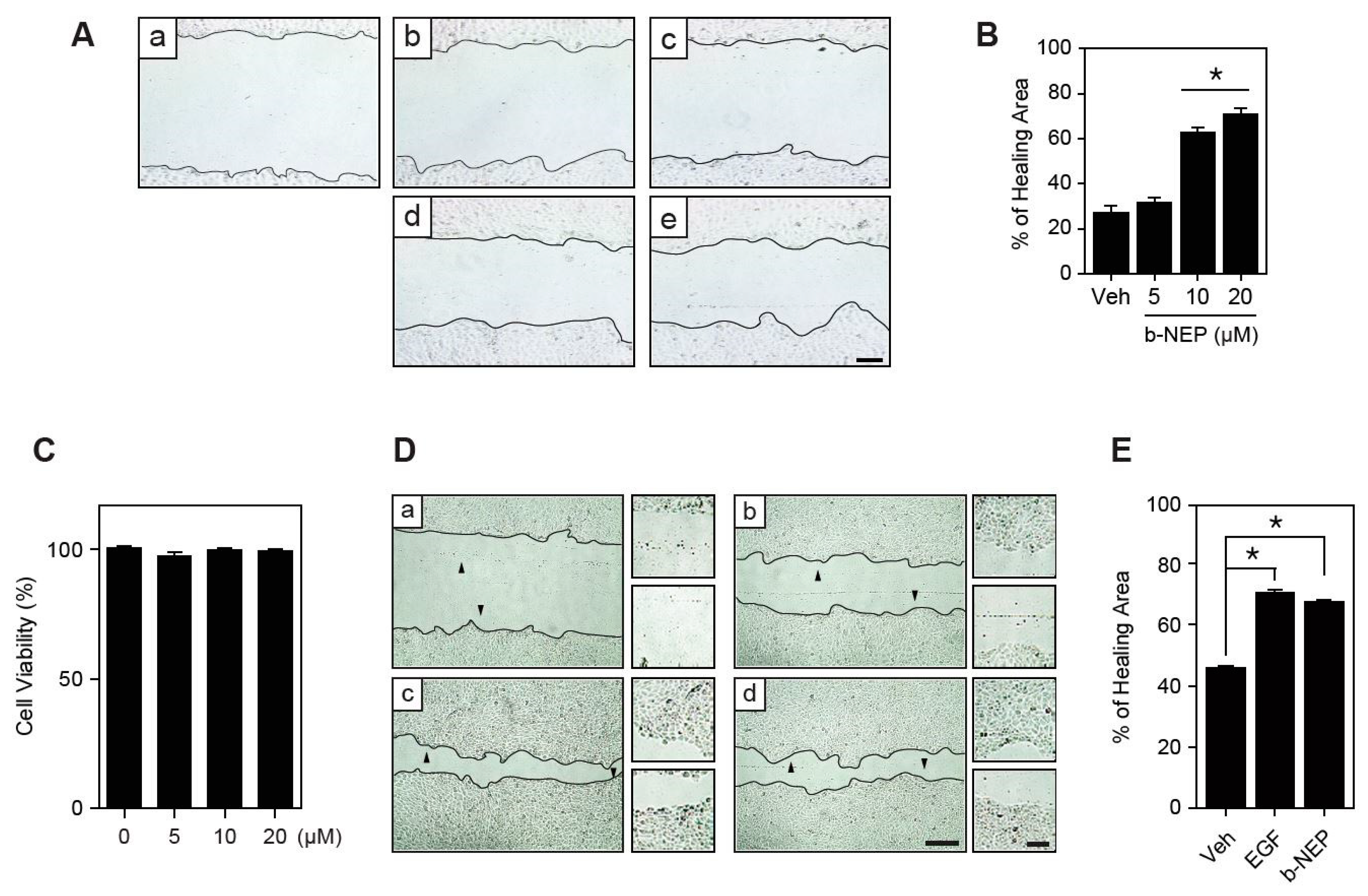
2.1. β-NEP Stimulates Wound Healing in Human Keratinocytes
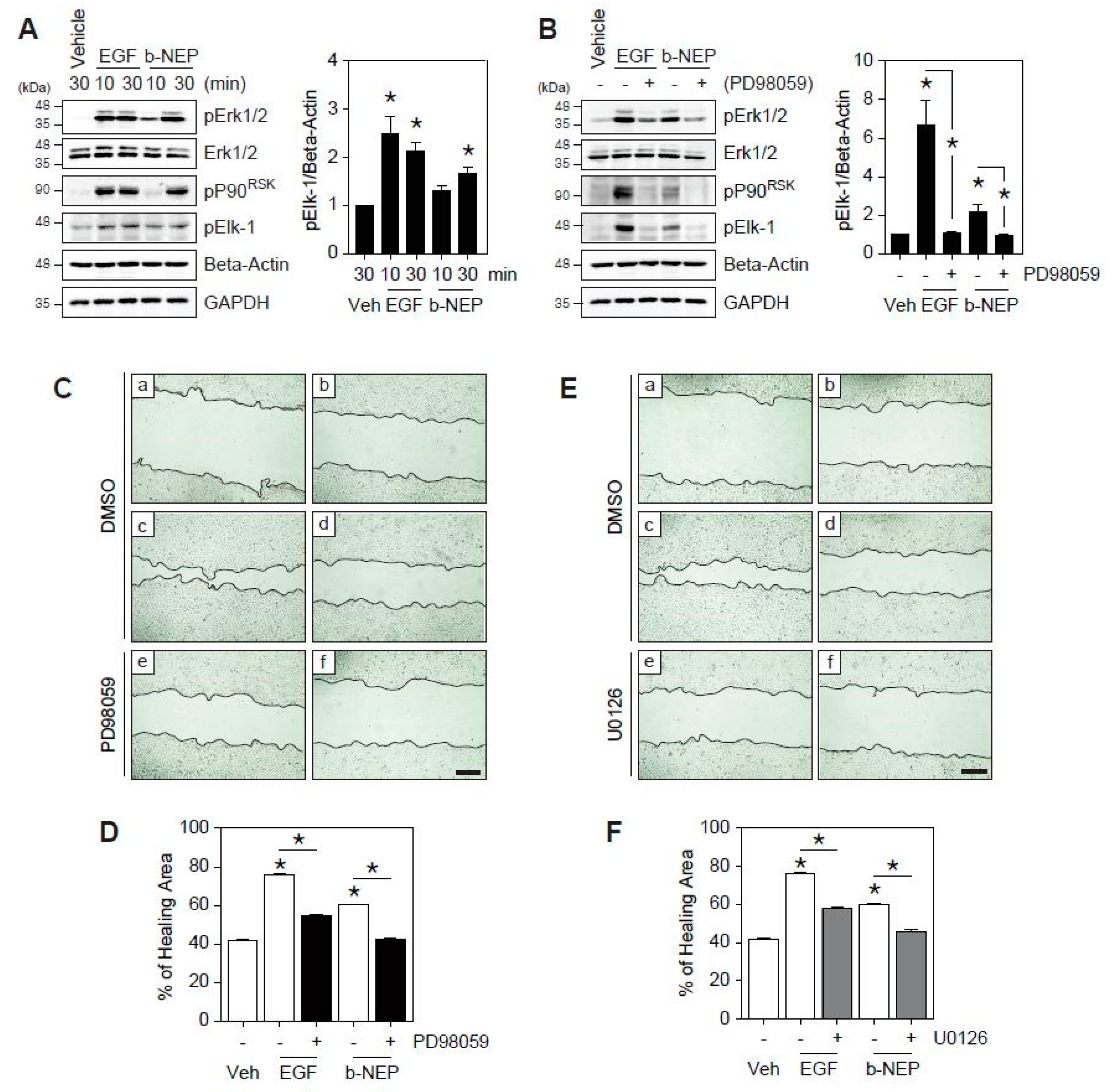
2.2. β-NEP Accelerates Wound Healing through Activation of Ekr1/2
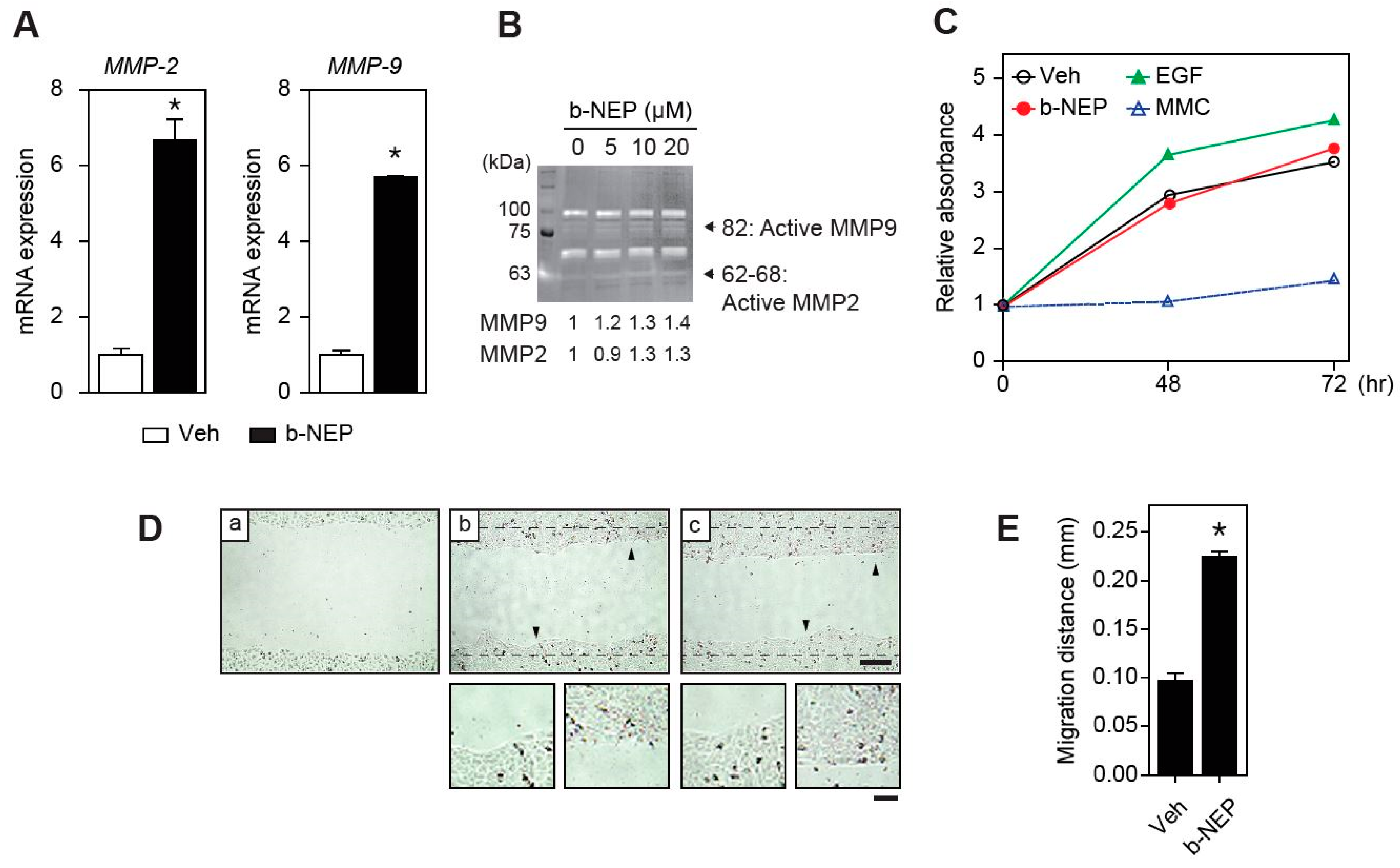
2.3. β-NEP Upregulates MMP-2 and -9 Expression in Human Keratinocytes
2.4. β-NEP Stimulates Fibroblast Migration
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagent
4.2. MTT Assay
4.3. Wound Healing and Migration Assays
4.4. Cell Proliferation Assays
4.5. Western Blot Analysis
4.6. Quantitative Real-time PCR
4.7. Gelatin Zymography Assay
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EGF | Epidermal growth factor |
| HaCaT | Human keratinocytes |
| DMSO | Dimethyl sulfoxide |
| Dyn | Dynorphin |
| MAPK | Mitogen-activated protein kinase |
| MEF | Mouse embryonic fibroblasts |
| MMC | Mitomycin C |
| MMP | Metalloproteinase |
| NEP | Neoendorphin |
| ProDyn | Prodynorphin |
| GPCR | G-protein coupled receptor |
| KO | Knock out |
| Veh | Vehicle |
| PKC | Protein kinase C |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| RIPA | Radioimmunoprecipitation assay |
| SDS | Sodium dodecyl sulfate |
| ANOVA | Analysis of variance |
References
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Slominski, A.; Theoharides, T.C.; Peters, E.M.; Paus, R. Neuroimmunology of stress: Skin takes center stage. J. Investig. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Brozyna, A.A.; Granese, J.; Pisarchik, A.; Szczesniewski, A.; Tobin, D.J. Regulated proenkephalin expression in human skin and cultured skin cells. J. Investig. Dermatol. 2011, 131, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Tobin, D.J.; Gaveriaux-Ruff, C.; Bigliardi-Qi, M. Opioids and the skin--where do we stand? Exp. Dermatol. 2009, 18, 424–430. [Google Scholar] [CrossRef]
- Ota, T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013, 37, 165–172. [Google Scholar] [CrossRef]
- Stein, C.; Kuchler, S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Nissen, J.B.; Lund, M.; Stengaard-Pedersen, K.; Kragballe, K. Enkephalin-like immunoreactivity in human skin is found selectively in a fraction of CD68-positive dermal cells: Increase in enkephalin-positive cells in lesional psoriasis. Arch. Dermatol. Res. 1997, 289, 265–271. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Buchner, S.; Rufli, T.; Bigliardi-Qi, M. Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. J. Recept. Signal Transduct. Res. 2002, 22, 191–199. [Google Scholar] [CrossRef]
- Bigliardi-Qi, M.; Gaveriaux-Ruff, C.; Zhou, H.; Hell, C.; Bady, P.; Rufli, T.; Kieffer, B.; Bigliardi, P. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation 2006, 74, 174–185. [Google Scholar] [CrossRef]
- Metze, D.; Reimann, S.; Beissert, S.; Luger, T. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J. Am. Acad. Dermatol. 1999, 41, 533–539. [Google Scholar] [PubMed]
- Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Wound healing: A paradigm for regeneration. Mayo Clin. Proc. 2013, 88, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., II; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef] [PubMed]
- Charbaji, N.; Schafer-Korting, M.; Kuchler, S. Morphine stimulates cell migration of oral epithelial cells by delta-opioid receptor activation. PLoS ONE 2012, 7, e42616. [Google Scholar] [CrossRef]
- Wolf, N.B.; Kuchler, S.; Radowski, M.R.; Blaschke, T.; Kramer, K.D.; Weindl, G.; Kleuser, B.; Haag, R.; Schafer-Korting, M. Influences of opioids and nanoparticles on in vitro wound healing models. Eur. J. Pharm. Biopharm. 2009, 73, 34–42. [Google Scholar] [CrossRef]
- Kuchler, S.; Radowski, M.R.; Blaschke, T.; Dathe, M.; Plendl, J.; Haag, R.; Schafer-Korting, M.; Kramer, K.D. Nanoparticles for skin penetration enhancement--a comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 243–250. [Google Scholar] [CrossRef]
- Kuchler, S.; Wolf, N.B.; Heilmann, S.; Weindl, G.; Helfmann, J.; Yahya, M.M.; Stein, C.; Schafer-Korting, M. 3D-wound healing model: Influence of morphine and solid lipid nanoparticles. J. Biotechnol. 2010, 148, 24–30. [Google Scholar] [CrossRef]
- Bigliardi-Qi, M.; Bigliardi, P.L.; Eberle, A.N.; Buchner, S.; Rufli, T. beta-endorphin stimulates cytokeratin 16 expression and downregulates mu-opiate receptor expression in human epidermis. J. Investig. Dermatol. 2000, 114, 527–532. [Google Scholar] [CrossRef]
- Nissen, J.B.; Kragballe, K. Enkephalins modulate differentiation of normal human keratinocytes in vitro. Exp. Dermatol. 1997, 6, 222–229. [Google Scholar] [CrossRef]
- Garzon, J.; Sanchez-Blazquez, P.; Hollt, V.; Lee, N.M.; Loh, H.H. Endogenous opioid peptides: Comparative evaluation of their receptor affinities in the mouse brain. Life Sci. 1983, 33, 291–294. [Google Scholar] [CrossRef]
- Oka, T.; Negishi, K. Evidence That Endogenous 6-(Arg or Lys)-Opioid Peptides Can Interact with Kappa-Receptors as Agonists. Life Sci. 1982, 31, 1707–1710. [Google Scholar] [CrossRef]
- Oka, T.; Negishi, K.; Kajiwara, M.; Watanabe, Y.; Ishizuka, Y.; Matsumiya, T. The choice of opiate receptor subtype by neo-endorphins. Eur. J. Pharmacol. 1982, 79, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi-Qi, M.; Gaveriaux-Ruff, C.; Pfaltz, K.; Bady, P.; Baumann, T.; Rufli, T.; Kieffer, B.L.; Bigliardi, P.L. Deletion of mu- and kappa-opioid receptors in mice changes epidermal hypertrophy, density of peripheral nerve endings, and itch behavior. J. Investig. Dermatol. 2007, 127, 1479–1488. [Google Scholar] [CrossRef]
- Klemke, R.L.; Cai, S.; Giannini, A.L.; Gallagher, P.J.; de Lanerolle, P.; Cheresh, D.A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997, 137, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Catling, A.D.; Webb, D.J.; Sankovic, M.; Walker, L.A.; Somlyo, A.V.; Weber, M.J.; Gonias, S.L. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J. Cell Biol. 1999, 146, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Ebisuya, M.; Honjoh, S.; Nishida, E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004, 14, 731–735. [Google Scholar] [CrossRef]
- Frijns, E.; Sachs, N.; Kreft, M.; Wilhelmsen, K.; Sonnenberg, A. EGF-induced MAPK signaling inhibits hemidesmosome formation through phosphorylation of the integrin {beta}4. J. Biol. Chem. 2010, 285, 37650–37662. [Google Scholar] [CrossRef]
- Faure, E.; Garrouste, F.; Parat, F.; Monferran, S.; Leloup, L.; Pommier, G.; Kovacic, H.; Lehmann, M. P2Y2 receptor inhibits EGF-induced MAPK pathway to stabilise keratinocyte hemidesmosomes. J. Cell Sci. 2012, 125, 4264–4277. [Google Scholar] [CrossRef]
- Kasza, A. Signal-dependent Elk-1 target genes involved in transcript processing and cell migration. Biochim. Biophys. Acta 2013, 1829, 1026–1033. [Google Scholar] [CrossRef]
- Hernandez-Perez, M.; Mahalingam, M. Matrix metalloproteinases in health and disease: Insights from dermatopathology. Am. J. Dermatopathol. 2012, 34, 565–579. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Wu, C.Y.; Yang, C.M. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia 2008, 56, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc. Res. 2010, 85, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wehrmann, M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. J. Cell. Mol. Med. 2007, 11, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Fujiwara, T.; Matsuzaki, S.; Shingaki, K.; Taniguchi, M.; Miyata, S.; Tohyama, M.; Sakai, Y.; Yano, K.; Hosokawa, K.; et al. bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS ONE 2010, 5, e12228. [Google Scholar] [CrossRef]
- Kohl, A.; Werner, A.; Buntrock, P.; Diezel, W.; Adrian, K.; Titov, M.I. The effect of the peptide dalargin on wound healing. Dermatol. Mon. 1989, 175, 561–572. [Google Scholar]
- Shekhter, A.B.; Solov’eva, A.I.; Spevak, S.E.; Titov, M.I. Effects of opioid peptide dalargin on reparative processes in wound healing. Biull. Eksp. Biol. Med. 1988, 106, 487–490. [Google Scholar] [CrossRef]
- Yang, D.J.; Lee, K.S.; Ko, C.M.; Moh, S.H.; Song, J.; Hur, L.C.; Cheon, Y.W.; Yang, S.H.; Choi, Y.H.; Kim, K.W. Leucine-enkephalin promotes wound repair through the regulation of hemidesmosome dynamics and matrix metalloprotease. Peptides 2016, 76, 57–64. [Google Scholar] [CrossRef]
- Mansour, A.; Hoversten, M.T.; Taylor, L.P.; Watson, S.J.; Akil, H. The cloned mu, delta and kappa receptors and their endogenous ligands: Evidence for two opioid peptide recognition cores. Brain Res. 1995, 700, 89–98. [Google Scholar] [CrossRef]
- Chavkin, C.; Bakhit, C.; Weber, E.; Bloom, F.E. Relative contents and concomitant release of prodynorphin/neoendorphin-derived peptides in rat hippocampus. Proc. Natl. Acad. Sci. USA 1983, 80, 7669–7673. [Google Scholar] [CrossRef]
- Ramsdell, C.D.; Meador-Woodruff, J.H. Expression of prodynorphin-derived peptides and mRNA in guinea-pig cortex. Neuropeptides 1993, 25, 131–138. [Google Scholar] [CrossRef]
- Evans, C.J.; Erdelyi, E.; Hunter, J.; Barchas, J.D. Co-localization and characterization of immunoreactive peptides derived from two opioid precursors in guinea pig adrenal glands. J. Neurosci. 1985, 5, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Silberring, J.; Castello, M.E.; Nyberg, F. Characterization of dynorphin A-converting enzyme in human spinal cord. An endoprotease related to a distinct conversion pathway for the opioid heptadecapeptide? J. Biol. Chem. 1992, 267, 21324–21328. [Google Scholar] [PubMed]
- Silberring, J.; Nyberg, F. A novel bovine spinal cord endoprotease with high specificity for dynorphin B. J. Biol. Chem. 1989, 264, 11082–11086. [Google Scholar] [PubMed]
- Kirfel, G.; Herzog, V. Migration of epidermal keratinocytes: Mechanisms, regulation, and biological significance. Protoplasma 2004, 223, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Jun, H.; Chung, H.; Yoon, C.; Kim, T.; Kwon, M.; Lee, S.; Jung, S.; Kim, M.; Park, J.H. Comparison of EGF with VEGF non-viral gene therapy for cutaneous wound healing of streptozotocin diabetic mice. Diabetes Metab. J. 2011, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Neumann, C.; Teo, Y.L.; Pant, A.; Bigliardi-Qi, M. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br. J. Pharmacol. 2015, 172, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sonnemann, K.J.; Bement, W.M. Wound repair: Toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 2011, 27, 237–263. [Google Scholar] [CrossRef]
- Keren, K.; Pincus, Z.; Allen, G.M.; Barnhart, E.L.; Marriott, G.; Mogilner, A.; Theriot, J.A. Mechanism of shape determination in motile cells. Nature 2008, 453, 475–480. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Huang, B.B.; Tian, H.S.; Chi, L.S.; Duan, Y.M.; Wang, X.; Zhu, Z.X.; Cai, W.H.; Zhu, Y.T.; Wei, T.M.; et al. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS ONE 2014, 9, e108182. [Google Scholar] [CrossRef]
- Frankowski, H.; Gu, Y.H.; Heo, J.H.; Milner, R.; Del Zoppo, G.J. Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods Mol. Biol. 2012, 814, 221–233. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the corresponding author on reasonable request. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.J.; Moh, S.H.; Choi, Y.-H.; Kim, K.W. β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte. Molecules 2020, 25, 4640. https://doi.org/10.3390/molecules25204640
Yang DJ, Moh SH, Choi Y-H, Kim KW. β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte. Molecules. 2020; 25(20):4640. https://doi.org/10.3390/molecules25204640
Chicago/Turabian StyleYang, Dong Joo, Sang Hyun Moh, Yun-Hee Choi, and Ki Woo Kim. 2020. "β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte" Molecules 25, no. 20: 4640. https://doi.org/10.3390/molecules25204640
APA StyleYang, D. J., Moh, S. H., Choi, Y.-H., & Kim, K. W. (2020). β-Neoendorphin Enhances Wound Healing by Promoting Cell Migration in Keratinocyte. Molecules, 25(20), 4640. https://doi.org/10.3390/molecules25204640





