Validated Reversed-Phase Liquid Chromatographic Method with Gradient Elution for Simultaneous Determination of the Antiviral Agents: Sofosbuvir, Ledipasvir, Daclatasvir, and Simeprevir in Their Dosage Forms
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Choice of Column and Temperature
2.1.2. Mobile Phase Composition and pH of Buffer
2.1.3. Choice of Detection Wavelength
2.1.4. Choice of Diluents
2.2. Method Validation
2.2.1. System Suitability
2.2.2. Specificity
2.2.3. Linearity and Range
2.2.4. Accuracy
2.2.5. Precision
2.2.6. Detection and Quantitation Limits
2.2.7. Robustness
2.3. Application of the Proposed Method for the Analysis of Commercial Tablets
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.W.; Finelli, L.; Alter, M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 2005, 5, 558–567. [Google Scholar] [CrossRef]
- Wilkins, T.; Malcolm, J.K.; Raina, D.; Schade, R.R. Hepatitis C: Diagnosis and treatment. Am. Fam. Physician 2010, 81, 1351–1357. [Google Scholar]
- Kandeel, A.; Genedy, M.; El-Refai, S.; Funk, A.L.; Fontanet, A.; Talaat, M. The prevalence of hepatitis C virus infection in Egypt 2015: Implications for future policy on prevention and treatment. Liver Int. 2017, 37, 45–53. [Google Scholar] [CrossRef]
- Rafiq, S.M.; Banik, G.R.; Khan, S.; Rashid, H.; Khandaker, G. Current Burden of Hepatitis C Virus Infection Among Injecting Drug Users: A Mini Systematic Review of Prevalence Studies. Infect. Disord. Drug Targets Disord. 2014, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.S.; Gilman, A.G.; Hardman, J.G.; Limbird, L.E.; Molinoff, P.B.; Ruddon, R.W.; Palmer, T.; Kunkel, E.; Rall, T.W. Goodman & Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill, Health Professions Division: New York, NY, USA, 1996. [Google Scholar]
- Kwon, H.J.; Xing, W.; Chan, K.; Niedziela-Majka, A.; Brendza, K.M.; Kirschberg, T.; Kato, D.; Link, J.O.; Cheng, G.; Liu, X.; et al. Direct binding of ledipasvir to HCV NS5A: Mechanism of resistance to an HCV antiviral agent. PLoS ONE 2015, 10, e0122844. [Google Scholar] [CrossRef]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef]
- Chukkapalli, V.; Berger, K.L.; Kelly, S.M.; Thomas, M.; Deiters, A.; Randall, G. Daclatasvir inhibits hepatitis C virus NS5A motility and hyper-accumulation of phosphoinositides. Virology 2015, 476, 168–179. [Google Scholar] [CrossRef]
- Shanmugam, S.; Nichols, A.K.; Saravanabalaji, D.; Welsch, C.; Yi, M. HCV NS5A dimer interface residues regulate HCV replication by controlling its self-interaction, hyperphosphorylation, subcellular localization and interaction with cyclophilin A. PLoS Pathog. 2018, 14, e1007177. [Google Scholar] [CrossRef]
- Sundaram, V.; Kowdley, K.V. Dual daclatasvir and sofosbuvir for treatment of genotype 3 chronic hepatitis C virus infection. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 13–20. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S. TMC-435, an NS3/4A protease inhibitor for the treatment of HCV infection. Curr. Opin. Investig. Drugs 2009, 10, 871–881. [Google Scholar] [PubMed]
- Raj Kumar, B.; Subrahmanyam, K.V. A new validated rp-hplc method for the simultaneous determination of simeprevir and sofosbuvir in pharmaceutical dosage form. Indo Am. J. Pharm. Res. 2016, 6, 4508–4520. [Google Scholar]
- Crittenden, N.E.; Buchanan, L.A.; Pinkston, C.M.; Cave, B.; Barve, A.; Marsano, L.; McClain, C.J.; Jones, C.M.; Marvin, M.R.; Davis, E.G.; et al. Simeprevir and sofosbuvir with or without ribavirin to treat recurrent genotype 1 hepatitis C virus infection after orthotopic liver transplantation. Liver Transplant. 2016, 22, 635–643. [Google Scholar] [CrossRef]
- Chakravarthy, V.A.; Sailaja, B.; Praveen Kumar, A. Method development and validation of ultraviolet-visible spectroscopic method for the estimation of hepatitis-c drugs-daclatasvir and sofosbuvir in active pharmaceutical ingredient form. Asian J. Pharm. Clin. Res. 2016, 9, 61–66. [Google Scholar] [CrossRef]
- Vejendla, R.; Subramanyam, C.V.S.; Veerabhadram, G. Estimation and validation of sofosbuvir in bulk and tablet dosage form by rp-hplc. Int. J. Pharm. 2016, 6, 121–127. [Google Scholar]
- Swain, D.; Samanthula, G.; Bhagat, S.; Bharatam, P.V.; Akula, V.; Sinha, B.N. Characterization of forced degradation products and in silico toxicity prediction of Sofosbuvir: A novel HCV NS5B polymerase inhibitor. J. Pharm. Biomed. Anal. 2016, 120, 352–363. [Google Scholar] [CrossRef]
- Nebsen, M.; Elzanfaly, E.S. Stability-Indicating Method and LC-MS-MS Characterization of Forced Degradation Products of Sofosbuvir. J. Chromatogr. Sci. 2016, 54, 1631–1640. [Google Scholar] [CrossRef]
- Rezk, M.R.; Basalious, E.B.; Amin, M.E. Novel and sensitive UPLC–MS/MS method for quantification of sofosbuvir in human plasma: Application to a bioequivalence study. Biomed. Chromatogr. 2016, 30, 1354–1362. [Google Scholar] [CrossRef]
- Madhavi, S.; Rani, A.P. Bioanalytical method development and validation for the determination of sofosbuvir from human plasma. Int. J. Pharm. Pharm. Sci. 2017, 9, 35–41. [Google Scholar] [CrossRef][Green Version]
- Semreen, M.H.; Alniss, H.Y.; Mousa, M.K.; Aboul-Enein, H.Y. Quick and Sensitive UPLC-ESI-MS/MS Method for Simultaneous Estimation of Sofosbuvir and Its Metabolite in Human Plasma. Molecules 2019, 24, 1302. [Google Scholar] [CrossRef]
- Ranjana, S.; Nitin, S.; Ganesh, T.; Gholve, S.B. Development and Validation of Simple UV Spectrophotometric Method for the Determination of Ledipasvir in Bulk Form and Stress Degradation Studies. Inventi Rapid Pharm. Anal. Qual. Assur. 2016, 3, 1–5. [Google Scholar]
- Devilal, J.; Durgaprasad, B.; Pal, N.; Rao Avanapu, S. New method development and validation for the determination of ledipasvir in bulk drug form by using reverse phase hplc technique. World J. Pharm. Pharm. Sci. 2016, 6, 1312–1321. [Google Scholar]
- Zhang, K.; Ma, X.-Q.; Li, Z.-H.; Zhang, Y.-L.; Song, J.-J. An UPLC-MS/MS Method for the Quantitation of Ledipasvir in Rat Plasma: Application to a Pharmacokinetic Study. Latin Am. J. Pharm. 2016, 35, 1116–1121. [Google Scholar]
- Chakravarthy, V.A.; Sailaja, B.B.V. Method development and validation of assay and dissolution methods for the estimation of daclatasvir in tablet dosage forms by reverse phase HPLC. Eur. J. Pharm. Med. Res. 2016, 3, 356–364. [Google Scholar]
- Nadig, S.; Jacob, J.T. Quantitative Estimation of Daclatasvir In Drug Substances and Formulated Drug Product By UPLC. Der Pharm. Lett. 2016, 8, 280–284. [Google Scholar]
- Sumathi, K.; Thamizhvanan, K.; Vijayraj, S. Development and validation of stability indicating RP-HPLC method for the estimation of Daclatasvir in bulk and formulation. Der Pharm. Lett. 2016, 8, 107–113. [Google Scholar]
- Saleh, H.; Ragab, G.H.; Othman, M.A. Stability Indicating HPLC Method Development and Validation for Determination of Daclatasvir in Pure and Tablets Dosage Forms. Indo Am. J. Pharm. Sci. 2016, 3, 1565–1572. [Google Scholar]
- Rezk, M.R.; Bendas, E.R.; Basalious, E.B.; Karim, I.A. Development and validation of sensitive and rapid UPLC-MS/MS method for quantitative determination of daclatasvir in human plasma: Application to a bioequivalence study. J. Pharm. Biomed. Anal. 2016, 128, 61–66. [Google Scholar] [CrossRef]
- Attia, K.A.M.; El-Abasawi, N.M.; El-Olemy, A.; Serag, A. Stability-indicating HPLC-DAD Method for the Determination of Simeprevir. Anal. Chem. Lett. 2017, 7, 43–51. [Google Scholar] [CrossRef]
- Nannetti, G.; Pagni, S.; Parisi, S.G.; Alberti, A.; Loregian, A.; Palu, G. Development of a simple HPLC-UV method for the determination of the hepatitis C virus inhibitor simeprevir in human plasma. J. Pharm. Biomed. Anal. 2016, 121, 197–203. [Google Scholar] [CrossRef]
- Vanwelkenhuysen, I.; de Vries, R.; Timmerman, P.; Verhaeghe, T. Determination of simeprevir: A novel. hepatitis C protease inhibitor in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 958, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, M.E.M.; Abdelrahman, M.M.; Mohamed, M.A. Assay and Dissolution Methods Development and Validation for Simultaneous Determination of Sofosbuvir and Ledipasvir by RP-HPLC Method in Tablet Dosage Forms. J. Forensic Sci. Crim. Inves 2017, 1, 555562. [Google Scholar] [CrossRef]
- Rezk, M.R.; Basalious, E.B.; Karim, I.A. Development of a sensitive UPLC-ESI-MS/MS method for quantification of sofosbuvir and its metabolite. GS-331007, in human plasma: Application to a bioequivalence study. J. Pharm. Biomed. Anal. 2015, 114, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Elkady, E.F.; Aboelwafa, A.A. A Rapid and Optimized LC-MS/MS Method for the Simultaneous Extraction and Determination of Sofosbuvir and Ledipasvir in Human Plasma. J. AOAC Int. 2016, 99, 1252–1259. [Google Scholar] [CrossRef]
- Zaman, B.; Siddique, F.; Hassan, W. RP-HPLC Method for Simultaneous Determination of Sofosbuvir and Ledipasvir in Tablet Dosage Form and Its Application to In Vitro Dissolution Studies. Chromatographia 2016, 79, 1605–1613. [Google Scholar] [CrossRef]
- Farid, N.F.; Abdelwahab, N.S. Chromatographic analysis of ledipasvir and sofosbuvir: New treatment for chronic hepatitis C infection with application to human plasma. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 327–332. [Google Scholar] [CrossRef]
- Abo-Talib, N.F.; El-Ghobashy, M.R.; Tammam, M.H. Spectrophotometric Methods for Simultaneous Determination of Sofosbuvir and Ledipasvir (HARVONI Tablet): Comparative Study with Two Generic Products. J. AOAC Int. 2017, 100, 976–984. [Google Scholar] [CrossRef]
- Ariaudo, A.; Favata, F.; De Nicolo, A.; Simiele, M.; Paglietti, L.; Boglione, L.; Cardellino, C.S.; Carcieri, C.; Di Perri, G.; D’Avolio, A. A UHPLC-MS/MS method for the quantification of direct antiviral agents simeprevir, daclatasvir, ledipasvir, sofosbuvir/GS-331007, dasabuvir, ombitasvir and paritaprevir, together with ritonavir, in human plasma. J. Pharm. Biomed. Anal. 2016, 125, 369–375. [Google Scholar] [CrossRef]
- ICH. ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology in pp. In ICH Guideline; ICH Secretariat: Geneva, Switzerland, 2005; Available online: http://www.ich.org (accessed on 27 September 2020).
- USP. USP 38—NF 33 the United States Pharmacopeia and National Formulary 2015: Main Edition Plus Supplements 1 and 2; Deutscher Apotheker Verlag: Baltimore, MD, USA, 2015. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

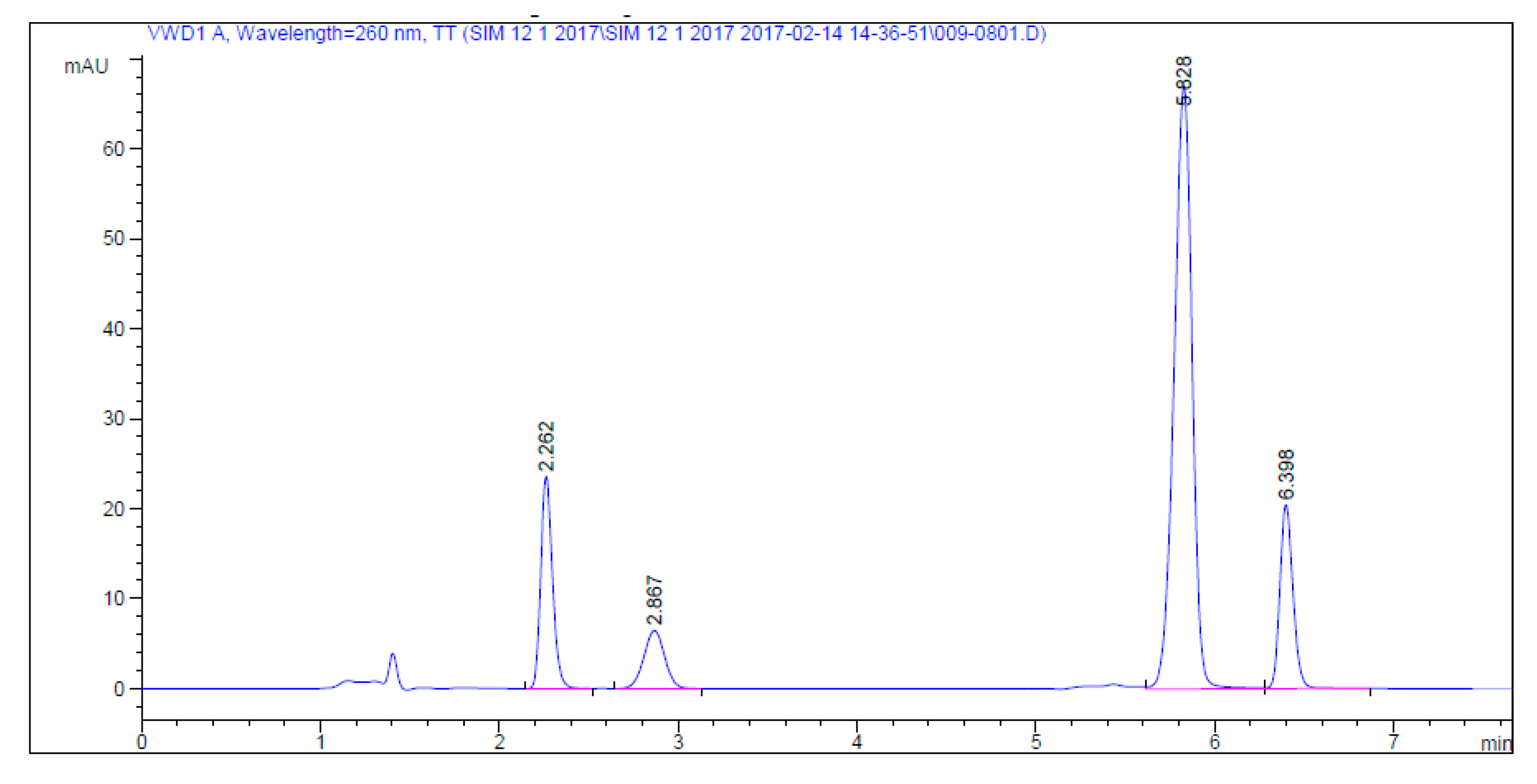
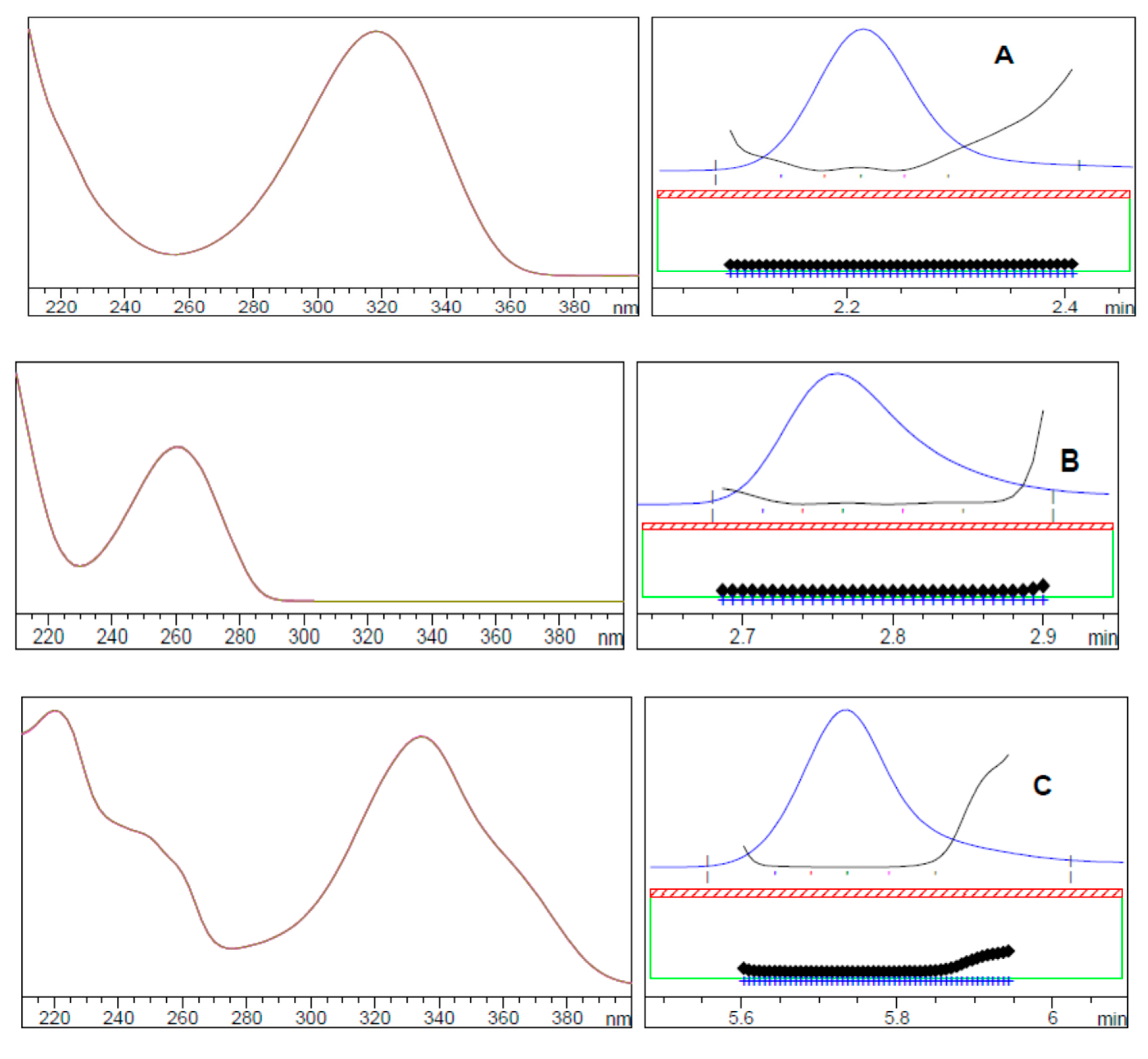
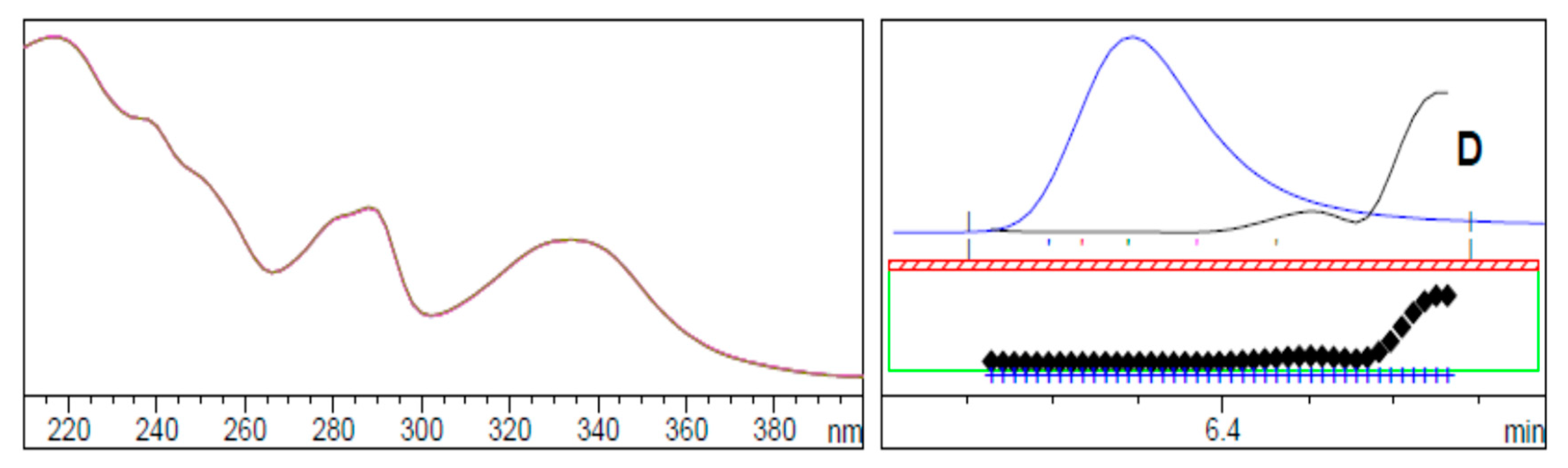
| Time (min) | Acetonitrile (%) | Buffer (%) | Wavelength (nm) |
|---|---|---|---|
| 0.0 | 47 | 53 | 260 |
| 3.5 | 47 | 53 | 260 |
| 3.6 | 70 | 30 | 330 |
| 9.5 | 70 | 30 | 330 |
| 9.7 | 47 | 53 | 260 |
| 10.0 | 47 | 53 | 260 |
| Parameters | DC | SF | LD | SM | Reference Value [41] | ||
|---|---|---|---|---|---|---|---|
| Obtained Value | |||||||
| * Resolution (R) | 2.73 | 12.57 | 4.46 | R > 1.5 | |||
| Tailing factor (T) | 0.81 | 1.13 | 1.63 | 0.87 | <1.5–2 (T = 1 for a typical symmetric peak) | ||
| Capacity factor (K) | 1.13 | 1.6 | 4.49 | 5.29 | 1.0–10.0 | ||
| ** Selectivity factor (α) | 1.22 | 2.21 | 1.14 | α > 1.5 | |||
| Number of theoretical plates (N) | 9546 | 1951 | 6651 | 37350 | More than 2000 | ||
| Height Equivalent to the Theoretical Plate (HETP) | 0.03 | 0.13 | 0.04 | 0.01 | Smaller values indicate higher column efficiencies | ||
| Parameter | Drugs | |||
|---|---|---|---|---|
| SF | LD | DC | SM | |
| Accuracy (Mean ± SD) | 98.05 ± 0.61 | 101.90 ± 0.31 | 99.78 ± 1.21 | 100.87 ± 0.9 |
| Precision: | ||||
| Repeatability a | 0.39 | 1.25 | 0.42 | 0.77 |
| Intermediate precision b | 1.32 | 1.57 | 0.70 | 1.45 |
| Linearity: | ||||
| Slope | 4.20 | 37.784 | 13.768 | 16.589 |
| Intercept | 0.081 | −0.6166 | −0.597 | 0.0124 |
| Correlation coefficient (r) | 0.9995 | 0.9993 | 0.9998 | 0.9996 |
| Range (µg/mL) | 1.0–20.0 | |||
| LOD (µg/mL) c | 0.034 | 0.039 | 0.022 | 0.030 |
| LOQ (µg/mL) d | 0.103 | 0.118 | 0.067 | 0.092 |
| Drug | Chromatographic Condition | |||||
|---|---|---|---|---|---|---|
| Mobile Phase Flow Rate mL/min | ||||||
| 1.9 | 2.0 | 2.1 | ||||
| Retention Time (min) ± SD | RSD% | Retention Time (min) ± SD | RSD% | Retention Time (min) ± SD | RSD% | |
| DC | 2.452 ± 0.012 | 0.469 | 2.254 ± 0.007 | 0.302 | 2.035 ± 0.010 | 0.474 |
| SF | 2.983 ± 0.005 | 0.177 | 2.854 ± 0.012 | 0.428 | 2.605 ±0.006 | 0.225 |
| LD | 6.007 ± 0.008 | 0.136 | 5.828 ± 0.003 | 0.043 | 5.652 ± 0.002 | 0.035 |
| SM | 6.561 ± 0.033 | 0.51 | 6.393 ± 0.005 | 0.071 | 6.102 ± 0.001 | 0.016 |
| pH | ||||||
| 6.4 | 6.5 | 6.6 | ||||
| DC | 2.257 ± 0.005 | 0.209 | 2.254 ± 0.007 | 0.302 | 2.233 ± 0.003 | 0.137 |
| SF | 2.862 ± 0.003 | 0.107 | 2.854 ± 0.012 | 0.428 | 2.861 ± 0.002 | 0.070 |
| LD | 5.829 ± 0.002 | 0.026 | 5.828 ± 0.003 | 0.043 | 5.831 ± 0.005 | 0.079 |
| SM | 6.393 ± 0.004 | 0.068 | 6.393 ± 0.005 | 0.071 | 6.399 ± 0.002 | 0.033 |
| Temperature | ||||||
| 39 °C | 40 °C | 41 °C | ||||
| DC | 2.259 ± 0.006 | 0.246 | 2.254 ± 0.007 | 0.302 | 2.255 ± 0.004 | 0.185 |
| SF | 2.883 ± 0.006 | 0.191 | 2.854 ± 0.012 | 0.428 | 2.829 ± 0.016 | 0.575 |
| LD | 5.832 ± 0.021 | 0.367 | 5.828 ± 0.003 | 0.043 | 5.852 ± 0.002 | 0.034 |
| SM | 6.390 ± 0.002 | 0.03 | 6.393 ± 0.005 | 0.071 | 6.391 ± 0.003 | 0.047 |
| Buffer Molarity | ||||||
| 0.018 M | 0.02 M | 0.022 M | ||||
| DC | 2.257 ± 0.008 | 0.355 | 2.254 ± 0.007 | 0.302 | 2.265 ± 0.010 | 0.444 |
| SF | 2.886 ± 0.019 | 0.666 | 2.854 ± 0.012 | 0.428 | 2.858 ± 0.024 | 0.855 |
| LD | 5.798 ± 0.023 | 0.397 | 5.828 ± 0.003 | 0.043 | 5.841 ± 0.034 | 0.581 |
| SM | 6.402 ± 0.023 | 0.364 | 6.393 ± 0.005 | 0.071 | 6.386 ± 0.005 | 0.072 |
| Dosage Form | Component in the Product | Taken (µg/mL) | Found % a ± SD | Pure Added (µg/mL) | Recovery % a | Mean ± SD |
|---|---|---|---|---|---|---|
| Harvoni tablet (400 mg SF and 90 mg LD) B.N. VCKSDI | SF (400 mg) | 8.0 | 98.37 ± 1.51 | 6.0 | 97.73 | 97.44 ± 0.26 |
| 8.0 | 97.38 | |||||
| 10.0 | 97.22 | |||||
| LD (90 mg) | 1.8 | 101.00 ± 0.70 | 1.2 | 99.01 | 99.27 ± 0.98 | |
| 1.8 | 100.36 | |||||
| 2.0 | 98.44 | |||||
| Daclavirocyrl B.N. 1631490 | DC (200 mg) | 1.2 | 100.81 ± 1.16 | 1.0 | 98.25 | 98.31 ± 0.45 |
| 1.20 | 97.89 | |||||
| 2.0 | 98.78 | |||||
| Olysio® B.N. FJ4H00 | SM (150 mg) | 3.0 | 101.19 ± 0.60 | 2.0 | 99.06 | 100.12 ± 0.99 |
| 3.0 | 100.27 | |||||
| 4.0 | 101.02 |
| Items | The Proposed HPLC Method | Reported Methods | ||||||
|---|---|---|---|---|---|---|---|---|
| SF | LD | DC | SM | SF a [38] | LD a [38] | DC b [27] | SM c [31] | |
| Mean ± SD * % | 98.37 ± 1.51 | 101.00 ± 0.70 | 100.81 ± 1.16 | 101.19 ± 0.60 | 99.19 ± 1.02 | 101.26 ± 0.79 | 100.54 ± 0.97 | 100.67 ± 0.951 |
| Variance | 2.280 | 0.490 | 1.346 | 0.360 | 1.040 | 0.624 | 0.941 | 0.904 |
| N | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 5 |
| Student’s t test | 1.102 (2.228) ** | 0.603 (2.228) ** | 0.372 (2.262) ** | 1.107 (2.262) ** | ||||
| F value | 2.19 (5.05) ** | 1.27 (5.05) ** | 1.43 (6.26) ** | 2.511 (5.19) ** | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzeldin, E.; Abo-Talib, N.F.; Tammam, M.H.; Asiri, Y.A.; Amr, A.E.-G.E.; Almehizia, A.A. Validated Reversed-Phase Liquid Chromatographic Method with Gradient Elution for Simultaneous Determination of the Antiviral Agents: Sofosbuvir, Ledipasvir, Daclatasvir, and Simeprevir in Their Dosage Forms. Molecules 2020, 25, 4611. https://doi.org/10.3390/molecules25204611
Ezzeldin E, Abo-Talib NF, Tammam MH, Asiri YA, Amr AE-GE, Almehizia AA. Validated Reversed-Phase Liquid Chromatographic Method with Gradient Elution for Simultaneous Determination of the Antiviral Agents: Sofosbuvir, Ledipasvir, Daclatasvir, and Simeprevir in Their Dosage Forms. Molecules. 2020; 25(20):4611. https://doi.org/10.3390/molecules25204611
Chicago/Turabian StyleEzzeldin, Essam, Nisreen F. Abo-Talib, Marwa H. Tammam, Yousif A. Asiri, Abd El-Galil E. Amr, and Abdulrahman A. Almehizia. 2020. "Validated Reversed-Phase Liquid Chromatographic Method with Gradient Elution for Simultaneous Determination of the Antiviral Agents: Sofosbuvir, Ledipasvir, Daclatasvir, and Simeprevir in Their Dosage Forms" Molecules 25, no. 20: 4611. https://doi.org/10.3390/molecules25204611
APA StyleEzzeldin, E., Abo-Talib, N. F., Tammam, M. H., Asiri, Y. A., Amr, A. E.-G. E., & Almehizia, A. A. (2020). Validated Reversed-Phase Liquid Chromatographic Method with Gradient Elution for Simultaneous Determination of the Antiviral Agents: Sofosbuvir, Ledipasvir, Daclatasvir, and Simeprevir in Their Dosage Forms. Molecules, 25(20), 4611. https://doi.org/10.3390/molecules25204611







