Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure
Abstract
1. Introduction
2. Results
2.1. Effect of Treatments on Turbidity Characterization
2.2. Quality Evolution During Storage
2.2.1. European Legal Requirements
2.2.2. Volatile Organic Compounds
2.2.3. Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Trials
4.2. Storage Conditions
4.3. Analyses
4.4. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EVOO | Extra virgin olive oil |
| VEVOO | veiled extra virgin olive oil |
| FEVOO | filtered extra virgin olive oil |
| HHP | olive oil treated with high hydrostatic pressure |
| FIL | filtered olive oil |
| F-HPP | olive oil filtered and treated with HHP |
| CON | not treated olive oil |
| LOX | Lipoxygenase |
| 3,4-DHPEA-EDA | dialdehydic form of decarboximethyl elenolic acid linked to hydroxytyrosol |
| 3,4-DHPEA-EA | oleuropein aglycones |
| 3,4-DHPEA | Hydroxytyrosol |
| p-HPEA-EDA | dialdehydic form of decarboxymethyl ligstroside aglycones |
| p-HPEA-EA | ligstroside aglycones |
| p-HPEA | Tyrosol |
| LOX | Lipoxygenase |
| YPD | Yeast Extract–Peptone–Dextrose Broth |
References
- European Commission Commission Implementing Regulation (EU) No 29/2012 of 13 January 2012 on marketing standards for olive oil (codification). Off. J. Eur. Union 2013, 39, 1–8.
- Aparicio, R.; Morales, M.T.; García-González, D.L. Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Kotti, F.; Cerretani, L.; Gargouri, M.; Chiavaro, E.; Bendini, A. Evaluation of the volatile fraction of commercial virgin olive oils from Tunisia and italy: Relation with olfactory attributes. J. Food Biochem. 2011, 35, 681–698. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences; European Food Safety Authority (EFSA): Parma, Italy, 2011. [Google Scholar]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [PubMed]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar]
- Koidis, A.; Triantafillou, E.; Boskou, D. Endogenous microflora in turbid virgin olive oils and the physicochemical characteristics of these oils. Eur. J. Lipid Sci. Technol. 2008, 110, 164–171. [Google Scholar] [CrossRef]
- Breschi, C.; Guerrini, L.; Domizio, P.; Ferraro, G.; Calamai, L.; Canuti, V.; Masella, P.; Parenti, A.; Fratini, E.; Fia, G.; et al. Physical, Chemical, and Biological Characterization of Veiled Extra Virgin Olive Oil Turbidity for Degradation Risk Assessment. Eur. J. Lipid Sci. Technol. 2019, 121, 1900195. [Google Scholar] [CrossRef]
- Lercker, G.; Frega, N.; Bocci, F.; Servidio, G. “Veiled” Extra-Virgin Olive Oils: Dispersion Response Related to Oil Quality. J. Am. Oil Chem. Soc. 1994, 71, 657–658. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Lercker, G. Effects of free fatty acids on oxidative stability of vegetable oil. J. Am. Oil Chem. Soc. 1999, 76, 325–329. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Georgiou, A.; Koidis, A.; Boskou, D. Loss of stability of “veiled” (cloudy) virgin olive oils in storage. Food Chem. 2005, 93, 377–383. [Google Scholar] [CrossRef]
- Koidis, A.; Boskou, D. The contents of proteins and phospholipids in cloudy (veiled) virgin olive oils. Eur. J. Lipid Sci. Technol. 2006, 108, 323–328. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Cerretani, L.; Bendini, A.; Gallina-Toschi, T.; Segura-Carretero, A.; Fernández-Gutiérrez, A. New Filtration Systems for Extra-Virgin Olive Oil: Effect on Antioxidant Compounds, Oxidative Stability, and Physicochemical and Sensory Properties. J. Agric. Food Chem. 2012, 60, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Zullo, B.A. Virgin olive oil yeasts: A review. Food Microbiol. 2018, 70, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Lavelli, V.; León, S.; Kapuralin, J.; Desamparados Salvador, M. Effect of filtration on virgin olive oil stability during storage. Eur. J. Lipid Sci. Technol. 2006, 108, 134–142. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.C. Sensory defects of virgin olive oil from a microbiological perspective. Trends Food Sci. Technol. 2015, 43, 227–235. [Google Scholar] [CrossRef]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Guerrini, L.; Masella, P.; Parenti, A. Shelf life and quality of olive oil filtered without vertical centrifugation. Eur. J. Lipid Sci. Technol. 2016, 118, 1213–1222. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Changes in chemical and sensory characteristics of Chemlali extra-virgin olive oil as depending on filtration. Eur. J. Lipid Sci. Technol. 2017, 119, 1–10. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Minnocci, A.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Sebastiani, L.; Servili, M. Compositional differences between veiled and filtered virgin oive oils during a simulated shelf life. LWT 2018, 94, 87–95. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Migliorini, M.; Cherubini, C.; Parenti, A. Addition of a steel pre-filter to improve plate filter-press performance in olive oil filtration. J. Food Eng. 2015, 157, 84–87. [Google Scholar] [CrossRef]
- Zeuthen, P.; Bøgh-Sørensen, L. Food Preservation Techniques; CRC Press: Boston MA, USA, 2003; p. 580. [Google Scholar]
- Guerrini, L.; Migliorini, M.; Giusti, M.; Parenti, A. The influence of crusher speed on extra virgin olive oil characteristics. Eur. J. Lipid Sci. Technol. 2017, 119, 1–7. [Google Scholar] [CrossRef]
- Guerrini, S.; Mari, E.; Migliorini, M.; Cherubini, C.; Trapani, S.; Zanoni, B.; Vincenzini, M.; Section, M. Investigation on microbiology of olive oil extraction process. Ital. J. Food Sci. 2015, 27, 236–247. [Google Scholar]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Migliorini, M.; Mugelli, M.; Cherubini, C.; Viti, P.; Zanoni, B. Influence of O2 on the quality of virgin olive oil during malaxation. J. Sci. Food Agric. 2006, 2146, 2140–2146. [Google Scholar] [CrossRef]
- Rallo, L.; El Riachy, M.; Priego-capote, F.; Leo, L.; Rabanales, C.; De Mutis, E.C.; Madrid-ca, C.; Rabanales, C.; De Curie, E.M.; Madrid-ca, C. Review Article Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar]
- Ciafardini, G.; Zullo, B.A. Survival of micro-organisms in extra virgin olive oil during storage. Food Microbiol. 2002, 19, 105–109. [Google Scholar] [CrossRef]
- Bakhouche, A.; Lozano-Sánchez, J.; Ballus, C.A.; Martínez-García, M.; Velasco, M.G.; Govantes, Á.O.; Gallina-Toschi, T.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Monitoring the moisture reduction and status of bioactive compounds in extra-virgin olive oil over the industrial filtration process. Food Control. 2013, 40, 292–299. [Google Scholar] [CrossRef]
- Zanoni, B. Which processing markers are recommended for measuring and monitoring the transformation pathways of main components of olive oil? Ital. J. Food Sci. 2014, 26, 3–12. [Google Scholar]
- Mossel, D.A.A.; Corry, J.E.L.; Strujik, C.B.; Baird, R.M. Essential of the Microbiology of Foods: A Textbook for Advanced Studies; John & Wiley Sons: Chichester, UK, 1995; p. 735. [Google Scholar]
- Xenakis, A.; Papadimitriou, V.; Sotiroudis, T.G. Colloidal structures in natural oils. Curr. Opin. Colloid Interface Sci. 2010, 15, 55–60. [Google Scholar] [CrossRef]
- Budilarto, E.S.; Kamal-Eldin, A. The Supramolecular Chemistry of Lipid Oxidation and Antioxidation in Bulk Oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef]
- BrkićBubola, K.; Lukić, M.; Mofardin, I.; Butumović, A.; Koprivnjak, O. Filtered vs. naturally sedimented and decanted virgin olive oil during storage: Effect on quality and composition. LWT 2017, 84, 370–377. [Google Scholar]
- Aparicio-Ruiz, R.; Garcia-Gonzalez, D.L.; Oliver-Pozo, C.; Tena, N.; Morales, M.; Aparicio, R. Phenolic profile of virgin olive oils with and without sensory defects: Oils with non-oxidative defects exhibit a considerable concentration of phenols. Eur. J. Lipid Sci. Technol. 2016, 118, 299–307. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical strategies to increase nutritional and sensory quality of virgin olive oil by modulating the endogenous enzyme activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Klen, T.J.; Wondra, A.G.; Sivilotti, P. Olive Fruit Phenols Transfer, Transformation, and -Partition Trail during Laboratory-Scale Olive Oil Processing. J. Agric. Food Chem. 2015, 63, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- Trapani, S.; Breschi, C.; Cecchi, L.; Guerrini, L.; Mulinacci, N.; Parenti, A.; Canuti, V.; Picchi, M.; Caruso, G.; Gucci, R.; et al. Indirect indices of oxidative damage to phenolic compounds for the implementation of olive paste malaxation optimization charts. J. Food Eng. 2017, 207, 24–34. [Google Scholar] [CrossRef]
- Zanoni, B.; Breschi, C.; Canuti, V.; Guerrini, L.; Masella, P.; Picchi, M.; Parenti, A. An original computer program (MalaxAction 1.0) to design and control olive paste malaxation under exposure to air. J. Food Eng. 2018, 234, 57–62. [Google Scholar] [CrossRef]
- Migliorini, M.; Cecchi, L.; Cherubini, C.; Trapani, S.; Cini, E.; Zanoni, B. Understanding degradation of phenolic compounds during olive oil processing by inhibitor addition. Eur. J. Lipid Sci. Technol. 2012, 114, 942–950. [Google Scholar] [CrossRef]
- Frankel, E.N. Recent advances in lipid oxidation. J. Sci. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Guerrini, L.; Luca Pantani, O.; Parenti, A. The impact of vertical centrifugation on olive oil quality. J. Food Process. Eng. 2017, 40, e12489. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Sánchez-Ortiz, A.; Pérez, A.G. Role of polyphenol oxidase and peroxidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2011, 44, 629–635. [Google Scholar] [CrossRef]
- European Union Commission implementing regulation (EC) No 1989/2003 of 6 November 2003 amending regulation No 2568/91 on the characertistics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2003, L295, 57–77.
- International Olive Council. Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of Virgin Olive Oil; International Olive Council: Madrid, Spain, 2018. [Google Scholar]
- Zullo, B.A.; Cioccia, G.; Ciafardini, G. Distribution of dimorphic yeast species in commercial extra virgin olive oil. Food Microbiol. 2010, 27, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. Determination of Biophenols in Olive Oils by HPLC; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Fiorini, D.; Boarelli, M.C.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; Gabbianelli, R.; Pacetti, D. Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int. 2018, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Calamai, L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta 2017, 165, 641–652. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Oil Samples | Degree of Turbidity (NTU) | Water Content (%w/w) | Solid Particle Content (%w/w) | Aw | Microbial Cell Count (log UFC/g) |
|---|---|---|---|---|---|
| CON | 1525 ± 108 | 0.25 ± 0.09 | 0.22 ± 0.06 | 0.76 ± 0.05 | 4.5 ± 0.2 |
| HHP | 0.0 ± 0.0 | ||||
| FIL | 17 ± 4 | 0.05 ± 0.01 | 0.00 ± 0.00 | 0.42 ± 0.02 | 0.0 ± 0.0 |
| F-HHP | 0.0 ± 0.0 |
| Storage Time (Months) | F-HHP | FIL | HHP | CON | p F | p HP | p T | p F × T | p F × HP | p HP × T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | |||||||
| Fruity | 5 ± 1 ax | 2 ± 1 bx | 2 ± 0 bx | 5 ± 1 ax | 2 ± 1 bx | 2 ± 1 bx | 3 ± 1 ay | 1 ± 1 by | 1 ± 1 by | 4 ± 1 ay | 1 ± 1 by | 1 ± 1 by | *** | ns | *** | ns | ns | ns |
| Bitter | 4 ± 0 | 2 ± 0 | 2 ± 1 | 5 ± 0 | 2 ± 0 | 2 ± 1 | 3 ± 0 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 2 ± 1 | 1 ± 1 | ** | ns | *** | * | ns | ns |
| Pungent | 5 ± 0 ax | 3 ± 1 bx | 2 ± 0 bx | 5 ± 1 ax | 4 ± 1 bx | 3 ± 1 bx | 4 ± 0 ay | 3 ± 1 by | 2 ± 1 by | 4 ± 1 ay | 2 ± 1 by | 1 ± 1 by | * | ns | *** | ns | ns | ns |
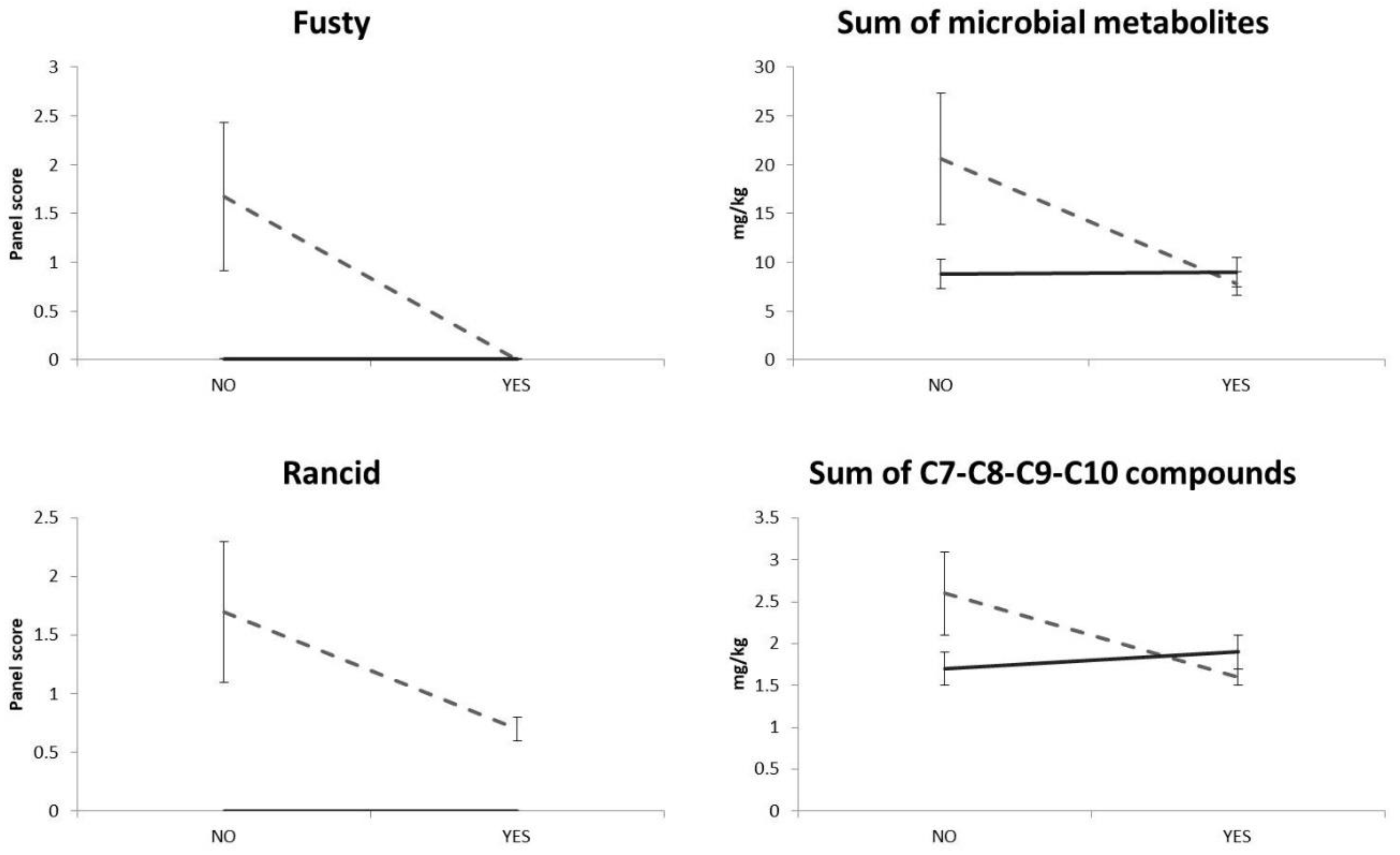
| Fusty | nd | nd | nd | nd | Nd | nd | nd | nd | nd | 1 ± 0 | 2 ± 1 | 2 ± 1 | *** | * | ns | ns | ** | ns |
| Rancid | nd | nd | nd | nd | Nd | nd | nd | 1 ± 0 | 1 ± 0 | nd | 2 ± 0 | 3 ± 1 | *** | * | *** | * | * | * |
| Storage Time (Months) | FIL-HHP | FIL | HHP | CON | p F | p HP | p T | p F × T | p F × HP | p HP × T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | |||||||
| Sum of C5 compounds | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.8 ± 0.3 | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.3 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.0 | 1.5 ± 0.1 | 2.4 ± 1.4 | ns | ns | ns | ns | Ns | ns |
| Sum of C6 compounds | 33.7 ± 7.1 | 33.8 ± 3.7 | 25.9 ± 2.5 | 35.0 ± 9.2 | 32.5 ± 3.1 | 22.9 ± 0.9 | 30.2 ± 5.8 | 29.8 ± 2.5 | 22.8 ± 2.5 | 34.1 ± 4.2 | 29.4 ± 7.5 | 21.7 ± 4.2 | ns | ns | * | ns | Ns | ns |
| E-2-hexenal | 30.2 ± 5.2 ax | 30.6 ± 5.7 ax | 22.6 ± 6.7 bx | 31.4 ± 8.7 ax | 29.3 ± 11.6ax | 19.7 ± 2.3 bx | 26.9 ± 3.1 ay | 27.1 ± 3.1 ay | 20.3 ± 8.8 by | 27.5 ± 2.3 ay | 19.8 ± 2.0 ay | 13.1 ± 0.4 by | ** | ns | *** | ns | Ns | ns |
| Sum of microbial metabolite compounds | 9.8 ± 2.6 | 8.3 ± 0.9 | 8.7 ± 0.6 | 9.7 ± 2.4 | 7.4 ± 1.1 | 9.2 ± 0.9 | 8.8 ± 2.0 | 6.6 ± 0.5 | 8.2 ± 0.5 | 7.6 ± 0.9 | 19.2 ± 9.5 | 35.2 ± 10.2 | ns | ns | ns | ns | * | ns |
| Sum of C7, C8, C9 and C10 compounds | 1.7 ± 0.1 | 1.7 ± 0.2 | 2.2 ± 0.3 | 1.4 ± 0.1 | 1.6 ± 0.2 | 2.2 ± 0.2 | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 4.2 ± 2.1 | 1.9 ± 0.1 | 1.8 ± 0.1 | ** | ** | ns | ns | ** | ns |
| Storage Time (Months) | FIL-HPP | FIL | HHP | CON | p F | p HP | p T | p F × T | p F × HP | p HP × T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | 0.5 | 1 | 6 | |||||||
| Sum of oleuropein and its derivatives | 333 ± 22 a | 306 ± 40 a | 309 ± 29 a | 346 ± 9 a | 317 ± 26 a | 290 ± 25 a | 218 ± 42 b | 228 ± 37 b | 248 ± 56 b | 229 ± 45 b | 229 ± 44 b | 241 ± 58 b | *** | ns | ns | ns | ns | ns |
| 3,4-DHPEA-EDA | 122 ± 14 a | 135 ± 16 a | 126 ± 25 a | 123 ± 7 a | 112 ± 17 a | 131 ± 15 a | 65 ± 22 b | 71 ± 23 b | 64 ± 21 b | 67 ± 24 b | 68 ± 11 b | 60 ± 22 b | *** | ns | ns | ns | ns | ns |
| Hydroxytyrosol | 1 ± 0 | 2 ± 0 | 3 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 | 6 ± 1 | 6 ± 2 | 15 ± 3 | 8 ± 3 | 7 ± 4 | 17 ± 3 | *** | ns | *** | *** | ns | ns |
| Sum of ligstroside and its derivatives | 106 ± 15 | 109 ± 15 | 128 ± 23 | 118 ± 22 | 109 ± 17 | 116 ± 13 | 124 ± 23 | 113 ± 22 | 135 ± 25 | 125 ± 26 | 115 ± 29 | 126 ± 28 | ns | ns | ns | ns | ns | ns |
| p-HPEA-EDA | 71 ± 6 | 68 ± 8 | 73 ± 14 | 73 ± 5 | 67 ± 12 | 75 ± 9 | 70 ± 12 | 65 ± 13 | 70 ± 14 | 74 ± 14 | 68 ± 21 | 64 ± 17 | ns | ns | ns | ns | ns | ns |
| Tyrosol | 2 ± 0 | 2 ± 0 | 3 ± 0 | 2 ± 0 | 2 ± 0 | 3 ± 0 | 2 ± 1 | 3 ± 0 | 6 ± 1 | 3 ± 1 | 4 ± 2 | 9 ± 6 | *** | ns | *** | *** | ns | ns |
| Total phenolic compounds | 448 ± 20 | 474 ± 42 | 484 ± 46 | 479 ± 19 | 468 ± 48 | 469 ± 37 | 418 ± 66 | 429 ± 52 | 481 ± 81 | 434 ± 68 | 420 ± 61 | 472 ± 72 | ns | ns | ns | ns | ns | ns |
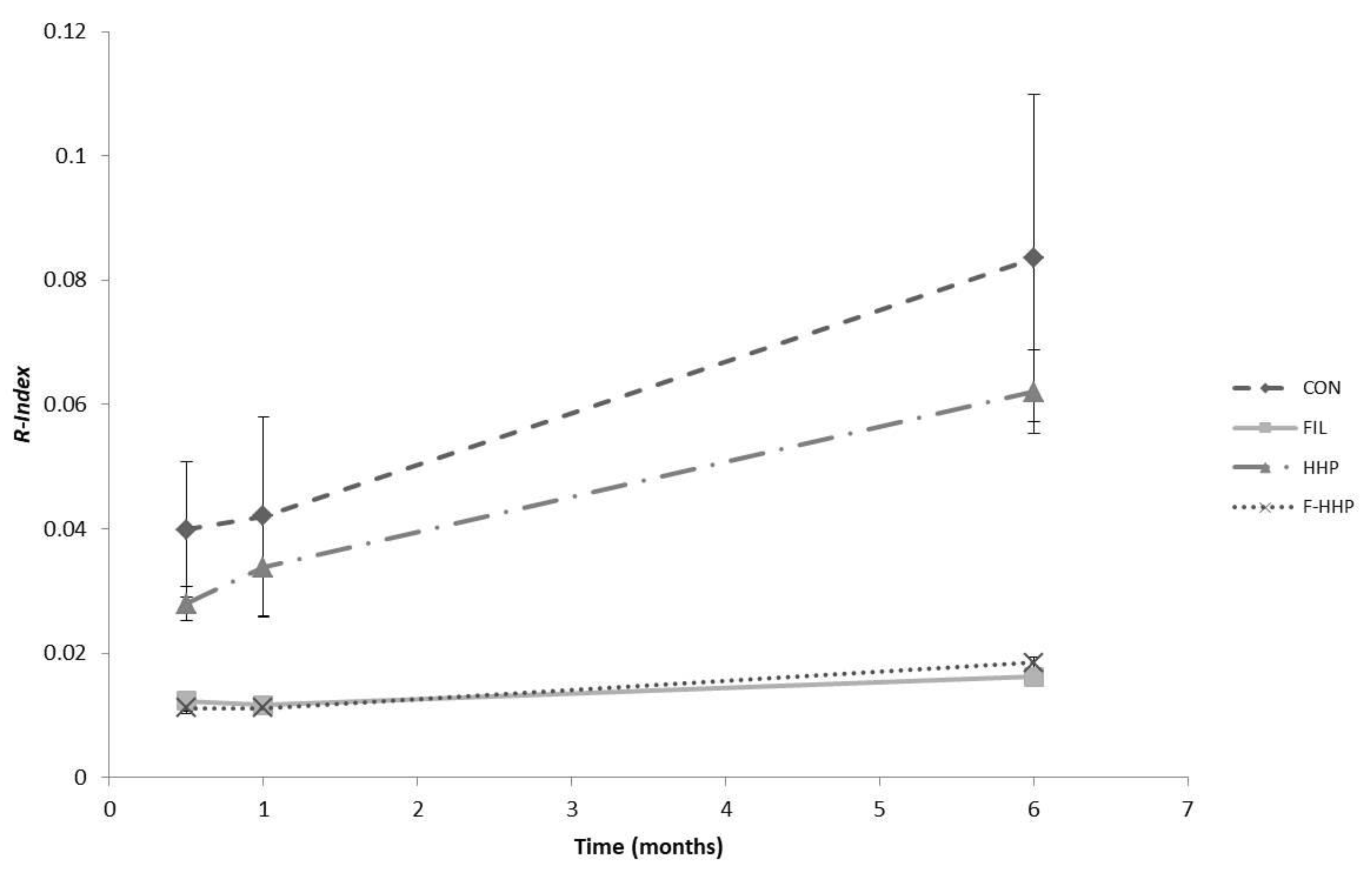
| R-Index (10−2) | 1 ± 0 | 1 ± 0 | 2 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 1 | 3 ± 2 | 6 ± 1 | 4 ± 2 | 4 ± 3 | 8 ± 5 | *** | ns | *** | *** | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, L.; Zanoni, B.; Breschi, C.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure. Molecules 2020, 25, 420. https://doi.org/10.3390/molecules25020420
Guerrini L, Zanoni B, Breschi C, Angeloni G, Masella P, Calamai L, Parenti A. Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure. Molecules. 2020; 25(2):420. https://doi.org/10.3390/molecules25020420
Chicago/Turabian StyleGuerrini, Lorenzo, Bruno Zanoni, Carlotta Breschi, Giulia Angeloni, Piernicola Masella, Luca Calamai, and Alessandro Parenti. 2020. "Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure" Molecules 25, no. 2: 420. https://doi.org/10.3390/molecules25020420
APA StyleGuerrini, L., Zanoni, B., Breschi, C., Angeloni, G., Masella, P., Calamai, L., & Parenti, A. (2020). Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure. Molecules, 25(2), 420. https://doi.org/10.3390/molecules25020420







