Immunomodulatory Drugs Alter the Metabolism and the Extracellular Release of Soluble Mediators by Normal Monocytes
Abstract
1. Introduction
2. Results
2.1. TLR4/LPS Activation Alters the Balance between Glycolysis and Oxidative Phosphorylation in the Direction of Glycolysis
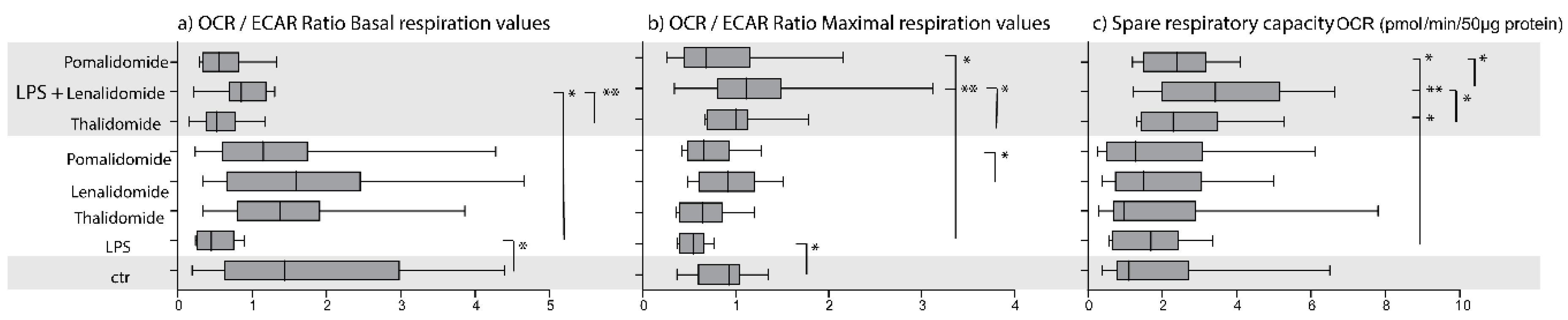
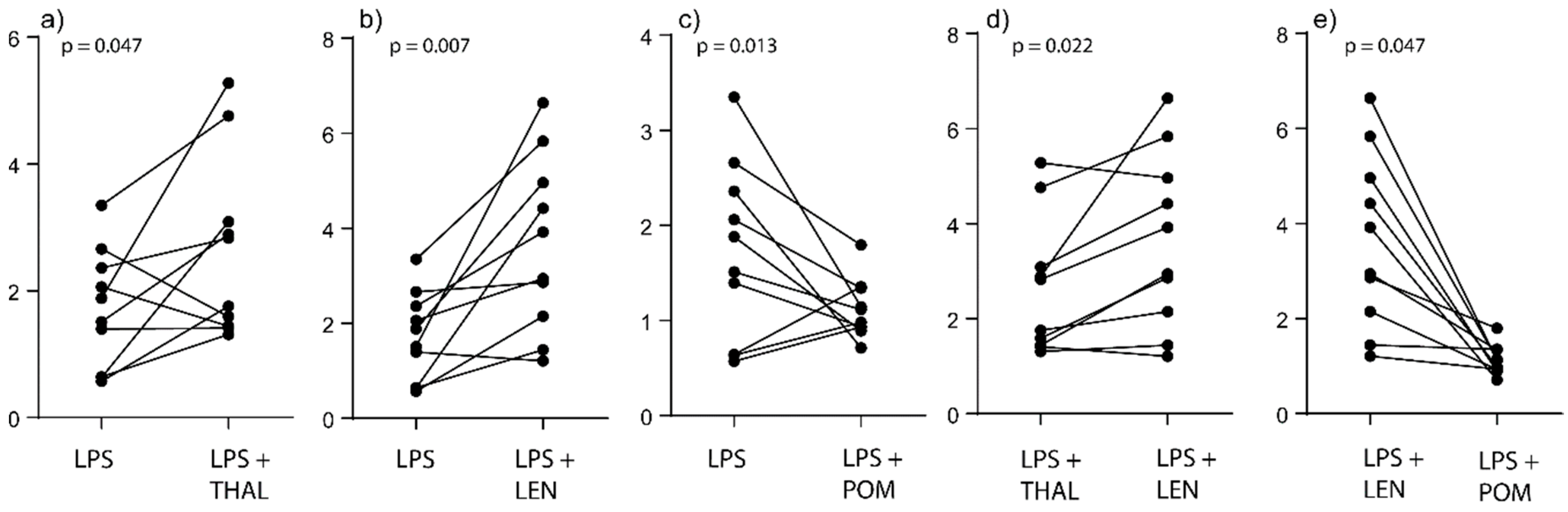
2.2. IMiDs Differ in Their Effects on the Balance between Glycolysis and Oxidative Phosphorylation; Especially Lenalidomide Increases Oxidative Phosphorylation
2.3. IMiDs Have Different Effects on the Spare Respiratory Capacity of Normal Monocytes
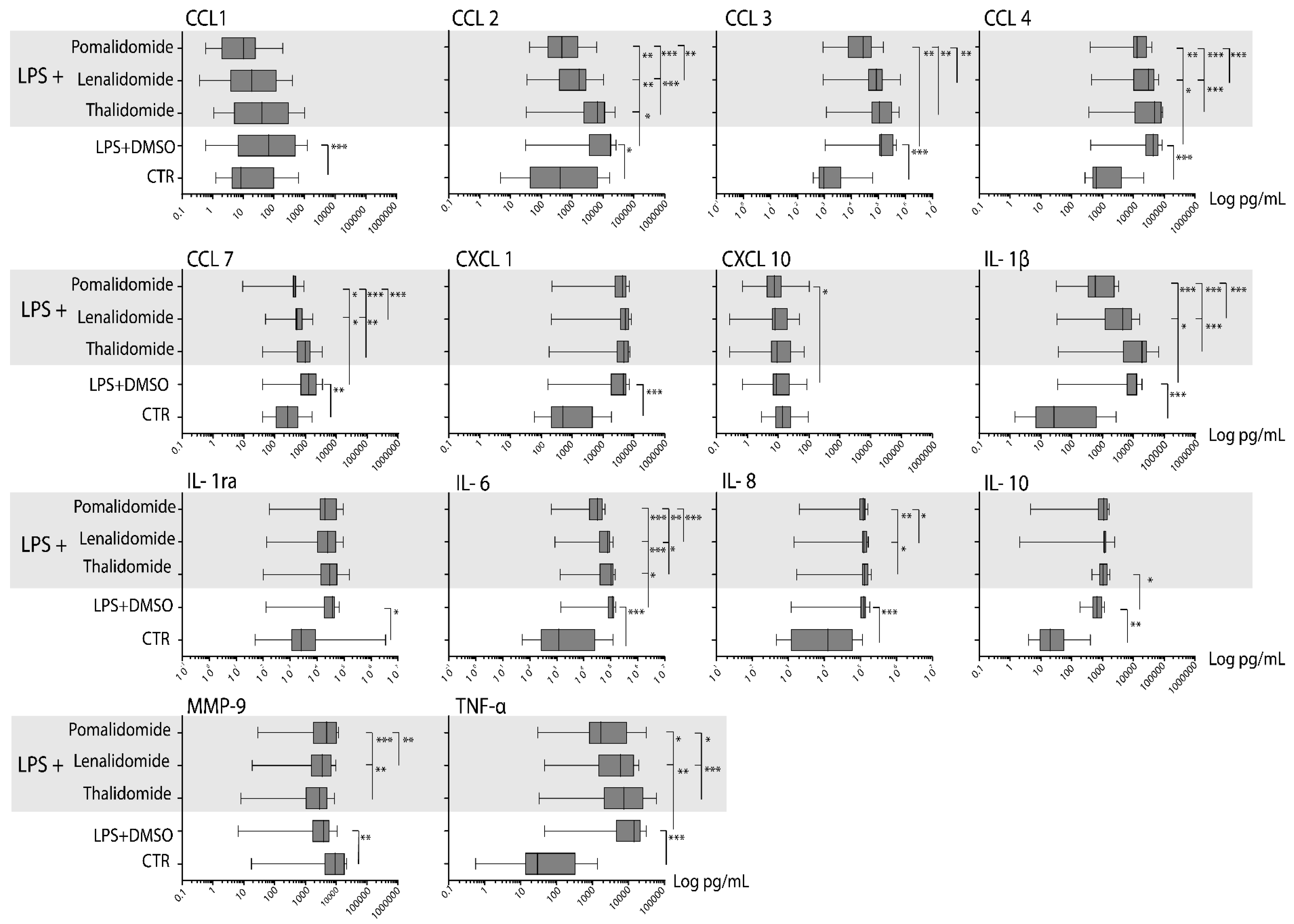
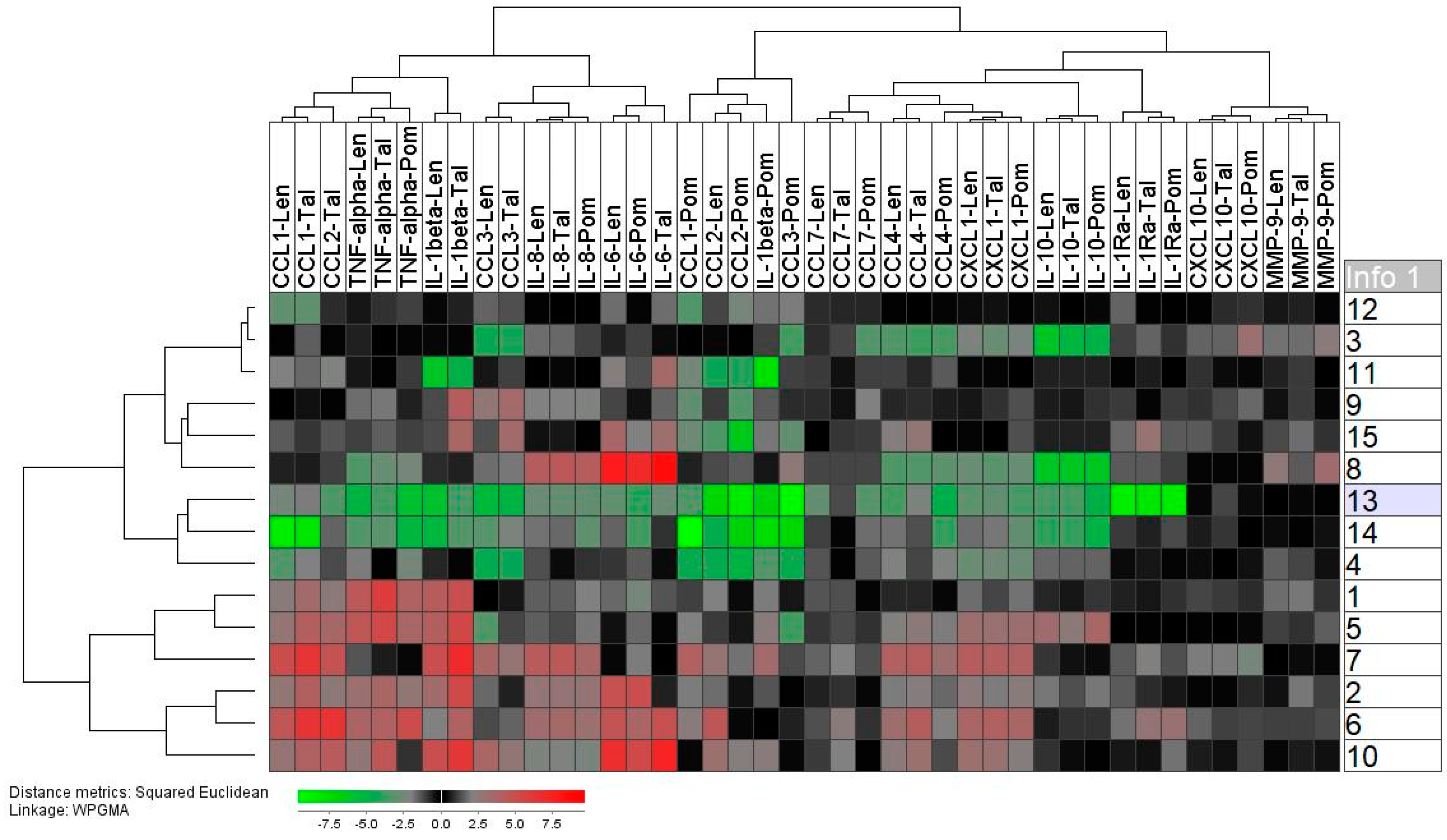
2.4. Healthy Individuals Differ in Their Spontaneous and TLR4/LPS-Induced Mediator Release
2.5. Thalidomide Shows a Weak, Lenalidomide an Intermediate, and Pomalidomide a Strong Inhibitory Effect on the TLR4/LPS-Induced Release of Soluble Mediators by Normal Monocytes
2.6. Lenalidomide and Pomalidomide Reduce TLR4/LPS-Induced Mediator Release by Normal Monocytes Also at Concentrations Corresponding to Their Therapeutic Serum Levels
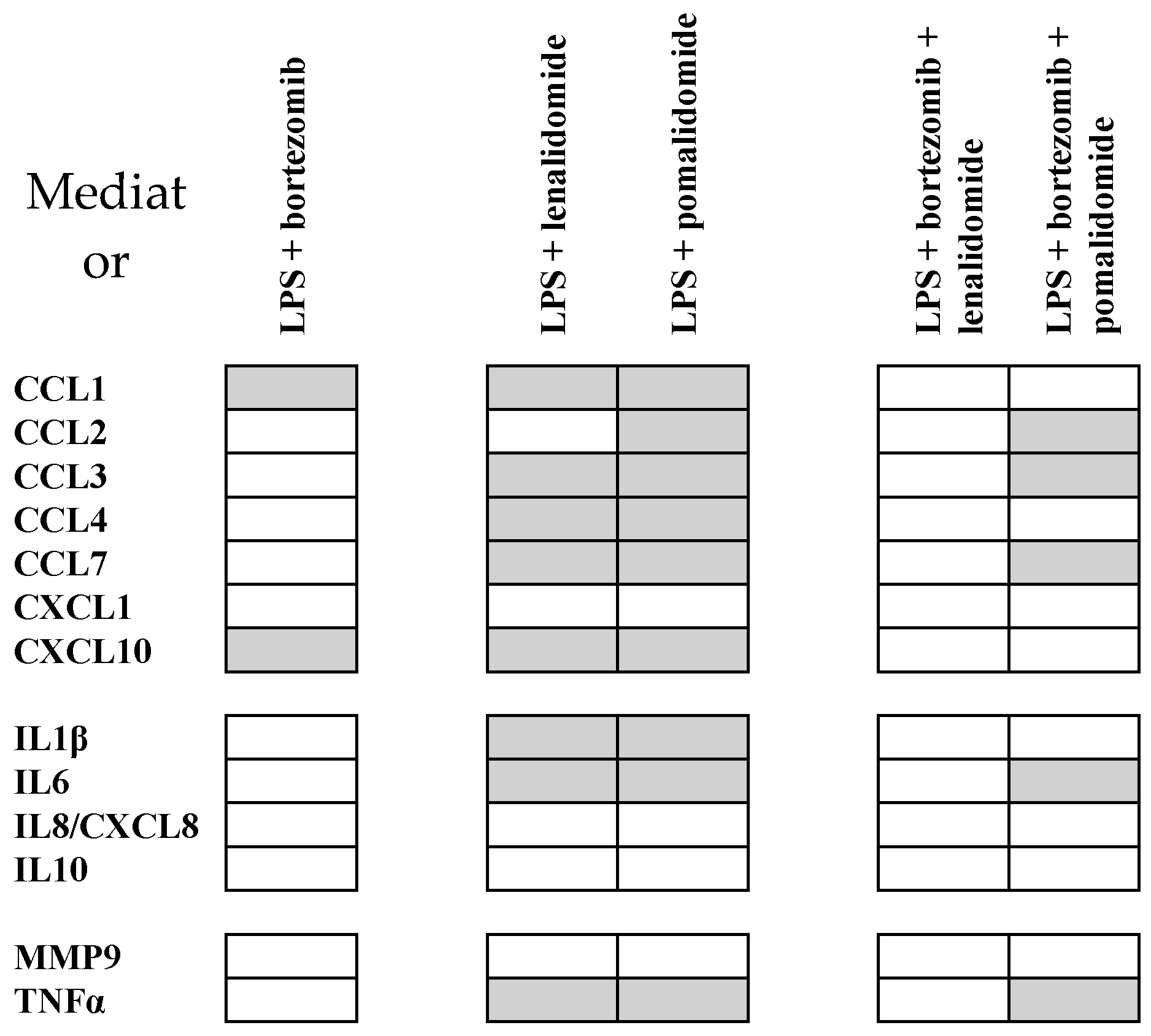
2.7. Pomalidomide Inhibits Soluble Mediator Release also in the Presence of Bortezomib
3. Discussion
4. Materials and Methods
4.1. Cell Donors
4.2. Reagents
4.3. Preparation of Enriched Normal Monocytes
4.4. Analysis of Monocyte Metabolism
4.5. Analysis of TLR4/LPS Induced Cytokine Release
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leufven, E.; Bruserud, O. Immunosuppression and Immunotargeted Therapy in Acute Myeloid Leukemia-The Potential Use of Checkpoint Inhibitors in Combination with Other Treatments. Curr. Med. Chem. 2019, 26, 5244–5261. [Google Scholar] [CrossRef]
- Ito, T.; Handa, H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int. J. Hematol. 2016, 104, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, O.; Aasebo, E.; Hernandez-Valladares, M.; Tsykunova, G.; Reikvam, H. Therapeutic targeting of leukemic stem cells in acute myeloid leukemia-the biological background for possible strategies. Expert Opin. Drug Dis. 2017, 12, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, A.; Trumpp, A. The bone marrow stem cell niche grows up: Mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011, 208, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Murphy, A.J.; Woollard, K.J. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat. Rev. Cardiol 2017, 14, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179. [Google Scholar] [CrossRef]
- Hoeksema, M.A.; de Winther, M.P.J. Epigenetic Regulation of Monocyte and Macrophage Function. Antioxid. Redox Sign. 2016, 25, 758–774. [Google Scholar] [CrossRef]
- Groh, L.; Keating, S.T.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 2018, 40, 203–214. [Google Scholar] [CrossRef]
- De Nardo, D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 2015, 74, 181–189. [Google Scholar] [CrossRef]
- Gao, D.; Li, W. Structures and recognition modes of toll-like receptors. Proteins 2017, 85, 3–9. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L.; et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef]
- Gautier, E.L.; Westerterp, M.; Bhagwat, N.; Cremers, S.; Shih, A.; Abdel-Wahab, O.; Lutjohann, D.; Randolph, G.J.; Levine, R.L.; Tall, A.R.; et al. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J. Exp. Med. 2013, 210, 339–353. [Google Scholar] [CrossRef]
- Wallace, C.; Keast, D. Glutamine and Macrophage Function. Metabolism 1992, 41, 1016–1020. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Shi, L.H.; Qin, X.Q.; Wang, H.J.; Xia, Y.H.; Li, Y.Y.; Chen, X.J.; Shang, L.; Tai, Y.T.; Feng, X.Y.; Acharya, P.; et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 2017, 8, 18792–18801. [Google Scholar] [CrossRef]
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; van Kessel, B.; van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.C.; Zweegman, S.; van Meerloo, J.; Musters, R.J.P.; et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Pugliese, M.; Di Salvo, E.; Ventura-Spagnolo, E.; Musolino, C.; Gangemi, S. Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 2822. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef]
- Vasileiou, S.; Baltadakis, I.; Delimpasi, S.; Karatza, M.H.; Liapis, K.; Garofalaki, M.; Tziotziou, E.; Poulopoulog, Z.; Karakasis, D.; Harhalakis, N. Ex Vivo Induction of Multiple Myeloma-specific Immune Responses by Monocyte-derived Dendritic Cells Following Stimulation by Whole-tumor Antigen of Autologous Myeloma Cells. J. Immunother. 2017, 40, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Millrud, C.R.; Bergenfelz, C.; Leandersson, K. On the origin of myeloid-derived suppressor cells. Oncotarget 2017, 8, 3649–3665. [Google Scholar] [CrossRef] [PubMed]
- Shortt, J.; Hsu, A.K.; Johnstone, R.W. Thalidomide-analogue biology: Immunological, molecular and epigenetic targets in cancer therapy. Oncogene 2013, 32, 4191–4202. [Google Scholar] [CrossRef] [PubMed]
- Corral, L.G.; Haslett, P.A.J.; Muller, G.W.; Chen, R.; Wong, L.M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 1999, 163, 380–386. [Google Scholar] [PubMed]
- Murray, P.J. Immune regulation by monocytes. Semin. Immunol. 2018, 35, 12–18. [Google Scholar] [CrossRef]
- Gay, F.; Engelhardt, M.; Terpos, E.; Wasch, R.; Giaccone, L.; Auner, H.W.; Caers, J.; Gramatzki, M.; van de Donk, N.; Oliva, S.; et al. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica 2018, 103, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Slade, D.; De Santo, C.; Wheat, R.; Ferguson, P.; Hodgkinson, A.; Brock, K.; Cavenagh, J.; Ingram, W.; Dennis, M.; et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J. Clin. Oncol. 2019, 37, 580–588. [Google Scholar] [CrossRef]
- Tamura, H. Immunopathogenesis and immunotherapy of multiple myeloma. Int. J. Hematol. 2018, 107, 278–285. [Google Scholar] [CrossRef]
- Li, L.; Wang, L. Multiple Myeloma: What Do We Do About Immunodeficiency? J. Cancer 2019, 10, 1675–1684. [Google Scholar] [CrossRef]
- Raza, S.; Safyan, R.A.; Lentzsch, S. Immunomodulatory Drugs (IMiDs) in Multiple Myeloma. Curr. Cancer Drug Targets 2017, 17, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ishida, T. Immunomodulatory drugs in the treatment of multiple myeloma. Jpn. J. Clin. Oncol. 2019, 49, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Prada, C.P.; Richardson, P.G. How I treat the young patient with multiple myeloma. Blood 2018, 132, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yang, J.; Xu, G. The interacting domains in cereblon differentially modulate the immunomodulatory drug-mediated ubiquitination and degradation of its binding partners. Biochem. Biophys. Res. Commun. 2018, 507, 443–449. [Google Scholar] [CrossRef]
- Gemechu, Y.; Millrine, D.; Hashimoto, S.; Prakash, J.; Sanchenkova, K.; Metwally, H.; Gyanu, P.; Kang, S.; Kishimoto, T. Humanized cereblon mice revealed two distinct therapeutic pathways of immunomodulatory drugs. Proc. Natl. Acad. Sci. USA 2018, 115, 11802–11807. [Google Scholar] [CrossRef]
- Latif, T.; Chauhan, N.; Khan, R.; Moran, A.; Usmani, S.Z. Thalidomide and its analogues in the treatment of Multiple Myeloma. Exp. Hematol. Oncol. 2012, 1, 27. [Google Scholar] [CrossRef]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C.; et al. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2019, 2019, 100616. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Stienstra, R.; Netea-Maier, R.T.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017, 26, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Estruch, M.; Bancells, C.; Beloki, L.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis 2013, 229, 356–362. [Google Scholar] [CrossRef]
- Bruserud, O.; Ehninger, G.; Hamann, W.; Pawelec, G. Secretion of IL-2, IL-3, IL-4, IL-6 and GM-CSF by CD4+ and CD8+ TCR alpha beta+ T-cell clones derived early after allogeneic bone marrow transplantation. Scand. J. Immunol. 1993, 38, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.K.; Colburn, W.A.; Tracewell, W.G.; Kook, K.A.; Stirling, D.I.; Jaworsky, M.S.; Scheffler, M.A.; Thomas, S.D.; Laskin, O.L. Clinical pharmacokinetics of thalidomide. Clin. Pharmacokinet. 2004, 43, 311–327. [Google Scholar] [CrossRef]
- Connarn, J.N.; Hwang, R.F.; Gao, Y.; Palmisano, M.; Chen, N.H. Population Pharmacokinetics of Lenalidomide in Healthy Volunteers and Patients with Hematologic Malignancies. Clin. Pharm. Drug Dev. 2018, 7, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.J.; Liu, L.G.; Wang, X.M.; Palmisano, M.; Zhou, S. Population Pharmacokinetics of Pomalidomide. J. Clin. Pharmacol. 2015, 55, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.G.; Wang, X.M.; Zhang, C.Y.; Reyes, J.; Hoffmann, M.; Palmisano, M.; Zhou, S. In Vivo Assessment of the Effect of CYP1A2 Inhibition and Induction on Pomalidomide Pharmacokinetics in Healthy Subjects. J. Clin. Pharmacol. 2018, 58, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, A.M.; Ernst, R.K.; Yi, J.; Yam, C.S.; Miller, S.I. Expression level of human TLR4 rather than sequence is the key determinant of LPS responsiveness. PLoS ONE 2017, 12, e0186308. [Google Scholar] [CrossRef] [PubMed]
- Dhangadamajhi, G.; Kar, A.; Rout, R.; Dhangadamajhi, P. A meta-analysis of TLR4 and TLR9 SNPs implicated in severe malaria. Rev. Soc. Bras. Med. Trop. 2017, 50, 153–160. [Google Scholar] [CrossRef]
- Jamshidi, M.; Fagerholm, R.; Khan, S.; Aittomaki, K.; Czene, K.; Darabi, H.; Li, J.; Andrulis, I.L.; Chang-Claude, J.; Devilee, P.; et al. SNP-SNP interaction analysis of NF-kappaB signaling pathway on breast cancer survival. Oncotarget 2015, 6, 37979–37994. [Google Scholar] [CrossRef]
- Rajasuriar, R.; Kong, Y.Y.; Nadarajah, R.; Abdullah, N.K.; Spelman, T.; Yuhana, M.Y.; Ponampalavanar, S.; Kamarulzaman, A.; Lewin, S.R. The CD14 C-260T single nucleotide polymorphism (SNP) modulates monocyte/macrophage activation in treated HIV-infected individuals. J. Transl. Med. 2015, 13, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, K.; Zhou, B.; Wang, Y.Y.; Rao, L.; Zhang, L. The TLR4 gene polymorphisms and susceptibility to cancer: A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Kumpf, O.; Giamarellos-Bourboulis, E.J.; Koch, A.; Hamann, L.; Mouktaroudi, M.; Oh, D.Y.; Latz, E.; Lorenz, E.; Schwartz, D.A.; Ferwerda, B.; et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: An observational study in three cohorts. Crit. Care 2010, 14, R103. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, O.; Ryningen, A.; Olsnes, A.M.; Stordrange, L.; Oyan, A.M.; Kalland, K.H.; Gjertsen, B.T. Subclassification of patients with acute myelogenous leukemia based on chemokine responsiveness and constitutive chemokine release by their leukemic cells. Haematologica 2007, 92, 332–341. [Google Scholar] [CrossRef]
- Blimark, C.; Holmberg, E.; Mellqvist, U.H.; Landgren, O.; Bjokholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 2015, 100, 107–113. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Zhu, W.Q.; Chen, X.L.; Zhou, H.B.; Chen, W.M. Peripheral Blood Lymphocyte-to-Monocyte Ratio as a Useful Prognostic Factor in Newly Diagnosed Multiple Myeloma. Biomed. Res. Int. 2018, 2018, 9434637. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Schwarzinger, I.; Simonitsch-Klupp, I.; Agis, H.; Oehler, R. Impaired efferocytosis by monocytes in multiple myeloma. Oncol. Lett. 2018, 16, 409–416. [Google Scholar] [CrossRef]
- Shinde, P.; Fernandes, S.; Melinkeri, S.; Kale, V.; Limaye, L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Sci. Rep. 2018, 8, 5705. [Google Scholar] [CrossRef]
- Romano, A.; Laura Parrinello, N.; Cerchione, C.; Letizia Consoli, M.; Parisi, M.; Calafiore, V.; Martino, E.; Conticello, C.; Di Raimondo, F.; Alberto Palumbo, G. The NLR and LMR ratio in newly diagnosed MM patients treated upfront with novel agents. Blood Cancer J. 2017, 7, 649. [Google Scholar] [CrossRef]
- Dosani, T.; Covut, F.; Beck, R.; Driscoll, J.J.; de Lima, M.; Malek, E. Significance of the absolute lymphocyte/monocyte ratio as a prognostic immune biomarker in newly diagnosed multiple myeloma. Blood Cancer J. 2017, 7, e579. [Google Scholar] [CrossRef]
- Bolzoni, M.; Ronchetti, D.; Storti, P.; Donofrio, G.; Marchica, V.; Costa, F.; Agnelli, L.; Toscani, D.; Vescovini, R.; Todoerti, K.; et al. IL21R expressing CD14(+)CD16(+) monocytes expand in multiple myeloma patients leading to increased osteoclasts. Haematologica 2017, 102, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Sponaas, A.M.; Moen, S.H.; Liabakk, N.B.; Feyzi, E.; Holien, T.; Kvam, S.; Groseth, L.A.; Stordal, B.; Buene, G.; Espevik, T.; et al. The proportion of CD16(+)CD14(dim) monocytes increases with tumor cell load in bone marrow of patients with multiple myeloma. Immun. Inflamm. Dis. 2015, 3, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Rundgren, I.M.; Ersvaer, E.; Ahmed, A.B.; Ryningen, A.; Bruserud, O. Circulating monocyte subsets in multiple myeloma patients receiving autologous stem cell transplantation-a study of the preconditioning status and the course until posttransplant reconstitution for a consecutive group of patients. BMC Immunol. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Compagno, N.; Malipiero, G.; Cinetto, F.; Agostini, C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front. Immunol. 2014, 5, 626. [Google Scholar] [CrossRef]
- Rundgren, I.M.; Bruserud, O.; Ryningen, A.; Ersvaer, E. Standardization of sampling and sample preparation for analysis of human monocyte subsets in peripheral blood. J. Immunol. Methods 2018, 461, 53–62. [Google Scholar] [CrossRef]
- Menck, K.; Behme, D.; Pantke, M.; Reiling, N.; Binder, C.; Pukrop, T.; Klemm, F. Isolation of Human Monocytes by Double Gradient Centrifugation and Their Differentiation to Macrophages in Teflon-coated Cell Culture Bags. JoVE-J. Vis. Exp. 2014, 91, e51554. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Mediator Classification | TLR4-Induced Release in Control Cultures | TLR4-Induced Release in the Presence of Thalidomide | p-Value | TLR4-Induced Release in the Presence of Lenalidomide | p-Value | TLR4-Induced Release in the Presence of Pomalidomide | p-Value |
|---|---|---|---|---|---|---|---|
| Chemokines | |||||||
| CCL1 | 63.8 (0.6–1229) | 40.7 (1.1–994) | n.s. | 22.2 (0.4–406) | n.s. | 10.4 (0.6–191) | n.s |
| CCL2 | 11,479 (30.3–26,405) | 6751 (32.4–24,724) | 0.036 | 1816 (34.5–10,349 | 0.003 | 461 (40.8–6173) | 0.001 |
| CCL3 | 973 (350–62,216) | 198,871 (1175–566,384) | n.s. | 84,338 (919–652,373) | n.s. | 26,298 (878–150,656) | n.s |
| CCL4 | 43,275 (409–86,040) | 48,452 (359–90,664) | n.s. | 30,375 (437–66,560) | 0.031 | 13,308 (394–31,896) | 0.003 |
| CCL7 | 1274 (39,7–3571) | 1088 (39.8–3473) | n.s. | 524 (50.7–1724) | 0.011 | 410 (9.1–485) | 0.003 |
| CXCL1 | 46,239 (161–70,248) | 47,546 (177–76,381) | n.s. | 56.508 (214–83,477) | n.s. | 43,350 (218–71,720) | n.s |
| CXCL10 | 8.3 (<0.1–86.3) | 9.3 (<0.1–69.7) | n.s. | 8.2 (<0.1–49.1) | n.s. | 7.5 (<0.1–102) | 0.019 |
| Interleukins | |||||||
| IL1β | 13,578 (34,9–19,091) | 20,310 (37.4–66,422) | n.s. | 6363 (33.4–15,877) | 0.02 | 580 (31.8–2442) | 0.001 |
| IL1RA | 97,396 (130–66,232) | 29,043 (102–158,380) | 23,839 (137–91,545) | n.s. | 19,870 (168–93,618) | n.s | |
| IL6 | 117,615 (2928–104.657) | 117,722 (1324–60,904) | 77,344 (865–125,094) | 0.0125 | 32,080 (615–61,886) | 0.0007 | |
| IL8/CXCL8 | 123,383 (2692–176,743) | 134,328 (1697–199,724) | n.s. | 135,616 (1428–165,045) | n.s. | 129,439 (1988–161,864) | n.s |
| IL10 | 931 (<2.1–2500) | 1255 (<2.1–1743) | 0.038 | 1082 (<2.1–1573) | n.s. | 1092 (<2.1–1573) | n.s |
| Other mediators | |||||||
| MMP9 | 3943 (50–10,680) | 2862 (8.2–8639) | n.s. | 2895 (18.5–9801) | n.s. | 4856 (28.3–11,681) | n.s |
| TNFα | 17,983 (46–21,758) | 7366 (33–60,688) | 0.0018 | 5997 (46–19,516) | n.s. | 1709 (30–31,550) | 0.0268 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rundgren, I.M.; Ryningen, A.; Anderson Tvedt, T.H.; Bruserud, Ø.; Ersvær, E. Immunomodulatory Drugs Alter the Metabolism and the Extracellular Release of Soluble Mediators by Normal Monocytes. Molecules 2020, 25, 367. https://doi.org/10.3390/molecules25020367
Rundgren IM, Ryningen A, Anderson Tvedt TH, Bruserud Ø, Ersvær E. Immunomodulatory Drugs Alter the Metabolism and the Extracellular Release of Soluble Mediators by Normal Monocytes. Molecules. 2020; 25(2):367. https://doi.org/10.3390/molecules25020367
Chicago/Turabian StyleRundgren, Ida Marie, Anita Ryningen, Tor Henrik Anderson Tvedt, Øystein Bruserud, and Elisabeth Ersvær. 2020. "Immunomodulatory Drugs Alter the Metabolism and the Extracellular Release of Soluble Mediators by Normal Monocytes" Molecules 25, no. 2: 367. https://doi.org/10.3390/molecules25020367
APA StyleRundgren, I. M., Ryningen, A., Anderson Tvedt, T. H., Bruserud, Ø., & Ersvær, E. (2020). Immunomodulatory Drugs Alter the Metabolism and the Extracellular Release of Soluble Mediators by Normal Monocytes. Molecules, 25(2), 367. https://doi.org/10.3390/molecules25020367






