Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols
Abstract
1. Introduction
2. Results and Discussion
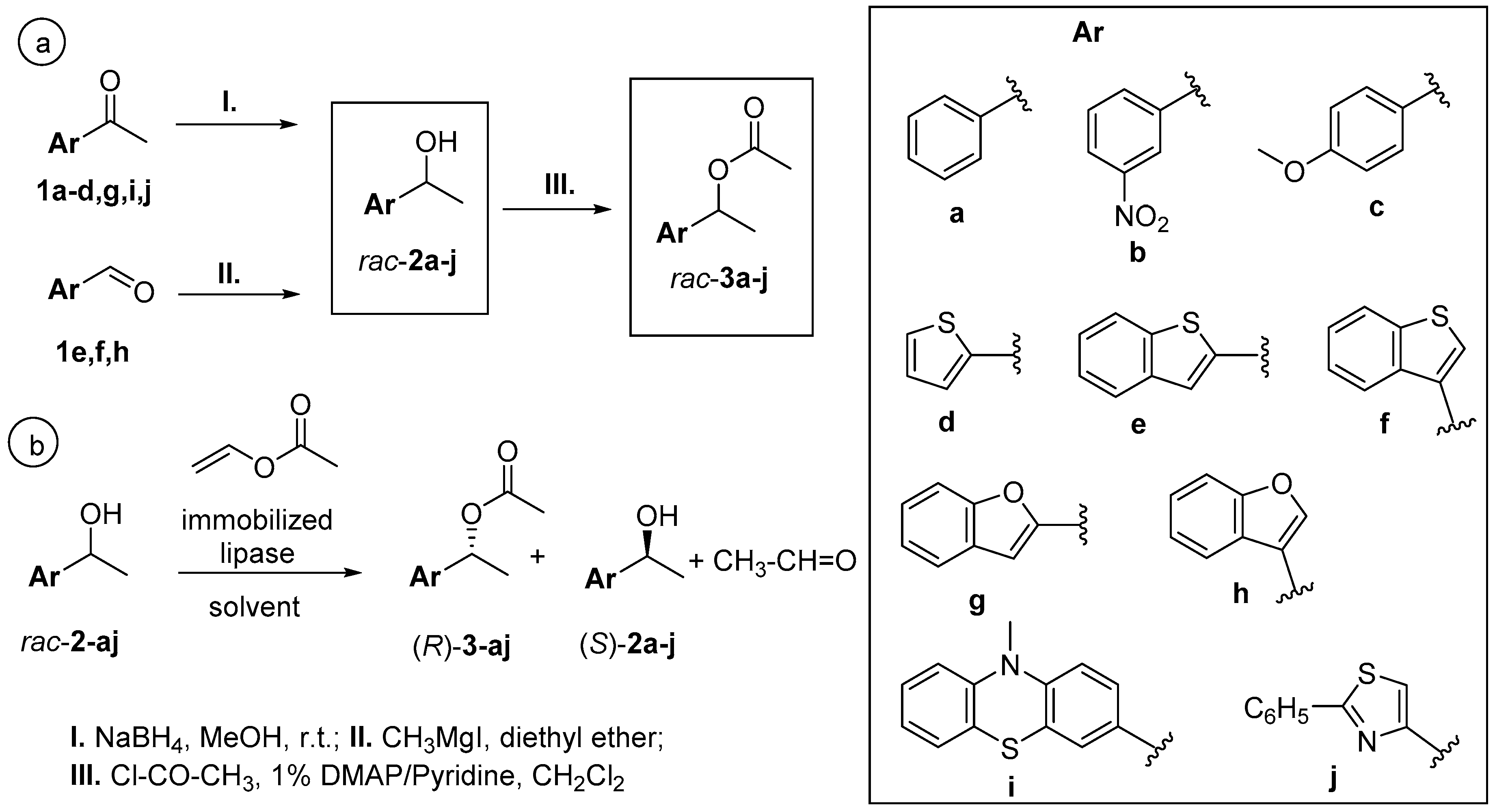
2.1. Chemical Synthesis of Racemic Heteroaryl-Ethanols rac-2a-i and Their Corresponding Acetates rac-3a–j
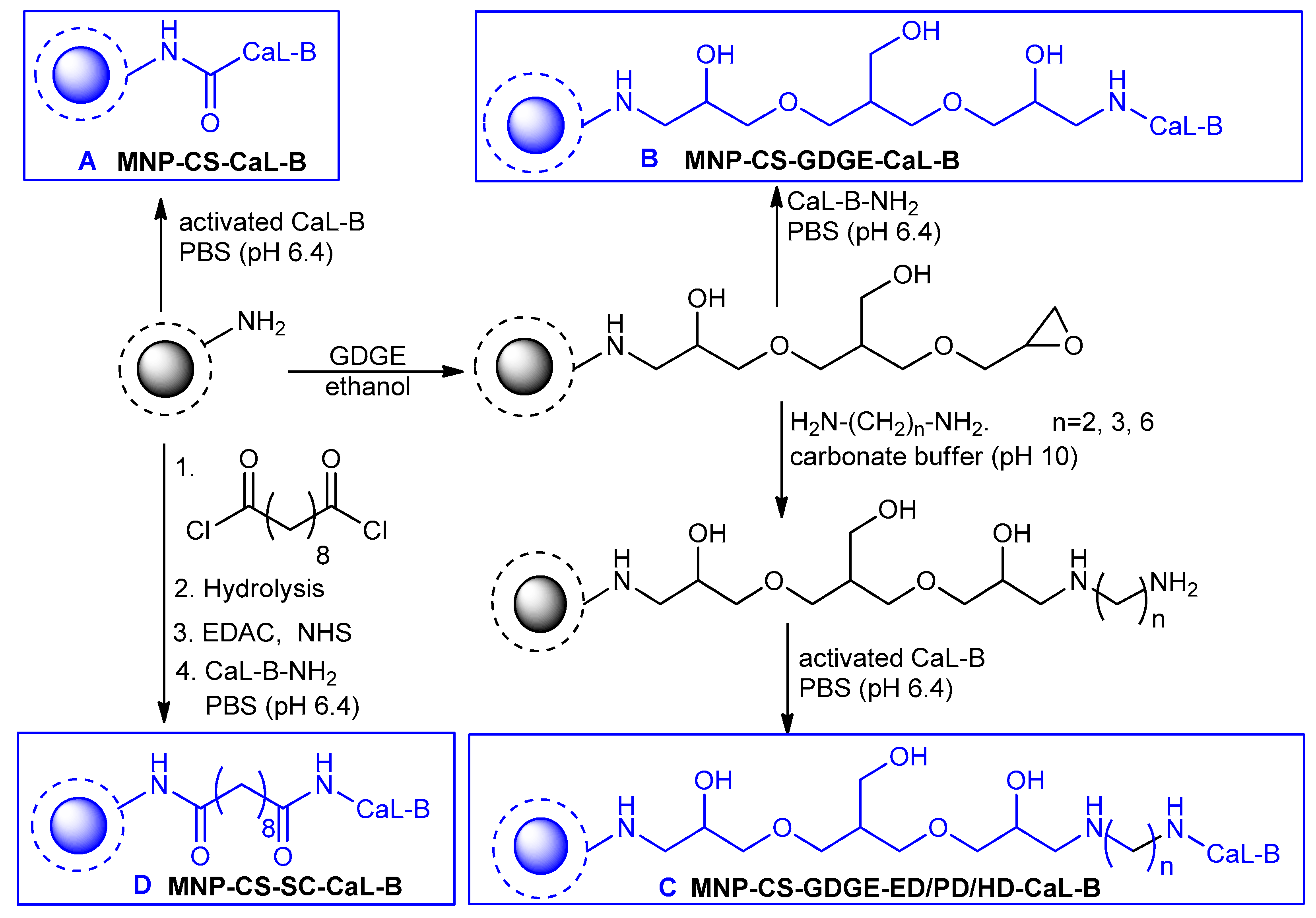
2.2. Covalent Immobilization of CaL-B on MNP-CS Supports
2.3. Enzyme Catalyzed Transesterification Studies
2.4. Analytical-Scale Lipase-Mediated O-Acylation of Racemic Heteroarylethanols rac-2a–j
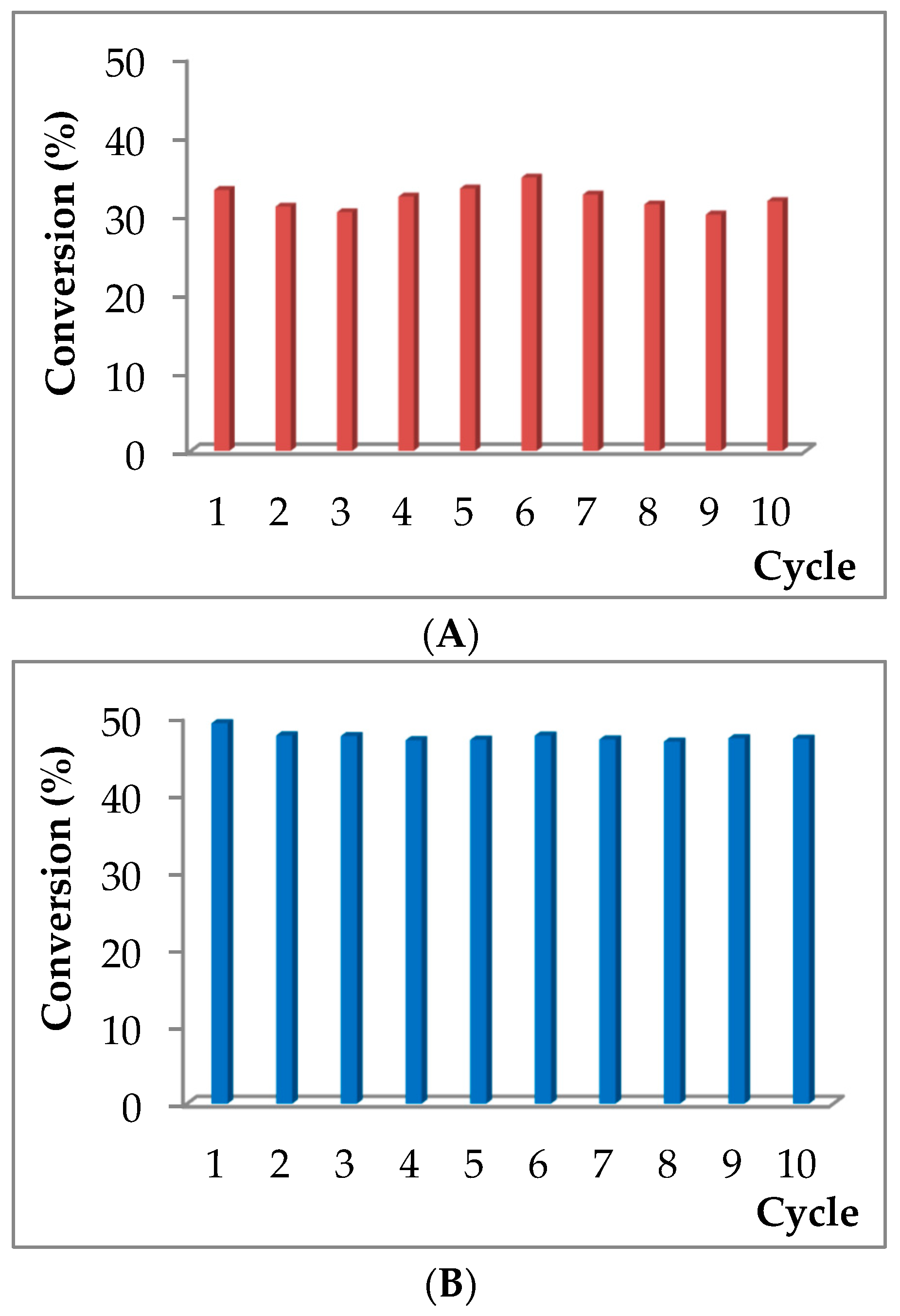
2.5. Reuse Experiments.
3. Materials and Methods
3.1. Chemical Synthesis
3.2. Immobilization of Lipase B from Candida Antarctica (CaL-B) on Magnetic Nanoparticles Coated with Chitosan (MNP-CS)
3.2.1. Synthesis of MNP-CS
3.2.2. Covalent Immobilization of CaL-B on MNP-CS
3.2.3. CaL-B Load on MNP-CS Based Supports
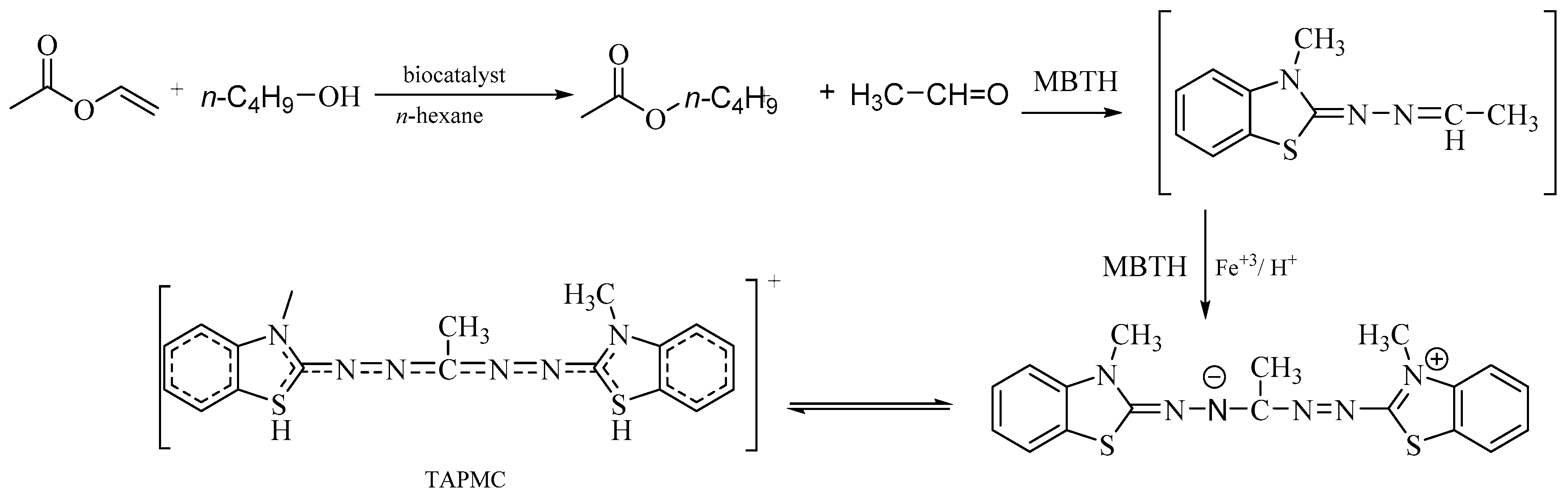
3.2.4. The Protocol for the Synthetic Activity
3.2.5. General Protocol for Enzymatic O-Transesterification
3.2.6. Enzyme Catalyzed Transesterification Studies
Determination of the Optimal Biocatalyst
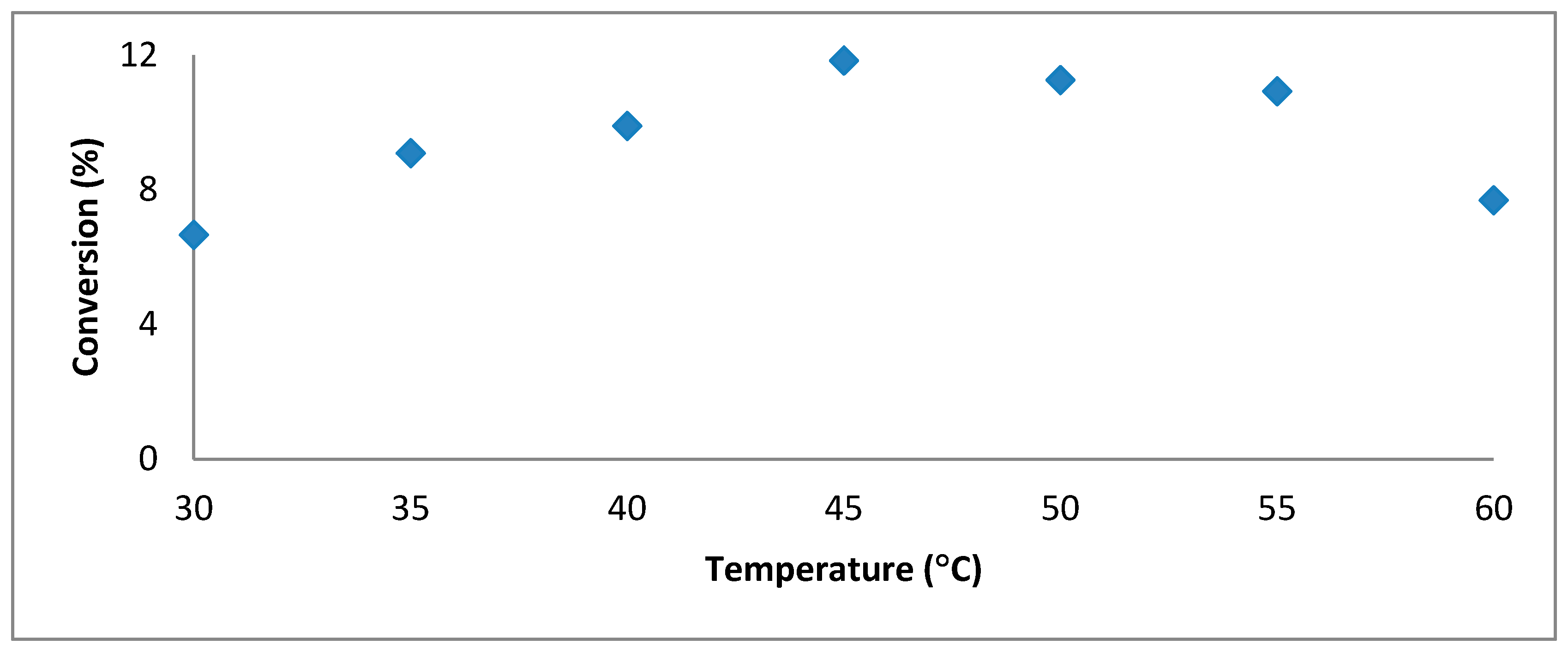
Establishing of the Optimal Temperature
Determination of the Optimal Solvent
Optimal Substrate-Enzyme Ratio
Optimal Acyl Donor
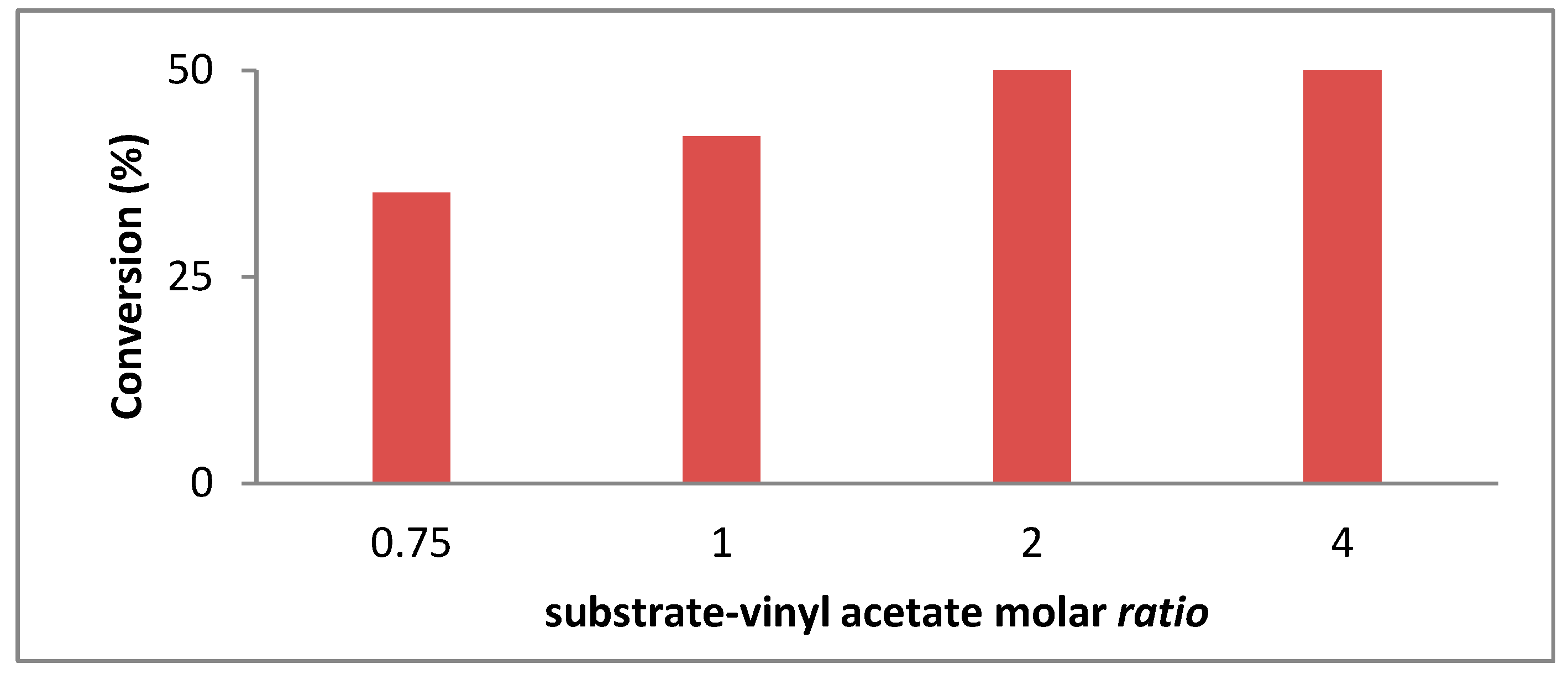
Optimal Molar Ratio Substrate-Vinyl Acetate
3.2.7. General Protocol for Recycling Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liese, A.; Seelbach, K.; Buchholz, A.; Haberland, J. Processes. In Industrial Biotransformations, 2nd ed.; Liese, A., Seelbach, K., Wandrey, C., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 273–315. [Google Scholar]
- Basso, A.; Serban, S. Industrial applications of immobilized ennzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Brito, E.; Cunha, D.A.; Bartkevihi, L.; Robert, J.M.; Cipolatti, E.P.; Ferreira, A.T.S.; Oliveira, D.M.P.; Gomes-Neto, F.; Almeida, R.V.; Fernandez-Lafuente, R.; et al. Structural differences of commercial and recombinant lipase B from Candida antarctica: An important implication on enzymes thermostability. Int. J. Biol. Macromol. 2019, 140, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Goto, S.; Sugiyama, J.; Iizuka, H. A Taxonomic Study of Antarctic Yeasts. Mycologia 1969, 61, 748–774. [Google Scholar] [CrossRef] [PubMed]
- Rogalskra, E.; Cudrey, C.; Ferrato, F.; Verger, R. Stereoselective Hydrolysis of Triglycerides by Animal and Microbial Lipases. Chirality 1993, 5, 24–30. [Google Scholar] [CrossRef]
- Solymár, M.; Fülöp, F.; Kanerva, L.T. Candida antarctica lipase A–A powerful catalyst for the resolution of heteroaromatic β-amino esters. Tetrahedron Asymmetry 2002, 13, 2383–2388. [Google Scholar] [CrossRef]
- Uppenberg, J.; Öhrner, N.; Norin, M.; Hult, K.; Kleyvegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and Molecular-Modeling Studies of Lipase B from Candida antarctica Reveal a Stereospecificity Pocket for Secondary Alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Xie, Y.; An, J.; Yang, G.; Wu, G.; Zhang, Y.; Cui, L.; Feng, Y. Enhanced Enzyme Kinetic Stability by Increasing Rigidity within the Active Site. J. Biol. Chem. 2014, 289, 7994–8006. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst—Many applications: The use of Candida antarctica lipase B in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Wu, Q.; Soni, P.; Reetz, M.T. Laboratory Evolution of Enantiocomplementary Candida antarctica Lipase B Mutants with Broad Substrate Scope. J. Am. Chem. Soc. 2013, 135, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Hapău, D.; Brem, J.; Moisă, M.; Toșa, M.-I.; Irimie, F.D.; Zaharia, V. Heterocycles 32. Efficient kinetic resolution of 1-(2-arylthiazol-4-yl) ethanols and their acetates using lipase B from Candida antarctica. J. Mol. Catal. B Enzym. 2013, 94, 88–94. [Google Scholar]
- Radadiya, A.; Shah, A. Bioactive benzofuran derivatives: An inside on lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Chimmiento, L.; Funicello, M.; Lupatteli, P.; Tramutola, F.; Berti, F.; Marino-Merlo, F. Synthesis and biological evaluation of novel small non-peptidic HIV-1Pls: The benzothiophene ring as an effective moiety. Bioorg. Med. Chem. Lett. 2012, 22, 2948–2950. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.G.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Role of Phenothiazines and Structurally Similar Compounds of Plant Origin in the Fight against Infections by Drug Resistant Bacteria. Antibiotics 2013, 2, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Paizs, C.; Toșa, M.; Bódai, V.; Szakács, G.; Kmecz, I.; Simándi, B.; Majdik, C.; Novák, L.; Irimie, F.-D.; Poppe, L. Kinetic resolution of 1-(benzofuran-2-yl) ethanols by lipase catalyzed enantiomer selective reactions. Tetrahedron Asymmetry 2003, 14, 1943–1949. [Google Scholar] [CrossRef]
- Toșa, M.; Pilbák, S.; Moldovan, P.; Paizs, C.; Szatzker, G.; Szakács, G.; Novák, L.; Irimie, F.-D.; Poppe, L. Lipase-catalyzed kinetic resolution of racemic 1-heteroarylethanols—Experimental and QM/MM study. Tetrahedron Asymmetry 2008, 19, 1844–1852. [Google Scholar] [CrossRef]
- Brem, J.; Toșa, M.-I.; Paizs, C.; Munceanu, A.; Matcović-Čalogović, D.; Irimie, F.-D. Lipase-catalyzed kinetic resolution of racemic 1-(10-alkyl-10H-phenothiazin-3-yl) ethanols and their butanoates. Tetrahedron Asymmetry 2010, 21, 1993–1998. [Google Scholar] [CrossRef]
- Hernández, J.G.; Frings, M.; Bolm, C. Mechanochemical Enzymatic Kinetic Resolution of Secondary Alcohols under Ball-Milling Conditions. ChemCatChem 2016, 8, 1769–1772. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Silva, M.J.A.; Klein, M.; Feddern, V.; Feltes, M.M.C.; Oliveira, J.V.; Ninow, J.L.; de Oliveira, D. Current status and trends in enzymatic nanoimmobilization. J. Mol. Catal. B Enzym. 2014, 99, 56–67. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Manoel, E.A.; Fernández-Lafuente, R.; Freire, D.M.G. Support engineering: Relation between development of new supports for immobilization of lipases and their applications. Biotechnol. Res. Innov. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Torres-Salas, P.; del Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Immobilized biocatalysts: Novel approaches and tools for binding enzymes to supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, P.; Lassalle, V.; Ferreiro, M.L. Development of a magnetic biocatalyst useful for the synthesis of ethyloleate. Bioprocess. Biosyst. Eng. 2014, 37, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Pospiskova, K.; Safariq, I. Low-cost, easy-to-prepare magnetic chitosan nanoparticles for enzyme immobilization. Carbohydr. Polym. 2013, 96, 545–548. [Google Scholar] [CrossRef]
- Ali, S.; Zafar, W.; Shafiq, S.; Manzoor, M. Enzymes immobilization: An overview of techniques, support materials and it’s applications. Int. J. Sci. Technol. Res. 2017, 6, 64–72. [Google Scholar]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Brady, D.; Jordan, J. Advances in enzyme immobilization. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianelllo, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, Y.-C.; Chang, C.-M.J.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimum conditions for lipase immnobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Ran, J.; Wu, S. Effects of static and magnetic field on activity and stability of immobilized α-amylase in chitosan bead. Catal. Commun. 2010, 11, 364–367. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Yilmaz, M.; Arica, M.Y. Preparation and characterization of epoxy-functionalized magnetic chitosan beads: Laccase immobilization for degradation of reactive dyes. Bioprocess. Biosyst. Eng. 2010, 33, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, S.; Gao, G. Immobilization of β-Galactosidase onto Magnetic Beads. Appl. Biochem. Biotechnol. 2010, 160, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Zhou, Z.D.; Li, Y.J.; Huang, K.L.; Zhong, M. Surface functionalization of chitosan-coated magnetic nanoparticles for covalent immobilization of yeast alcohol dehydrogenase from Saccharomyces cerevisiae. J. Magn. Magn. Mater. 2010, 322, 3862–3868. [Google Scholar] [CrossRef]
- Lee, D.G.; Ponvel, K.M.; Kim, M.; Hwang, S.; Ahn, I.S.; Lee, C.H. Immobilization of lipase on hydrophobic nano-sized magnetite particles. J. Mol. Catal. B Enzym. 2009, 57, 62–66. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Zhu, Y.; Che, L.; Shen, Q.; Zhang, J.; Li, X. Oligoamines conjugated chitosan derivatives: Synthesis, characterization in vitro and in vivo biocompatibility evaluations. Carbohydr. Polym. 2011, 83, 1153–1161. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilization: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan nanofibers as a green polymer-Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; Román, J.S.; Goya, G.F.; Mijangos, C. Chitosan nanoparticles for combinated drug delivery and magnetic hyperthermia: From preparation to in vivo studies. Carbohydr. Plolym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-Ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Tarczykowska, A.; Sroka, W.D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipase immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformations. J. Mol. Catal. B Enzym. 2016, 134, 43–50. [Google Scholar] [CrossRef]
- Ghasemi, S.; Heidary, M.; Faramarzi, M.A.; Habibi, Z. Immobilization of lipase on Fe3O4/ZnO core/shell magnetic nanoparticles and catalysis of Michael-type addition to chalcone derivatives. J. Mol. Catal. B Enzym. 2014, 100, 121–128. [Google Scholar] [CrossRef]
- Kress, J.; Zanaletti, R.; Amour, A.; Ladlow, M.; Frey, J.G.; Bradley, M. Enzyme accessibility and solid supports: Which molecular weight enzymes can be used on solid supports? An investigation using confocal Raman microscopy. Chem. Eur. J. 2002, 8, 3769–3772. [Google Scholar] [CrossRef]
- Cui, J.D.; Li, L.L.; Bian, H.J. Immobilization of cross-linked phenylalanine ammonia lyase aggregates in microporous silica gel. PLoS ONE 2013, 8, e80581. [Google Scholar] [CrossRef]
- Bartha-Vári, J.H.; Toșa, M.-I.; Irimie, F.-D.; Weisser, D.; Boros, Z.; Vértessy, B.G.; Paizs, C.; Poppe, L. Immobilization of phenylalanine ammonia-lyase on single-walled carbon nanotubes for stereoselective biotranformations in batch and continuous flow modes. ChemCatChem 2015, 7, 1122–1128. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Siódmiak, T.; Sikora, A.; Marzsałł, H.K. Synthesis of new chitosan coated magnetic nanoparticles with surface modified with long-distanced amino groups as a support for bioligands binding. Mater. Lett. 2014, 132, 63–65. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, H.M. Chitosan nanoparticle hydrogel based sebacoyl moiety with remarkable capability for metal ion removal from aqueous systems. Int. J. Biol. Macromol. 2019, 122, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Saeki, D.; Matsuyama, H. A novel strategy to immobilize enzymes on microporous membranes via dicarboxylic acid halides. RSC Adv. 2017, 7, 48199–48207. [Google Scholar] [CrossRef]
- Brem, J.; Pilbák, S.; Paizs, C.; Bánóczi, G.; Irimie, F.-D.; Toșa, M.-I.; Poppe, L. Lipase-catalyzed kinetic resolution of racemic 1-(10-ethyl-10H-phenothiazin-1,2 and 4-yl)ethanols and their acetates. Tetrahedron Asymmetry 2011, 22, 916–923. [Google Scholar] [CrossRef]
- Zheng, J.; Fu, X.; Ying, X.; Zhang, Y.; Wang, Z. A sensitive colorimetric high-throughput screening method for lipase synthetic activity assay. Anal. Biochem. 2014, 452, 13–15. [Google Scholar] [CrossRef]
- Pace, V.; Sinisterra, J.V.; Alcántara, A.R. Celite-Supported Reagents in Organic Synthesis: An Overview. Curr. Org. Chem. 2010, 14, 2384–2408. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernández-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Kazlauskas, R.J.; Weissflock, A.N.E.; Rappaport, A.T.; Cuccia, L.A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991, 56, 2656–2665. [Google Scholar] [CrossRef]
- Nagaraja, S.; Ravindra, V.G.; Abdullah, M.A.; Bandar, E.A.-D.; Katharigatta, N.V. Immobilization studies of Candida Antarctica lipase B on gallic acid resin-grafted magnetic iron oxide nanoparticles. Int. J. Nanomed. 2019, 4, 3235–3244. [Google Scholar] [CrossRef]
- Ottoson, J.; Hult, K. Influence of acyl chain length on the enantioselectivity of Candida antarctica lipase B and its thermodynamic components in kinetic resolution of sec-alcohols. J. Mol. Catal. B Enzym. 2001, 11, 1025–1028. [Google Scholar] [CrossRef]
- Chen, C.-S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analysis of biochemical kinetic resolution of enantiomers. J. Am. Chem. Soc. 1982, 14, 7294–7299. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Biocatalyst | Synthetic Activity a | |
|---|---|---|---|
| (mmol/min genzyme preparate) | (µmol/min mgCaL-B) | ||
| 1 | Lyophilized CaL-B | 0.44 | 0.44 |
| 2 | Novozym 435 | 1.96 | 32.53 |
| 3 | MNP-CS-CaL-B | 0.13 | 0.84 |
| 4 | MNP-CS-GDGE-CaL-B | 0.13 | 0.78 |
| 5 | MNP-CS-GDGE-ED-CaL-B | 0.21 | 1.1 |
| 6 | MNP-CS-GDGE-PD-CaL-B | 0.20 | 1.02 |
| 7 | MNP-CS-GDGE-HD-CaL-B | 0.09 | 0.45 |
| 8 | MNP-CS-SC-CaL-B | 1.95 | 7.08 |
| Entry | Acylation Agent | Time (h) | eeS (%) a | eeP (%) a | c (%) a |
|---|---|---|---|---|---|
| 1 | Vinyl acetate | 13 | >99.9 | >99.9 | 50 |
| 2 | Vinyl butyrate | 79 | >99.9 | >99.9 | 50 |
| 3 | Vinyl decanoate | 24 | 77.6 | 85 | 43.7 |
| Entry | Substrate | Chromatographic Chiral Separation | Original Results b | Literature Data c | |||
|---|---|---|---|---|---|---|---|
| Column | n-Hexane:IPA | c a (%)/Reaction Time (h) | eeS (%) | C (%)/Reaction Time (h) | Ref. | ||
| 1 | rac-2a | LUX-3 | 98:2 | 50/13 | >99.9 | 37/3 | 19 |
| 2 | rac-2b | AS-H | 95:5-15 min; 85:15 | 50/5 | >99.9 | - | - |
| 3 | rac-2c | LUX-3 | 95:5 | 49.7/5 | 98.9 | 48/3 | 19 |
| 4 | rac-2d | LUX-3 | 95:5 | 50/4.5 | >99.9 | 49/3 | 19 |
| 5 | rac-2e | LUX-5 | 95:5 | 49.4/4 | 97.6 | 49.8d/24 | 17 |
| 6 | rac-2f | LUX-3 | 99:1-10 min; 90:10 | 49.2/16 | 96.9 | 42.2/24 | 17 |
| 7 | rac-2g | LUX-3 | 90:10 | 49/8 | 96.1 | 49/8 | 16 |
| 8 | rac-2h | LUX-3 | 90:10 | 49.6/12 | 98.2 | 48/16 | 17 |
| 9 | rac-2i | LUX-3 | 70:30 | 49.2/10 | 96.8 | 49e/24 | 18 |
| 10 | rac-2j | LUX-3 | 90:10 | 50/3 | >99.9 | 51/5 | 21 |
| Entry | Method | Biocatalyst | Enzyme Load (mgenz/mgenzyme preparate) | Immobilization Yield (%) |
|---|---|---|---|---|
| 1 | A | MNP-CS-CaL-B | 0.15 | 54.90 |
| 2 | B | MNP-CS-GDGE-CaL-B | 0.16 | 59.79 |
| 3 | C, n=2 | MNP-CS-GDGE-ED-CaL-B | 0.19 | 66.88 |
| 4 | C, n=3 | MNP-CS-GDGE-PD-CaL-B | 0.19 | 68.86 |
| 5 | C, n=6 | MNP-CS-GDGE-HD-CaL-B | 0.19 | 69.75 |
| 6 | D | MNP-CS-SC-CaL-B | 0.27 | 95.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spelmezan, C.G.; Bencze, L.C.; Katona, G.; Irimie, F.D.; Paizs, C.; Toșa, M.I. Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules 2020, 25, 350. https://doi.org/10.3390/molecules25020350
Spelmezan CG, Bencze LC, Katona G, Irimie FD, Paizs C, Toșa MI. Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules. 2020; 25(2):350. https://doi.org/10.3390/molecules25020350
Chicago/Turabian StyleSpelmezan, Cristina Georgiana, László Csaba Bencze, Gabriel Katona, Florin Dan Irimie, Csaba Paizs, and Monica Ioana Toșa. 2020. "Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols" Molecules 25, no. 2: 350. https://doi.org/10.3390/molecules25020350
APA StyleSpelmezan, C. G., Bencze, L. C., Katona, G., Irimie, F. D., Paizs, C., & Toșa, M. I. (2020). Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules, 25(2), 350. https://doi.org/10.3390/molecules25020350








