Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells
Abstract
1. Introduction
2. Results
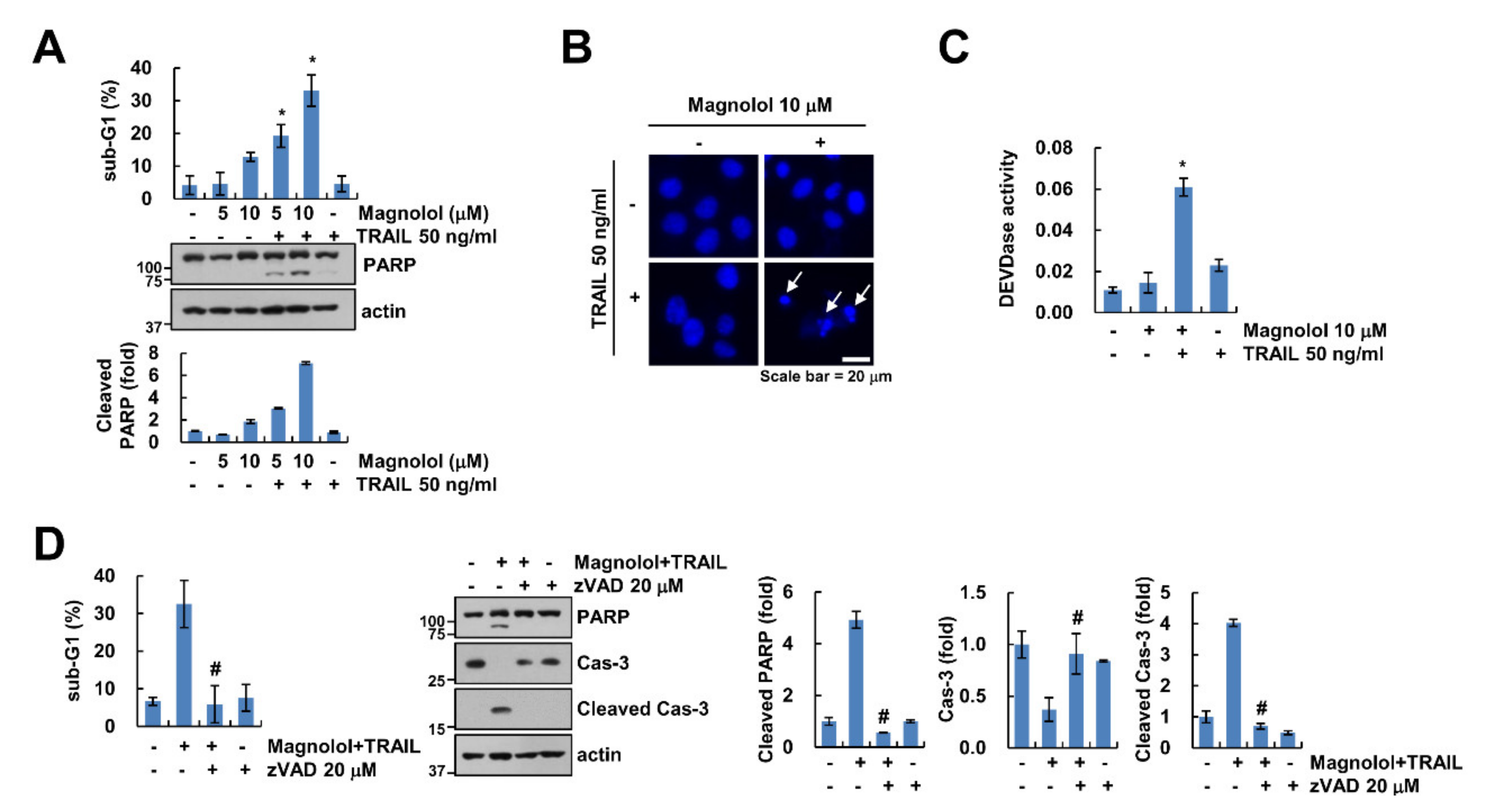
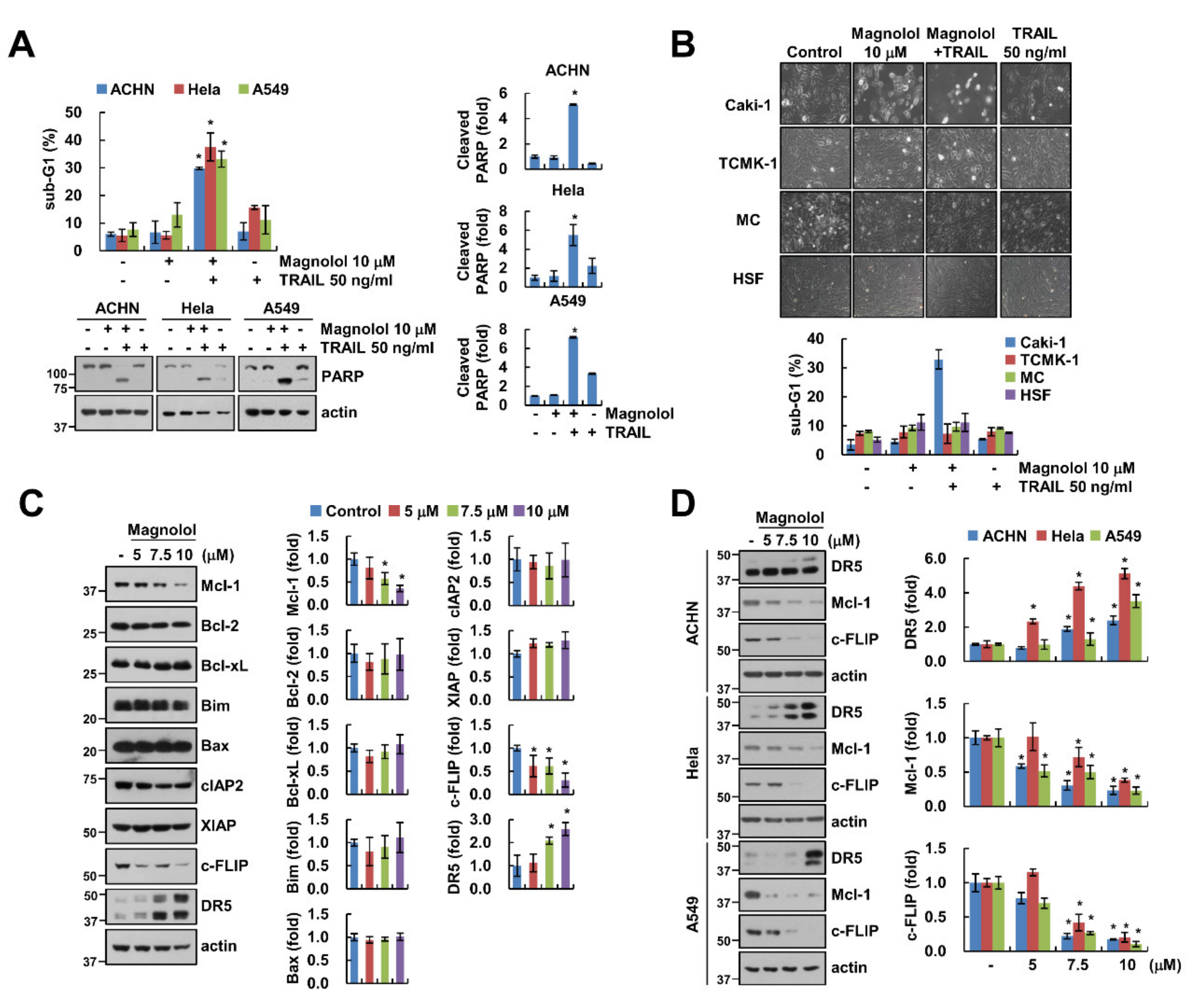
2.1. Magnolol Enhances Sensitivity to TRAIL in Human Renal Carcinoma Caki-1 Cells
2.2. Magnolol Downregulates Mcl-1 and c-FLIP Expression and Upregulates DR5 Expression
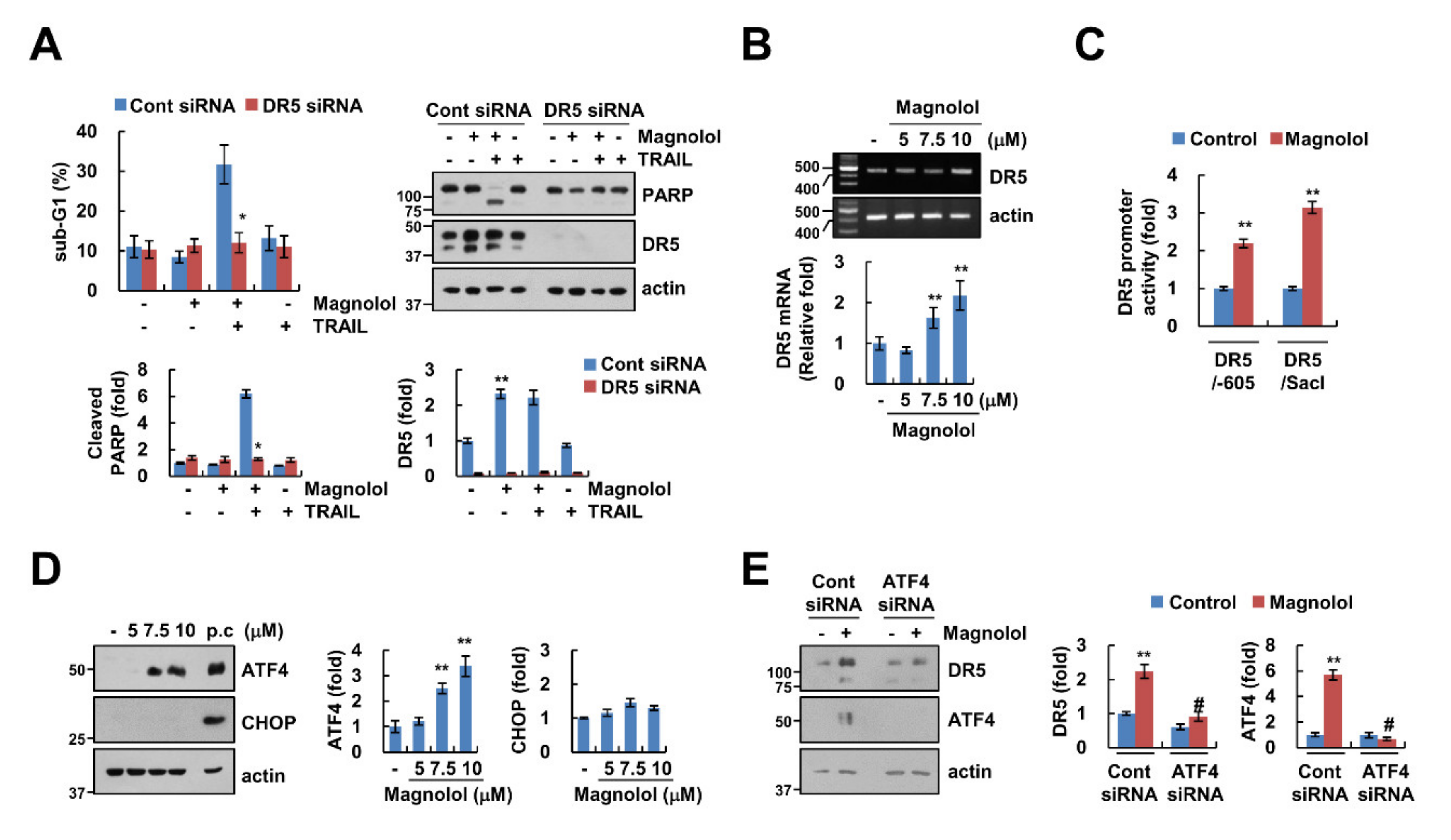
2.3. ATF4-Mediated DR5 Upregulation Contributes to Magnolol Plus TRAIL-Induced Apoptosis
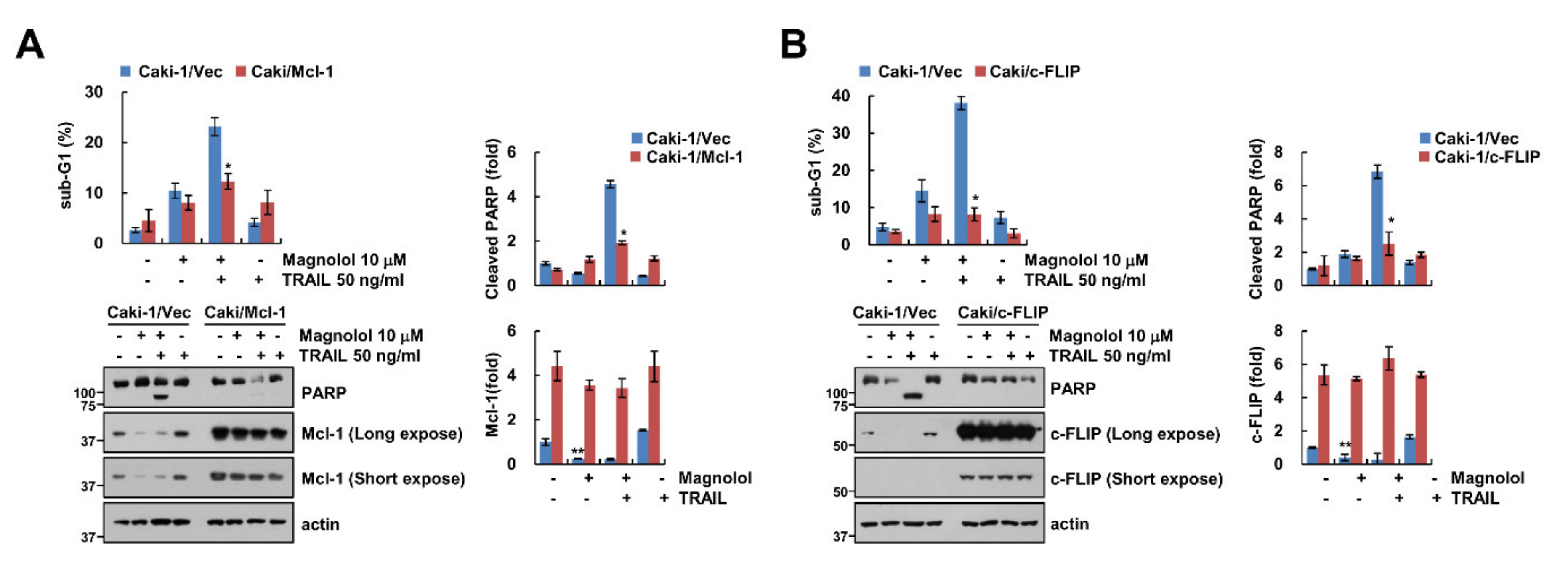
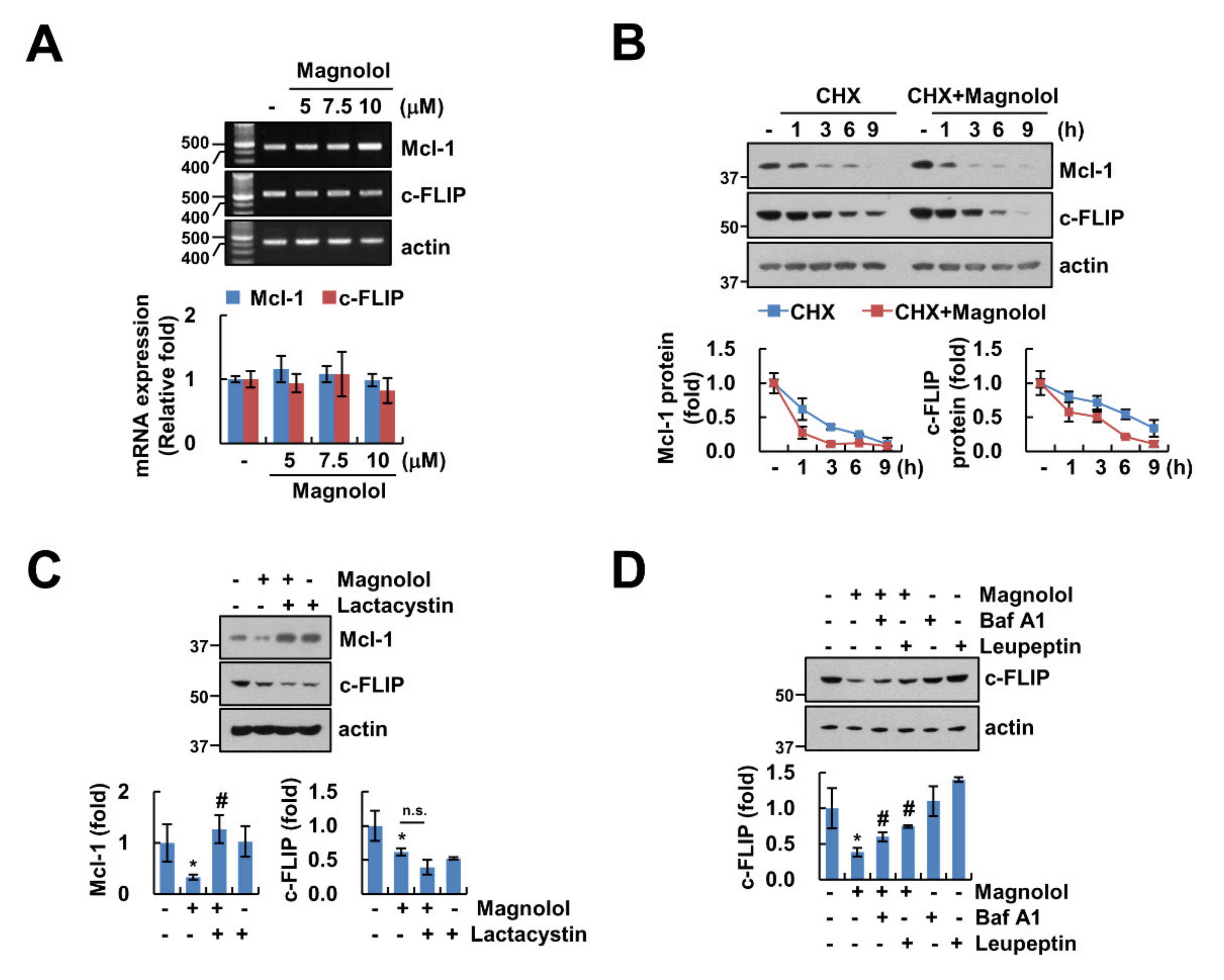
2.4. Downregulation of c-FLIP and Mcl-1 by Magnolol Is Involved in TRAIL-Mediated Apoptosis
2.5. Magnolol Induces Downregulation of Mcl-1 and c-FLIP Protein Expression at the Post-Translation Level
2.6. Generation of ROS Is Not Associated with Magnolol Plus TRAIL-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Reagents and Antibodies
4.3. FACS Analysis and DAPI Staining
4.4. Western Blotting
4.5. DEVDase (Caspase-3) Activity
4.6. Knockdown of Genes Using siRNA
4.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative PCR (qPCR)
4.8. Promoter Activity Assay
4.9. Measurement of Reactive Oxygen Species
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guru, S.K.; Pathania, A.S.; Kumar, A.; Bhushan, S.; Malik, F. Autophagy triggered by magnolol derivative negatively regulates angiogenesis. Cell Death Dis. 2013, 4, e889. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Ikeda, K.; Sakai, Y.; Nagase, H. Inhibitory effect of magnolol on tumour metastasis in mice. Phytother. Res. 2003, 17, 933–937. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Kubo, M.; Harada, K. The search for, and chemistry and mechanism of, neurotrophic natural products. J. Nat. Med. 2020, 74, 648–671. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.; Zhang, B.; Liu, Y.Q.; Wang, Z.Y.; Wu, Y.P.; Yu, X.J.; Zhang, X.D.; Ming, P.H.; Zhou, G.B.; et al. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci. Rep. 2013, 3, 3098–3106. [Google Scholar] [CrossRef]
- Hwang, E.S.; Park, K.K. Magnolol suppresses metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 961–967. [Google Scholar] [CrossRef]
- Zhang, F.H.; Ren, H.Y.; Shen, J.X.; Zhang, X.Y.; Ye, H.M.; Shen, D.Y. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-kappaB signaling pathway. Biomed. Pharmacother. 2017, 94, 474–480. [Google Scholar] [CrossRef]
- Zhou, S.; Wen, H.; Li, H. Magnolol induces apoptosis in osteosarcoma cells via G0/G1 phase arrest and p53-mediated mitochondrial pathway. J. Cell Biochem. 2019, 120, 17067–17079. [Google Scholar] [CrossRef]
- Wen, H.; Zhou, S.; Song, J. Induction of apoptosis by magnolol via the mitochondrial pathway and cell cycle arrest in renal carcinoma cells. Biochem. Biophys. Res. Commun. 2019, 508, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Khan, M.; Zhang, K.; Iqbal, F.; Ma, T.; Yang, H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012, 40, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bi, Y.; Yang, C.; Yang, J.; Jiang, Y.; Meng, F.; Yu, B.; Khan, M.; Ma, T.; Yang, H. Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Pharmazie 2013, 68, 755–762. [Google Scholar] [PubMed]
- Chen, L.C.; Liu, Y.C.; Liang, Y.C.; Ho, Y.S.; Lee, W.S. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J. Agric. Food Chem. 2009, 57, 7331–7337. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Cho, Y.H.; Park, K.; Kim, E.J.; Jung, K.H.; Park, S.S.; Kim, W.J.; Moon, S.K. Magnolol elicits activation of the extracellular signal-regulated kinase pathway by inducing p27KIP1-mediated G2/M-phase cell cycle arrest in human urinary bladder cancer 5637 cells. Biochem. Pharmacol. 2008, 75, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.F.; Lee, T.S.; Lin, S.Y.; Hsu, S.P.; Juan, S.H.; Hsu, Y.H.; Zhong, W.B.; Lee, W.S. Involvement of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of p21 and cell-cycle arrest in colon cancer cells. Mol. Carcinog. 2007, 46, 275–283. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Y.; Yang, X.; Li, F.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Hu, M.; et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol. Res. 2016, 111, 113–125. [Google Scholar] [CrossRef]
- Chen, Y.S.; Sun, R.; Chen, W.L.; Yau, Y.C.; Hsu, F.T.; Chung, J.G.; Tsai, C.J.; Hsieh, C.L.; Chiu, Y.M.; Chen, J.H. The In Vivo Radiosensitizing Effect of Magnolol on Tumor Growth of Hepatocellular Carcinoma. In Vivo 2020, 34, 1789–1796. [Google Scholar] [CrossRef]
- Chen, J.H.; Chiang, I.T.; Hsu, F.T. Protein Kinase B Inactivation Is Associated with Magnolol-Enhanced Therapeutic Efficacy of Sorafenib in Hepatocellular Carcinoma In Vitro and In Vivo. Cancers (Basel) 2019, 12, 87. [Google Scholar] [CrossRef]
- De Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef]
- Kretz, A.L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing Strategies for Cancer Treatment. Cancers (Basel) 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Chawla-Sarkar, M.; Bae, S.I.; Reu, F.J.; Jacobs, B.S.; Lindner, D.J.; Borden, E.C. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004, 11, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, T.; Liang, Y.; Li, P.; Chen, X.; Zhang, J.; Ma, Y.; Hao, Q.; Wang, J.; Zhang, P.; et al. Neddylation Inhibition Activates the Extrinsic Apoptosis Pathway through ATF4-CHOP-DR5 Axis in Human Esophageal Cancer Cells. Clin. Cancer. Res. 2016, 22, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Su, L.; Hao, X.; Zhong, N.; Zhong, D.; Singhal, S.; Liu, X. Salermide up-regulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J. Cell. Mol. Med. 2012, 16, 1618–1628. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chang, Y.T.; Liu, J.D.; Yu, C.H.; Ho, Y.S.; Lee, Y.H.; Lee, W.S. Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol. Carcinog. 2001, 32, 73–83. [Google Scholar] [CrossRef]
- Wang, Y.D.; Sun, X.J.; Yang, W.J.; Li, J.; Yin, J.J. Magnolol exerts anticancer activity in hepatocellular carcinoma cells through regulating endoplasmic reticulum stress-mediated apoptotic signaling. Onco Targets Ther. 2018, 11, 5219–5226. [Google Scholar] [CrossRef]
- Su, C.M.; Weng, Y.S.; Kuan, L.Y.; Chen, J.H.; Hsu, F.T. Suppression of PKCdelta/NF-kappaB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 3527. [Google Scholar] [CrossRef]
- Burger, A.M.; Seth, A.K. The ubiquitin-mediated protein degradation pathway in cancer: Therapeutic implications. Eur. J. Cancer 2004, 40, 2217–2229. [Google Scholar] [CrossRef]
- Min, K.J.; Shahriyar, S.A.; Kwon, T.K. Arylquin 1, a potent Par-4 secretagogue, induces lysosomal membrane permeabilization-mediated non-apoptotic cell death in cancer cells. Toxicol. Res. 2020, 36, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Woo, S.M.; Kim, M.W.; Lee, H.S.; Kim, S.H.; Kang, S.C.; Lee, E.W.; Min, K.J.; Kwon, T.K. Cathepsin K inhibition-induced mitochondrial ROS enhances sensitivity of cancer cells to anti-cancer drugs through USP27x-mediated Bim protein stabilization. Redox Biol. 2020, 30, 101422–101434. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Seo, S.U.; Kubatka, P.; Min, K.J.; Kwon, T.K. Honokiol Enhances TRAIL-Mediated Apoptosis through STAMBPL1-Induced Survivin and c-FLIP Degradation. Biomolecules 2019, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Shahriyar, S.A.; Seo, S.U.; Min, K.J.; Kubatka, P.; Min, D.S.; Chang, J.S.; Kim, D.E.; Woo, S.M.; Kwon, T.K. Upregulation of DR5 and Downregulation of Survivin by IITZ-01, Lysosomotropic Autophagy Inhibitor, Potentiates TRAIL-Mediated Apoptosis in Renal Cancer Cells via Ubiquitin-Proteasome Pathway. Cancers (Basel) 2020, 12, 2363. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.M.; Min, K.-j.; Kwon, T.K. Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells. Molecules 2020, 25, 4591. https://doi.org/10.3390/molecules25194591
Woo SM, Min K-j, Kwon TK. Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells. Molecules. 2020; 25(19):4591. https://doi.org/10.3390/molecules25194591
Chicago/Turabian StyleWoo, Seon Min, Kyoung-jin Min, and Taeg Kyu Kwon. 2020. "Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells" Molecules 25, no. 19: 4591. https://doi.org/10.3390/molecules25194591
APA StyleWoo, S. M., Min, K.-j., & Kwon, T. K. (2020). Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells. Molecules, 25(19), 4591. https://doi.org/10.3390/molecules25194591





