Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland
Abstract
1. Introduction
2. Results
- early to medium—”Krasnaja Płodorodnaja” and “Montmorency”,
- medium—”Demesova”, Granatnaja”, “Paraszt Meggy”, “Turgieniewka” and “Zagoriewskaja”,
- medium to late—”Dradem”, “Lucyna”, “Marasca”, “Mołodiożnaja”, “Sokówka Nowotomyska” and “Wróble”,
- late—”Grosenkirch”, “Malinówka”, “Meteor”, “Nana”, “Sokówka nr 9”, “Suda Hardy” and “Wieluń 17”,
- very late—”Sokówka nr 6”.
2.1. Sugars
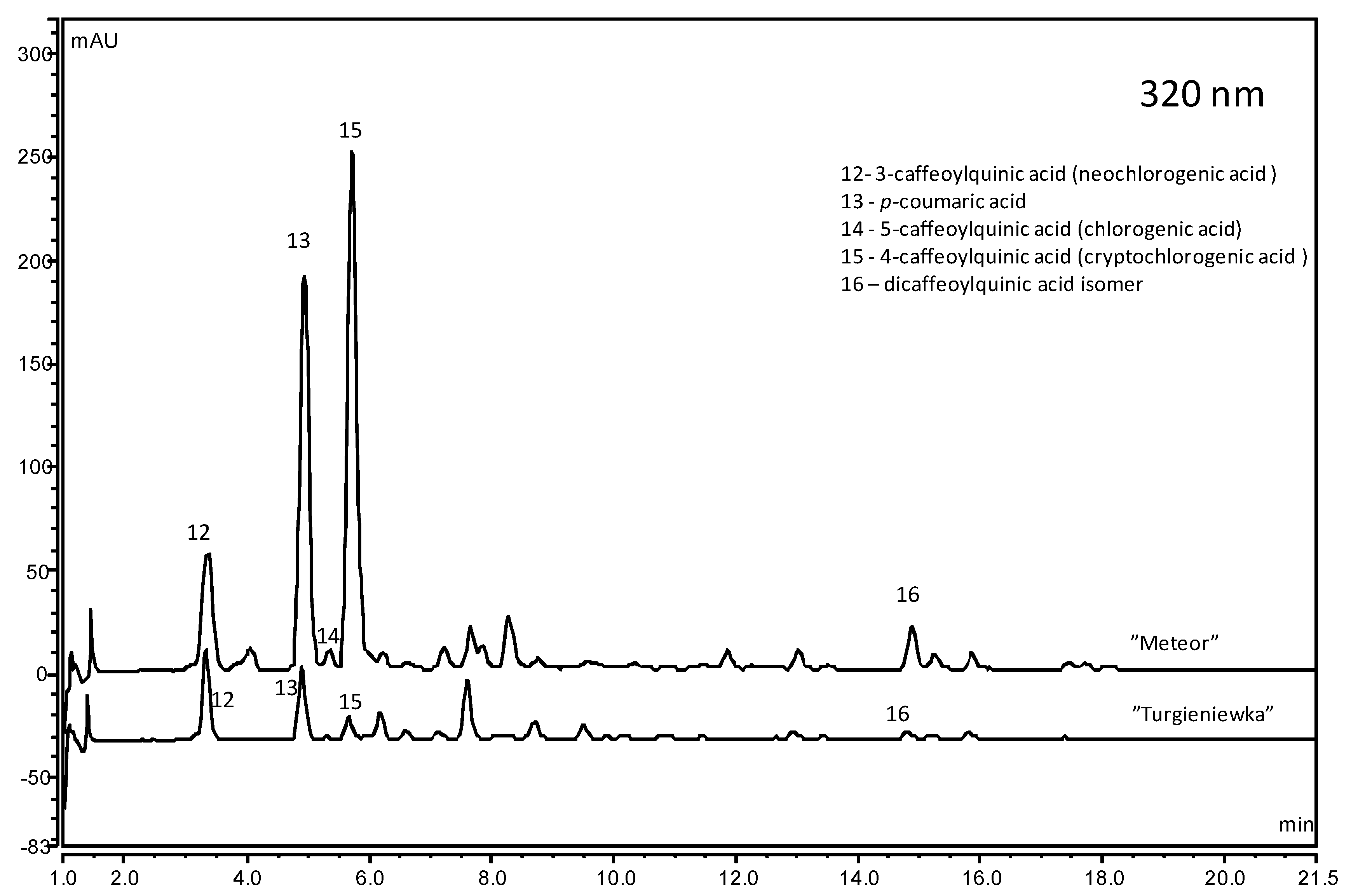
2.2. Organic Acids
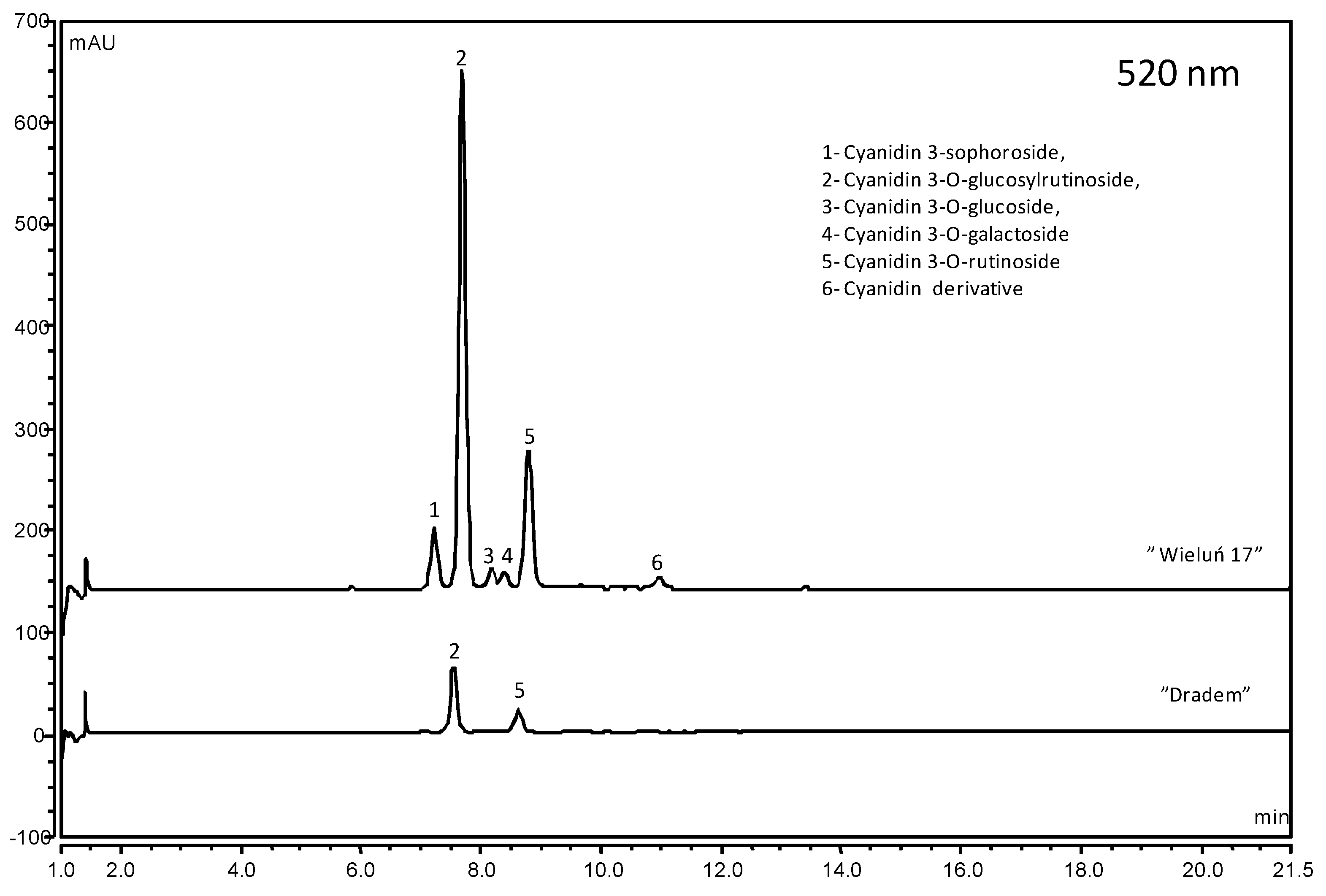
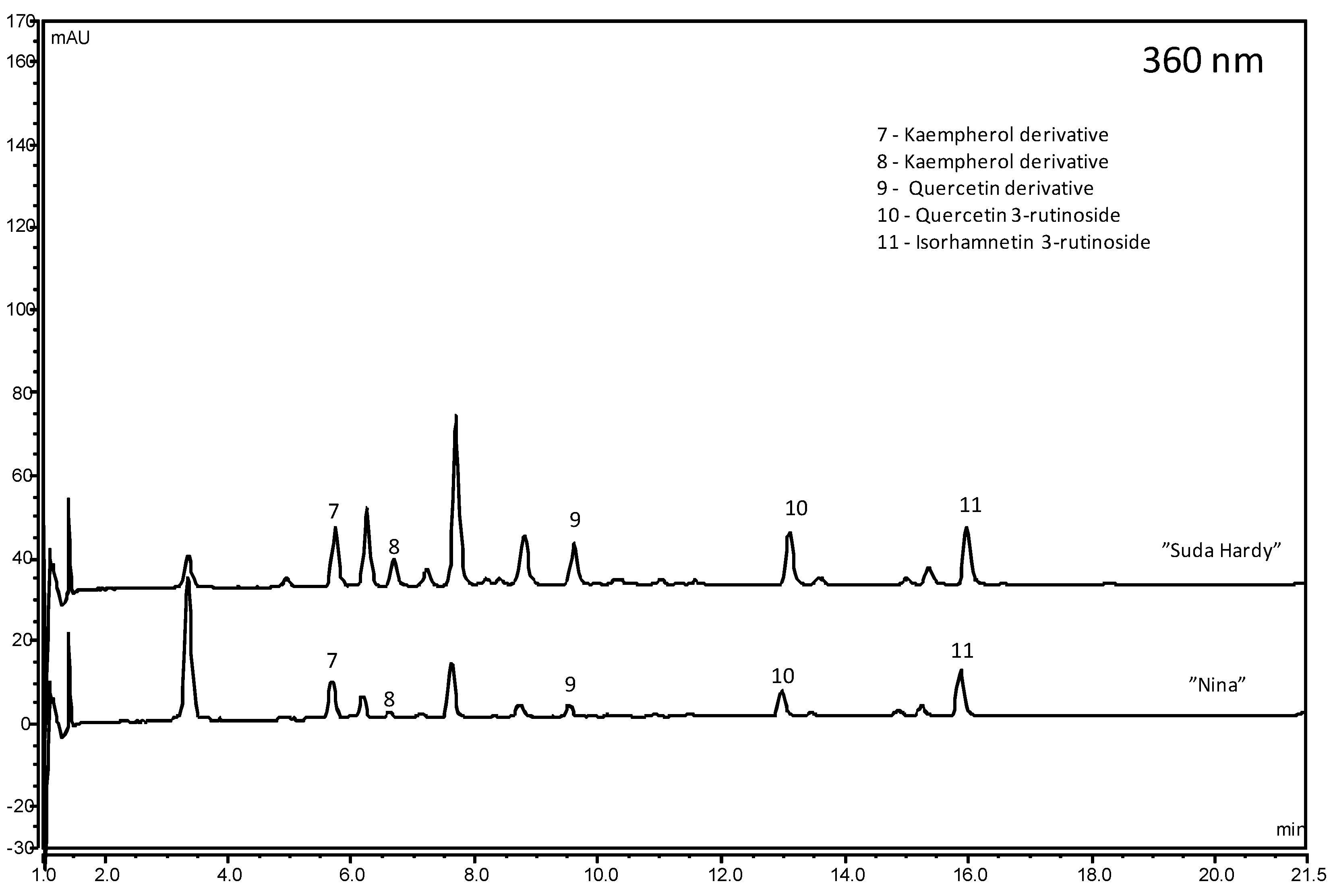
2.3. Phenolic Compounds
2.4. Antioxidant Capacity
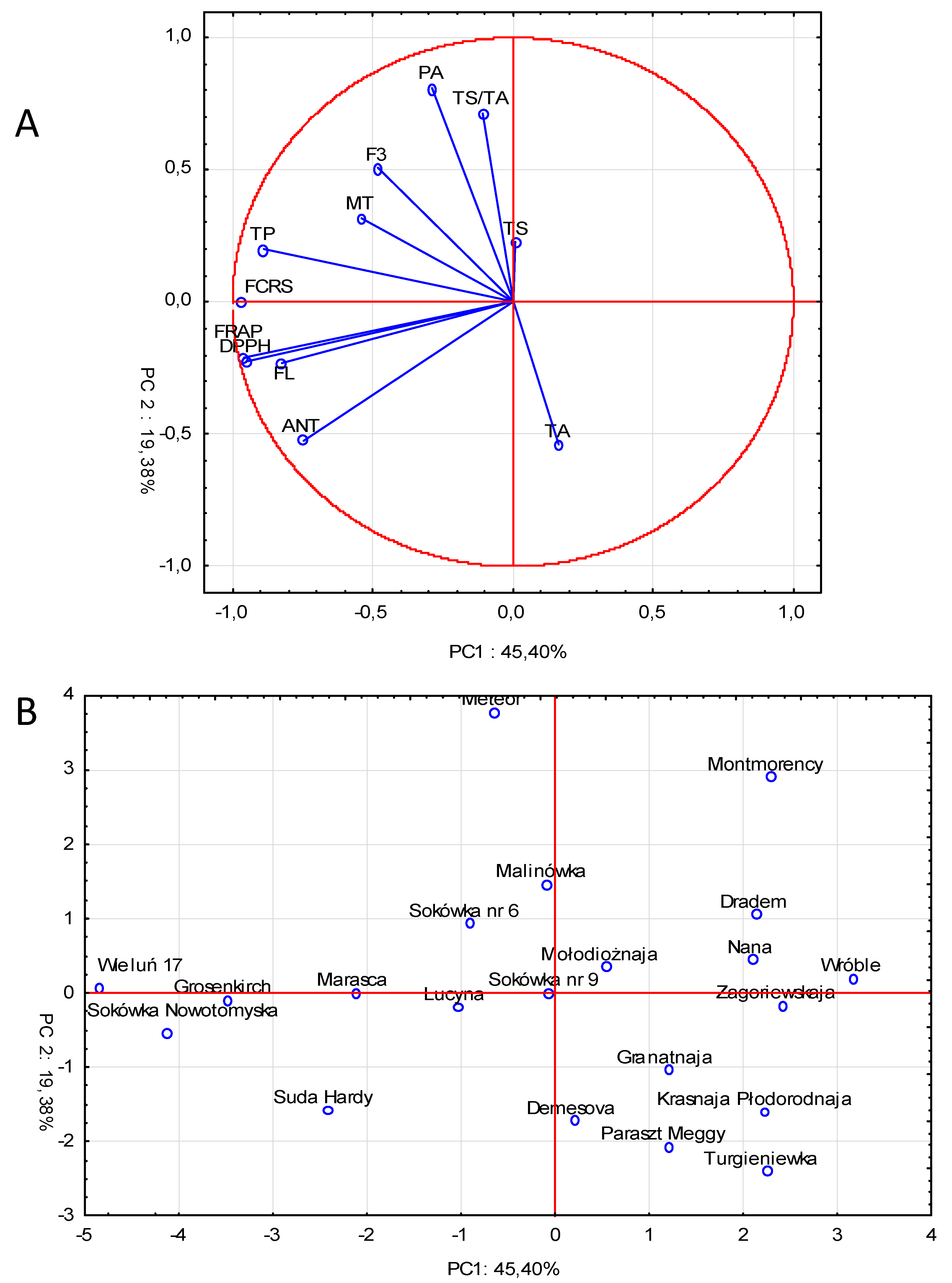
2.5. Principal Component Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.2.1. Location
3.2.2. Plant Material Characteristics
3.3. HPLC
3.3.1. Preparation of Samples
3.3.2. Samples for Phenolic Compounds and Antioxidant Capacity Determination
3.3.3. Samples for the Determination of Sugars and Organic Acids
3.3.4. Phenolic Compounds
3.3.5. Sugars
3.3.6. Organic Acids
3.4. Antioxidant Activity
3.4.1. Content of Substances Which React with the Folic–Ciocalteu Reagent
3.4.2. DPPH
3.4.3. FRAP
3.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alba, C.M.A.; Daya, M.; Franck, C. Tart cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2019, 59, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Kelley, S.D.; Adkins, Y.; Laugero, D.K. A Review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Viljevac-Vuletić, M.; Dugalić, K.; Mihaljević, I.; Tomaš, V.; Vuković, D.; Zdunić, Z.; Puškar, B.; Jurković, Z. Season, location and cultivar influence on bioactive compounds of sour cherry fruits. Plant Soil Env. 2017, 63, 389–395. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Khoo, G.M.; Clausen, M.R.; Pedersen, B.H.; Larsen, E. Bioactivity and total phenolic content of 34 sour cherry cultivars. J. Food Compos. Anal. 2011, 24, 772–776. [Google Scholar] [CrossRef]
- Najafzadeh, R.; Arzani, K.; Bouzari, N.; Hashemi, J. Identification of new Iranian sour cherry genotypes with enhanced fruit quality parameters and high antioxidant properties. N. Z. J. Crop Hortic. Sci. 2014, 42, 275–287. [Google Scholar] [CrossRef]
- Feldmane, D. Investigation of the biochemical composition of sour cherry (Prunus cerasus L.) fruits grown in Latvia. In Proceedings of the International Scientific Conference Sustainable Fruit Growing: From Plant To Product, Jūrmala–Dobele, Latvia, 28–31 May 2008; pp. 258–264. Available online: http://www.lvai.lv/Konference/ (accessed on 7 October 2020).
- Alrgei, H.O.S.; Dabić, D.Č.; Natić, M.M.; Rakonjac, V.S.; Milojković-Opsenica, D.; Tešić, Ž.L.; Fotirić Akšić, M.M. Chemical profile of major taste- and health-related compounds of Oblačinska sour cherry. J. Sci. Food Agric. 2016, 96, 1241–1251. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 August 2020).
- Papp, N.; Szilvássy, B.; Abrankó, L.; Szabó, T.; Pfeiffer, P.; Szabó, Z.; Nyéki, J.; Ercisli, S.; Stefanovits-Bányai, É.; Hegedűs, A. Main quality attributes and antioxidants in Hungarian sour cherries: Identification of genotypes with enhanced functional properties. Int. J. Food Sci. Technol. 2010, 45, 395–402. [Google Scholar] [CrossRef]
- Grafe, C.; Schuster, M. Physicochemical characterization of fruit quality traits in a German sour cherry collection. Sci. Hortic. 2014, 180, 24–31. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N. Morphological physical & chemical, and antioxidant profiles of polish varieties of cornelian cherry fruit (Cornus mas L.). Zywnosc-Nauka Technol. Jakosc 2011, 18, 78–89. [Google Scholar]
- Kucharska, A.Z. Związki Aktywne Owoców Derenia (Cornus mas L.); Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wroclaw, Poland, 2020. [Google Scholar]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and Health: A Review. Crit. Rev. Food Sci. Nutr. 2010, 51, 1–12. [Google Scholar] [CrossRef]
- Borowy, A.; Chrzanowska, E.; Kapłan, M. Comparison of three sour cherry cultivars grown in central-eastern Poland. Acta Sci. Pol. Hortorum Cultus 2018, 17, 63–73. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Adamska, A. Charakterystyka owoców pigwowca japońskiego oraz ich zastosowanie w przemyśle spożywczym. ŻYWNOŚĆ Nauka Technol. Jakość 2017, 24, 5–15. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Milosevic, T.; Milosevic, N.; Mladenovic, J. Tree vigor, yield, fruit quality, and antioxidant capacity of apple (Malus × domestica Borkh.) influenced by different fertilization regimes: Preliminary results. Turk. J. Agric. For. 2019, 43, 48–57. [Google Scholar] [CrossRef]
- Mratinić, E.; Popovski, B.; Milošević, T.; Popovska, M. Impact of harvest time on the main agronomic and fruit quality traits of three apricot cultivars. Int. J. Fruit Sci. 2012, 12, 427–436. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, X.; Shi, L.; Shalimu, D.; Ma, C.; Liu, Y. Phenolic profiles and antioxidant activities of six Chinese pomegranate (Punica granatum L.) cultivars. Int. J. Food Prop. 2017, 20, S94–S107. [Google Scholar] [CrossRef]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and cultivar-dependent traits of Prunus avium and Prunus cerasus polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Šeruga, B.; Novak, I.; Medvidović-Kosanović, M. Phenolic compound composition and antioxidant activity of fruits of Rubus and Prunus species from Croatia. Int. J. Food Sci. Technol. 2009, 44, 860–868. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdylo, A.; Lech, K.; Figiel, A. Chemical composition, antioxidant capacity, and sensory quality of dried sour cherry fruits pre-dehydrated in fruit concentrates. Food Bioprocess Technol. 2015, 8, 2076–2095. [Google Scholar] [CrossRef]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour Cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef]
- Šimunić, V.; Kovač, S.; Gašo-Sokač, D.; Pfannhauser, W.; Murkovic, M. Determination of anthocyanins in four Croatian cultivars of sour cherries (Prunus cerasus). Eur. Food Res. Technol. 2005, 220, 575–578. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Blando, F.; Gerardi, C.; Nicoletti, I. Sour Cherry (Prunus cerasus L) anthocyanins as ingredients for functional foods. J. Biomed. Biotechnol. 2004, 2004, 314938. [Google Scholar] [CrossRef]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Filimon, R.V.; Beceanu, D.; Niculaua, M.; Arion, C. Study on the anthocyanin content of some sour cherry varieties grown in Iaşi area, Romania. Cercet. Agron. Mold. 2011, 44, 81–91. [Google Scholar] [CrossRef][Green Version]
- Levaj, B.; Dragović-Uzelac, V.; Ganić, K.K.; Banović, M.; Kovačević, D.B. Polyphenols and volatiles in fruits of two sour cherry cultivars, some berry fruits and their jams. Food Technol. Biotechnol. 2010, 48, 538–547. [Google Scholar]
- Karaaslan, M.; Yılmaz, F.M.; Karaaslan, A.; Vardin, H. Synthesis and accumulation of anthocyanins in sour cherries during ripening in accordance with antioxidant capacity development and chalcone synthase expression. Eur. Food Res. Technol. 2016, 242, 189–198. [Google Scholar] [CrossRef]
- Kim, D.-O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Szumny, A.; Wińska, K.; Nawirska-Olszańska, A. Phenolic composition stability and antioxidant activity of sour cherry liqueurs. Molecules 2018, 23, 2156. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
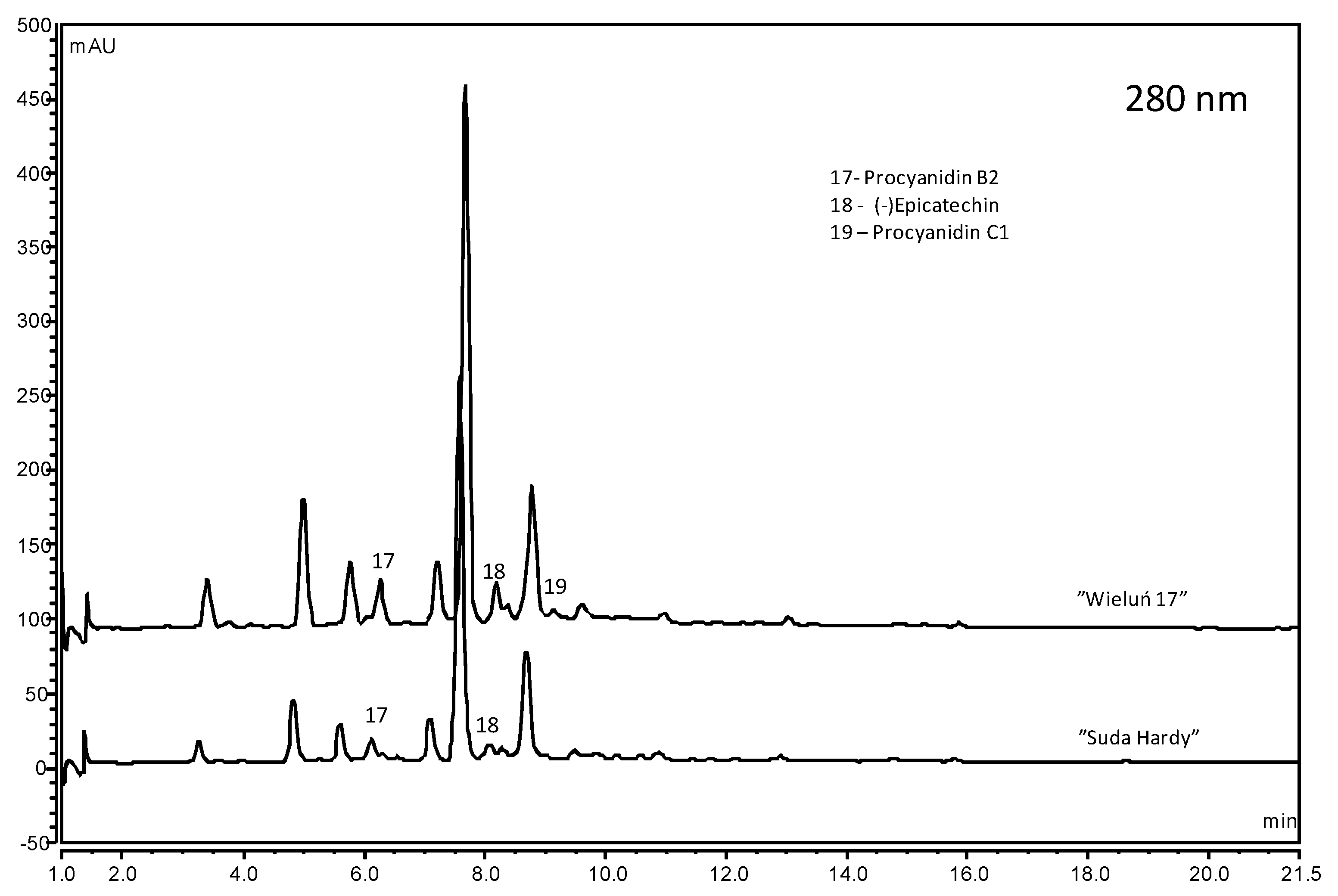
| Cultivar | Glucose | Fructose | Sorbitol | Saccharose | Galactose | Xylose | Total |
|---|---|---|---|---|---|---|---|
| Demesova | 4.85 ± 0.08 hg | 4.00 ± 0.06 ed | 2.74 ± 0.01 dc | 1.41 ± 0.03 ba | nd | nd * | 13.00 ± 0.33 d |
| Dredem | 5.37 ± 0.02 cb | 3.99 ± 0.08 ed | 2.40 ± 0.02 ih | 1.35 ± 0.01 edc | nd | nd | 13.11 ± 0.1 d |
| Granatnaja | 3.10 ± 0.01 m | 2.74 ± 0.01 k | 2.07 ± 0.01 k | 1.38 ± 0.01 dcb | nd | 1.36 ± 0.02 a | 10.65 ± 0.20 ih |
| Grosenkirch | 4.39 ± 0.07 kj | 3.60 ± 0.10 hg | 2.45 ± 0.03 hg | nd | nd | nd | 10.44 ± 0.15 i |
| Krasnaja Płodorodnaja | 4.43 ± 0.03 kj | 3.71 ± 0.03 gf | 2.42 ± 0.07 hg | 1.38 ± 0.03 cba | nd | nd | 11.93 ± 0.35 f |
| Lucyna | 5.68 ± 0.12 a | 4.36 ± 0.03 b | 2.47 ± 0.03 hgf | 1.38 ± 0.01 cba | 1.50 ± 0.00 a | nd | 15.38 ± 0.53 a |
| Malinówka | 4.91 ± 0.15 gfe | 4.00 ± 0.11 fe | 2.16 ± 0.07 k | 1.28 ± 0.04 hg | nd | nd | 12.34 ± 0.26 fe |
| Marasca | 4.74 ± 0.07 hgf | 3.89 ± 0.08 f | 2.34 ± 0.03 ji | nd | nd | nd | 10.97 ± 0.36 g |
| Meteor | 3.91 ± 0.01 l | 4.88 ± 0.15 a | 3.12 ± 0.08 a | 1.28 ± 0.02 hg | 1.39 ± 0.03 b | nd | 14.58 ± 0.05 b |
| Mołodiożnaja | 5.07±0.07 fed | 4.06 ± 0.13 d | 2.61 ± 0.04 fe | 1.40 ± 0.04 ba | 1.53 ± 0.01 a | nd | 14.67 ± 0.46 ba |
| Montmorency | 3.32 ± 0.09 m | 4.22 ± 0.13 dc | 2.75 ± 0.08 edc | nd | Nd * | nd | 10.29 ± 0.04 i |
| Nana | 4.20 ± 0.12 k | 3.55 ± 0.07 h | 1.93 ± 0.05 l | 1.03 ± 0.00 j | nd | nd | 10.71 ± 0.27 hg |
| Paraszt Meggy | 4.74 ± 0.08 ih | 4.09 ± 0.08 dc | 2.85 ± 0.03 c | nd | nd | nd | 11.68 ± 0.05 g |
| Sokówka Nowotomyska | 4.80 ± 0.16 hg | 3.31 ± 0.08 i | 2.29 ± 0.04 j | 1.32 ± 0.00 fed | nd | 1.31 ± 0.04 a | 13.03 ± 0.17 d |
| Sokówka nr 6 | 5.18 ± 0.16 edcb | 4.09 ± 0.12 ed | 2.31 ± 0.01 ij | 1.31 ± 0.00 fe | nd | nd | 12.90 ± 0.02 de |
| Sokówka nr 9 | 4.63 ± 0.11 ij | 3.87 ± 0.07 f | 2.52 ± 0.08 gf | 1.25 ± 0.01 h | nd | nd | 12.27 ± 0.27 fe |
| Suda Hardy | 5.29 ± 0.07 b | 4.35 ± 0.04 cb | 3.00 ± 0.03 b | 1.31 ± 0.04 fe | nd | nd | 13.95 ± 0.20 c |
| Turgieniewka | 3.67 ± 0.06 l | 3.01 ± 0.00 j | 1.93 ± 0.03 b | nd | nd | nd | 8.62 ± 0.12 j |
| Wieluń 17 | 2.81 ± 0.00 n | 3.68 ± 0.09 gf | 2.36 ± 0.02 ij | 1.40 ± 0.02 a | nd | nd | 10.25 ± 0.22 i |
| Wróble | 5.02 ± 0.08 edc | 4.32 ± 0.05 cb | 2.71 ± 0.00 fed | 1.32 ± 0.02 gf | nd | 1.30 ± 0.04 b | 14.67 ± 0.38 b |
| Zagoriewskaja | 5.22 ± 0.01 dcb | 3.94 ± 0.11 f | 2.69 ± 0.09 edc | 1.24 ± 0.02 h | nd | nd | 13.09 ± 0.20 d |
| Min-max | 2.81–5.68 | 2.74–4.88 | 1.93–3.12 | 1.03–1.41 | 1.39–153 | 1.30–1.36 | 8.62–15.38 |
| Average | 4.54 | 3.89 | 2.48 | 1.32 | 1.47 | 1.33 | 12.31 |
| Median | 4.74 | 3.99 | 2.45 | 1.32 | 1.50 | 1.31 | 12.34 |
| Cultivar | Malic | Malonic | Oxalic | Shikimic | Fumaric | Total |
|---|---|---|---|---|---|---|
| Demesova | 1858.77 ± 7.83 e | 270.64 ± 2.11 ji | 13.93 ± 0.32 b | 4.08 ± 0.01 a | 0.21 ± 0.00 e | 2147.63 ± 10.10 c |
| Dredem | 1810.91 ± 2.51 g | 313.44 ± 1.75 fe | 11.03 ± 0.26 e | 2.22 ± 0.02 h | 0.23 ± 0.00 d | 2137.82 ± 6.53 d |
| Granatnaja | 1602.67 ± 6.66 l | 299.88 ± 9.97 fe | 9.33 ± 0.39 g | 2.37 ± 0.01 f | 0.17 ± 0.01 ih | 1914.43 ± 7.30 j |
| Grosenkirch | 1684.85 ± 5.75 j | 295.01 ± 5.63 hg | 9.13 ± 0.16 h | 1.95 ± 0.07 j | 0.24 ± 0.01 d | 1991.17 ± 2.27 i |
| Krasnaja Poodorodnaja | 1976.15 ± 7.73 a | 258.40 ± 0.18 k | 6.74 ± 0.09 l | 1.21 ± 0.03 o | 0.15 ± 0.00 lkj | 2242.66 ± 4.11 b |
| Lucyna | 1636.94 ± 1.30 k | 372.38 ± 4.48 b | 8.54 ± 0.28 i | 1.89 ± 0.00 kj | 0.29 ± 0.01 b | 2020.03 ± 1.20 g |
| Malinówka | 1219.56 ± 1.03 p | 333.46 ± 2.36 c | 4.75 ± 0.12 o | 1.92 ± 0.05 jl | 0.35 ± 0.00 a | 1560.04 ± 5.87 n |
| Marasca | 1176.83 ± 1.63 r | 302.28 ± 0.43 gf | 5.22 ± 0.04 n | 1.74 ± 0.02 k | 0.15 ± 0.01 l | 1486.22 ± 2.65 o |
| Meteor | 1589.80 ± 4.88 ml | 406.53 ± 4.17 a | 9.08 ± 0.02 h | 2.72 ± 0.00 e | 0.16 ± 0.01 lkj | 2008.29 ± 6.66 h |
| Mołodiożnaja | 1798.26 ± 8.9 h | 318.22 ± 3.71 ed | 11.76 ± 0.01 d | 2.32 ± 0.02 gf | 0.19 ± 0.00 f | 2130.75 ± 3.69 d |
| Montmorency | 1027.35 ± 2.75 t | 260.13 ± 4.32 kj | 5.03 ± 0.02 n | 1.67 ± 0.04 l | 0.16 ± 0.01 kji | 1294.35 ± 2.63 p |
| Nana | 1599.93 ± 1.60 l | 279.38 ± 3.46 ih | 5.91 ± 0.17 m | 1.05 ± 0.06 p | 0.19 ± 0.00 g | 1886.46 ± 5.14 k |
| Paraszt Meggy | 1963.60 ± 3.79 b | 322.60 ± 0.23 dc | 10.16 ± 0.30 f | 3.86 ± 0.07 b | 0.26 ± 0.00 c | 2300.47 ± 1.37 a |
| Sokówka Nowotomyska | 1784.40 ± 3.80 h | 235.65 ± 5.42 l | 9.03 ± 0.12 h | 1.44 ± 0.03 n | 0.18 ± 0.00 hg | 2030.71 ± 0.10 g |
| Sokówka nr 6 | 1579.90 ± 7.59 m | 317.01 ± 2.24 ed | 7.23 ± 0.10 k | 2.01 ± 0.11 i | 0.24 ± 0.01 d | 1906.39 ± 6.51 j |
| Sokówka nr 9 | 1491.26 ± 3.70 o | 290.46 ± 1.23 gf | 23.44 ± 0.39 a | 2.26 ± 0.07 dg | 0.26 ± 0.00 c | 1807.67 ± 5.46 l |
| Suda Hardy | 1915.88 ± 4.74 d | 369.96 ± 3.14 b | 10.33 ± 0.27 f | 3.12 ± 0.02 c | 0.23 ± 0.00 d | 2299.52 ± 1.25 a |
| Turgieniewka | 1825.73 ± 6.33 f | 231.52 ± 6.88 l | 7.81 ± 0.10 j | 2.08 ± 0.00 ih | 0.20 ± 0.00 e | 2067.35 ± 9.93 f |
| Wieluń 17 | 1528.21 ± 2.65 n | 260.08 ± 1.01 kj | 8.84 ± 0.23 i | 2.06 ± 0.05 ih | 0.20 ± 0.01f | 1799.38 ± 5.08 m |
| Wróble | 1939.57 ± 3.65 c | 337.84 ± 5.81 c | 12.74 ± 0.20 c | 2.97 ± 0.02 d | 0.16 ± 0.01lk | 2293.28 ± 1.02 a |
| Zagoriewskaja | 1748.39 ± 0.90 i | 330.58 ± 2.10 c | 6.84 ± 0.07 l | 1.55 ± 0.07 m | 0.16 ± 0.01 ji | 2087.52 ± 4.24 e |
| Min-max | 1027.35–1976.15 | 231.52–406.53 | 4.75–23.44 | 1.05–4.08 | 0.15–0.35 | 1294.35–2300.47 |
| Average | 1655.19 | 305.02 | 9.38 | 2.21 | 0.21 | 1972.01 |
| Median | 1684.85 | 302.28 | 9.03 | 2.06 | 0.20 | 2020.03 |
| Cultivar | Anthocyanins | Flavonols | Phenolic Acids | Flavan 3-ols | Total |
|---|---|---|---|---|---|
| Demesova | 128.17 ± 1.06 b | 18.12 ± 0.24 e | 26.53 ± 0.09 n | 154.69 ± 0.97 f | |
| Dradem | 17.97 ± 0.16 p | 15.84 ± 0.42 f | 44.00 ± 0.25 h | 40.10 ± 0.11 b | 117.91 ± 0.72 m |
| Granatnaja | 81.50 ± 1.46 h | 15.61 ± 0.11 gf | 35.10 ± 0.08 j | 2.87 ± 0.04 i | 119.45 ± 1.50 j |
| Grosenkirch | 121.38 ± 0.23 c | 19.65 ± 0.25 d | 76.25 ± 0.29 b | 35.44 ± 0.27 c | 252.72 ± 0.50 b |
| Krasnaja Płodorodnaja | 80.85 ± 0.55 h | 13.19 ± 0.23 i | 32.33 ± 0.04 k | 126.37 ± 0.74 l | |
| Lucyna | 107.91 ± 0.56 e | 19.51 ± 0.21 d | 30.94 ± 0.06 l | 9.45 ± 0.23 g | 167.81 ± 0.52 g |
| Malinówka | 85.39 ± 1.47 g | 14.09 ± 0.54 ih | 57.13 ± 0.07 d | 1656.61 ± 1.94 h | |
| Marasca | 112.95 ± 0.99 d | 21.60 ± 0.17 c | 44.45 ± 0.10 h | 178.99 ± 1.06 e | |
| Meteor | 51.28 ± 0.71 l | 15.50 ± 0.10 hgf | 126.99 ± 0.94 a | 40.81 ± 1.15 b | 219.07 ± 2.80 d |
| Mołodiożnaja | 84.14 ± 1.52 g | 13.30 ± 0.18 i | 49.28 ± 0.81 f | 8.73 ± 0.15 g | 155.45 ± 2.37 h |
| Montmorency | 30.35 ± 0.59 o | 14.18 ± 2.62 ih | 73.35 ± 0.86 c | 26.94 ± 1.36 e | 144.82 ± 2.53 i |
| Pamięci Wawilowa | 33.38 ± 0.87 n | 11.09 ± 0.24 j | 46.54 ± 0.92 g | 5.55 ± 0.10 h | 96.56 ± 2.14 p |
| Paraszt Meggy | 100.30 ± 0.48 f | 16.16 ± 0.03 f | 28.27 ± 0.05 m | 144.73 ± 0.55 i | |
| Sokówka Nowotomyska | 130.14 ± 0.13 ab | 23.18 ± 0.08 b | 53.71 ± 0.28 e | 33.82 ± 0.27 d | 240.85 ± 0.06 c |
| Sokówka nr 6 | 67.72 ± 0.57 k | 14.39 ± 0.20 ihg | 44.84 ± 0.63 h | 26.69 ± 0.07 e | 153.64 ± 1.47 h |
| Sokówka nr 9 | 81.02 ± 1.27 h | 14.06 ± 0.28 i | 35.91 ± 0.57 j | 130.99 ± 1.57 k | |
| Suda Hardy | 107.06 ± 0.90 e | 25.08 ± 0.00 a | 37.42 ± 0.12 i | 169.55 ± 1.02 g | |
| Turgeniewka | 74.61 ± 1.1 i | 14.32 ± 0.09 ihg | 16.56 ± 0.06 o | 105.50 ± 1.25 n | |
| Wieluń 17 | 131.28 ± 0.91 a | 20.73 ± 0.14 dc | 53.90 ± 0.19 e | 63.08 ± 0.54 a | 268.98 ± 0.32 a |
| Wróble | 48.49 ± 1.64 m | 10.81 ± 0.29 j | 29.13 ± 0.19 m | 13.31 ± 1.57 f | 101.74 ± 0.55 o |
| Zagoriewskaja | 72.28 ± 1.44 j | 10.41 ± 0.21 j | 44.23 ± 0.44 h | 126.92 ± 2.09 l | |
| Min-max | 17.97–131.28 | 10.41–25.08 | 16.56–126.99 | 0.00–63.08 | 96.56–268.98 |
| Average | 83.24 | 16.23 | 46.99 | 14.61 | 161.07 |
| Median | 81.47 | 15.50 | 44.23 | 5.55 | 153.64 |
| Cultivar | FCRS mg GAE/100 g | DPPH µmol TE/100 g | FRAP µmol TE/100 g |
|---|---|---|---|
| Demesova | 267.00 ± 0.81 ghij | 647.09 ± 0.64 e | 1461.00 ± 5.04 gf |
| Dredem (Maćkowiaka) | 262.75 ± 17.68 hij | 695.39 ± 1.72 j | 1673.66 ± 22.94 k |
| Granatnaja | 250.46 ± 11.47 hg | 675.22 ± 13.51 f | 1681.80 ± 17.59 h |
| Grosenkirch | 495.20 ± 5.31 b | 984.81 ± 11.95 b | 3065.78 ± 36.24 b |
| Krasnaja Płodorodnaja | 239.35 ± 18.21 hij | 552.32 ± 9.09 h | 1183.87 ± 7.80 i |
| Lucyna | 244.17 ± 2.89 e | 739.90 ± 4.92 c | 1853.96 ± 2.41 d |
| Malinówka | 238.44 ± 1.31 f | 634.20 ± 6.63 e | 1539.87 ± 54.43 f |
| Marasca | 432.29 ± 10.95 cd | 910.45 ± 11.08 b | 2650.47 ± 42.80 c |
| Meteor | 245.36 ± 3.51 f | 631.65 ± 1.36h g | 1472.12 ± 29.10 i |
| Mołodiożnaja | 412.35 ± 6.92 f | 907.86 ± 11.02 e | 2613.64 ± 0.31 g |
| Montmorency | 309.18 ± 3.08 ijk | 655.14 ± 5.77 k | 1542.40 ± 28.06 kl |
| Nana | 340.16 ± 16.41 g | 826.61 ± 17.55 h | 2174.62 ± 4.42 ij |
| Paraszt Meggy | 227.71 ± 0.94 ghi | 510.62 ± 0.69 gf | 1111.10 ± 88.16 h |
| Sokówka Nowotomyska | 220.22 ± 3.81 a | 607.84 ± 3.12 a | 1444.57 ± 55.15 a |
| Sokówka nr 6 | 376.70 ± 14.46 d | 804.12 ± 6.00 cd | 2197.94 ± 52.67 d |
| Sokówka nr 9 | 475.68 ± 0.09 e | 975.62 ± 8.56 d | 3028.11 ± 24.87 e |
| Suda Hardy | 306.71 ± 16.67 bc | 749.08 ± 17.19 b | 1930.53 ± 59.23 b |
| Turgieniewka | 342.96 ± 5.87 ghi | 802.01 ± 7.78 h | 2082.88 ± 33.92 ij |
| Wieluń 17 | 205.71 ± 0.66 a | 520.98 ± 29.49 a | 1045.68 ± 31.84 a |
| Wróble | 392.83 ± 19.05 k | 891.29 ± 6.52 k | 2501.18 ± 1.27 l |
| Zagoriewskaja | 298.34 ± 12.74 k | 726.10 ± 3.95 i | 1792.50 ± 23.78 j |
| Min-max | 205.71–495.20 | 510.62–984.81 | 1045.68–3065.78 |
| Average | 313.50 | 735.63 | 1907.03 |
| Median | 298.34 | 726.10 | 1792.50 |
| ANT | FL | PA | F3 | TP | FCRS | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|
| ANT | 1.00 | |||||||
| FL | 0.70 | 1.00 | ||||||
| PA | −0.18 | 0.05 | 1.00 | |||||
| FS | −0.02 | 0.26 | 0.58 | 1.00 | ||||
| SP | 0.64 | 0.68 | 0.57 | 0.65 | 1.00 | |||
| FCRS | 0.66 | 0.77 | 0.24 | 0.48 | 0.80 | 1.00 | ||
| DPPH | 0.83 | 0.80 | 0.04 | 0.23 | 0.73 | 0.94 | 1.00 | |
| FRAP | 0.83 | 0.81 | 0.06 | 0.30 | 0.76 | 0.96 | 0.99 | 1.00 |
| Cultivar | Origin | Fruits | |||||
|---|---|---|---|---|---|---|---|
| Parents | Country ** | Harvest Maturity | Weight [g] | Skin Colour | Juice Colour | ||
| “Demesova” | unknown | probably the Czech Republic | medium | 12–16.07 | 4.5–5.0 | black | black red |
| “Dradem” | “Łutówka” x “Northstar” | Poland | medium to late | 15–20.07 | 5.0–5.5 | light red | colourless |
| “Granatnaja” | unknown | former USSR | medium | 05–15.07 | 5.3–5.6 | red | red |
| “Grosenkirch” | unknown | probably. the Netherlands | late | 16–22.07 | 5.5–5.9 | red | red |
| “Krasnaja Płodorodnaja” | unknown | former USSR | early to medium | 02–09.07 | 4.6–4.9 | dark red | dark red |
| “Lucyna” | “Łutówka” x “Schirpotreb” | Poland | medium to late | 10–20.07 | 5.1–5.6 | dark red | red |
| “Malinówka” | unknown | former USSR | late | 15–25.07 | 4.8–5.3 | light red | red |
| “Marasca” | unknown | former Yugoslavia | medium to late | 10–20.07 | 2.7–3.2 | dark red | dark red |
| “Meteor” | “Montmorency” x (“Vladimir” x “Shubianka”) | USA | late | 15–25.07 | 5.2–5.6 | light red | pink |
| “Mołodiożnaja” | unknown | Ukraine | medium to late | 05–20.07 | 4.6–5.0 | dark red | dark red |
| “Montmorency” | unknown | USA | early to medium | 01–15.07 | 4.5–5.0 | light red | colourless |
| “Nana” | “Crisana” x “Morella Neagra” | Romania | late | 15–25.07 | 4.9–5.3 | red | red |
| “Paraszt Meggy” | unknown | probably Hungary | medium | 03–15.07 | 4.6–5.0 | dark red | dark red |
| “Sokówka Nowotomyska” | unknown | Poland | medium to late | 10–25.07 | 3.8–4.2 | dark red | dark red |
| “Sokówka nr 6” * | unknown | Poland | late to very late | 20–28.07 | 4.5–4.9 | dark red | red |
| “Sokówka nr 9” * | unknown | Poland | late | 15–25.07 | 4.0–4.4 | red | red |
| “Suda Hardy” | probably seedling cv “Łutówka” | USA | late | 15–25.07 | 4.6–4.9 | red | red |
| “Turgieniewka” | seedling cv “Żukowskaja” | Russia | medium | 05–15.07 | 5.2–5.6 | red | red |
| “Wieluń 17” * | unknown | Poland | late | 15–25.07 | 2.9–3.7 | dark red | dark red |
| “Wróble” | unknown | prob. the Chech Republic | medium to late | 10–20.07 | 5.0–5.5 | light red | light red |
| “Zagoriewskaja” | unknown | prob. Russia | medium | 05–15.07 | 4.8–5.6 | dark red | light red |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokół-Łętowska, A.; Kucharska, A.Z.; Hodun, G.; Gołba, M. Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland. Molecules 2020, 25, 4587. https://doi.org/10.3390/molecules25194587
Sokół-Łętowska A, Kucharska AZ, Hodun G, Gołba M. Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland. Molecules. 2020; 25(19):4587. https://doi.org/10.3390/molecules25194587
Chicago/Turabian StyleSokół-Łętowska, Anna, Alicja Z. Kucharska, Grzegorz Hodun, and Marta Gołba. 2020. "Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland" Molecules 25, no. 19: 4587. https://doi.org/10.3390/molecules25194587
APA StyleSokół-Łętowska, A., Kucharska, A. Z., Hodun, G., & Gołba, M. (2020). Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland. Molecules, 25(19), 4587. https://doi.org/10.3390/molecules25194587