Antiproliferative and Antimigration Activities of Beauvericin Isolated from Isaria sp. on Pancreatic Cancer Cells
Abstract
1. Introduction
2. Results
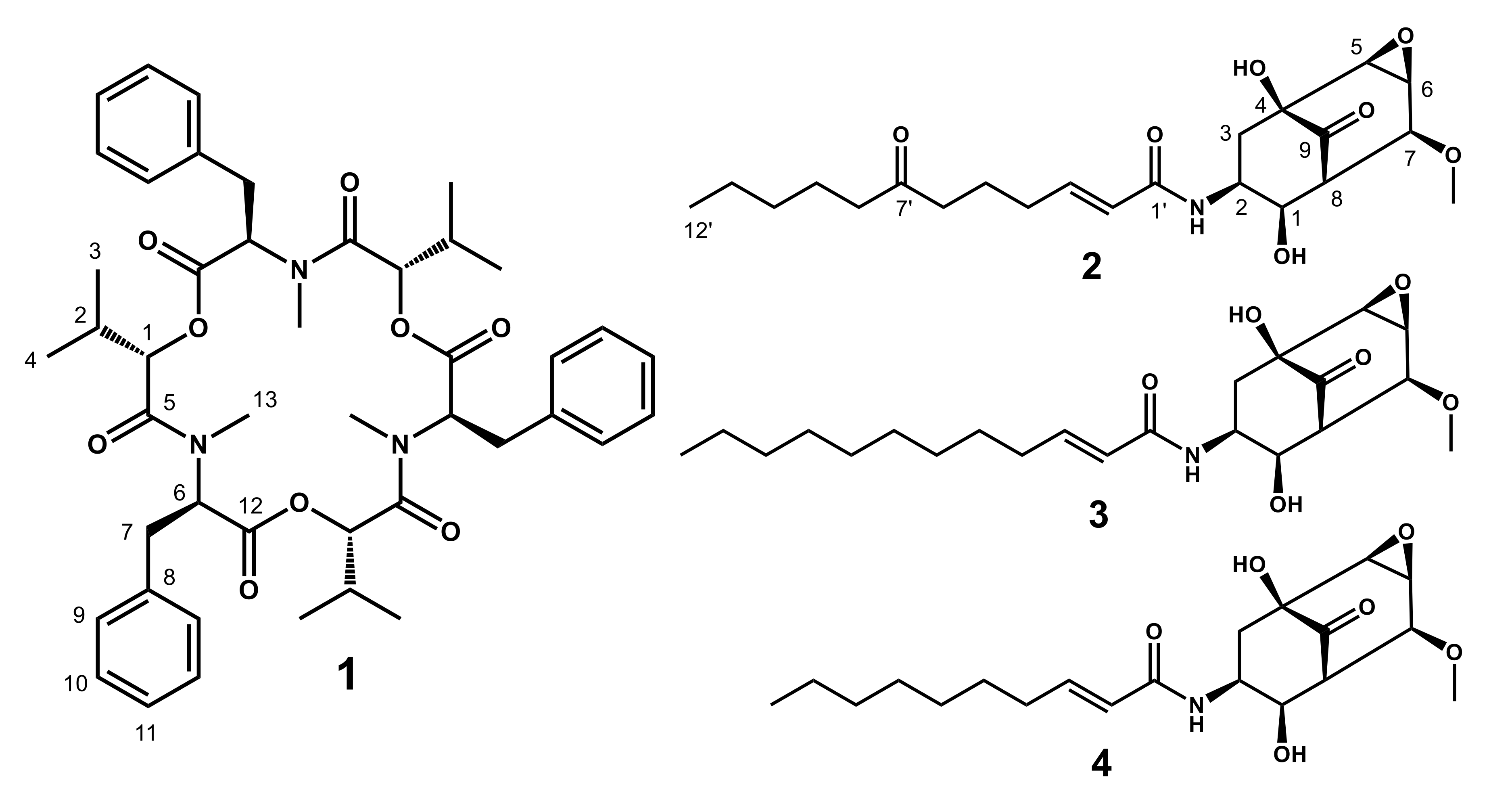
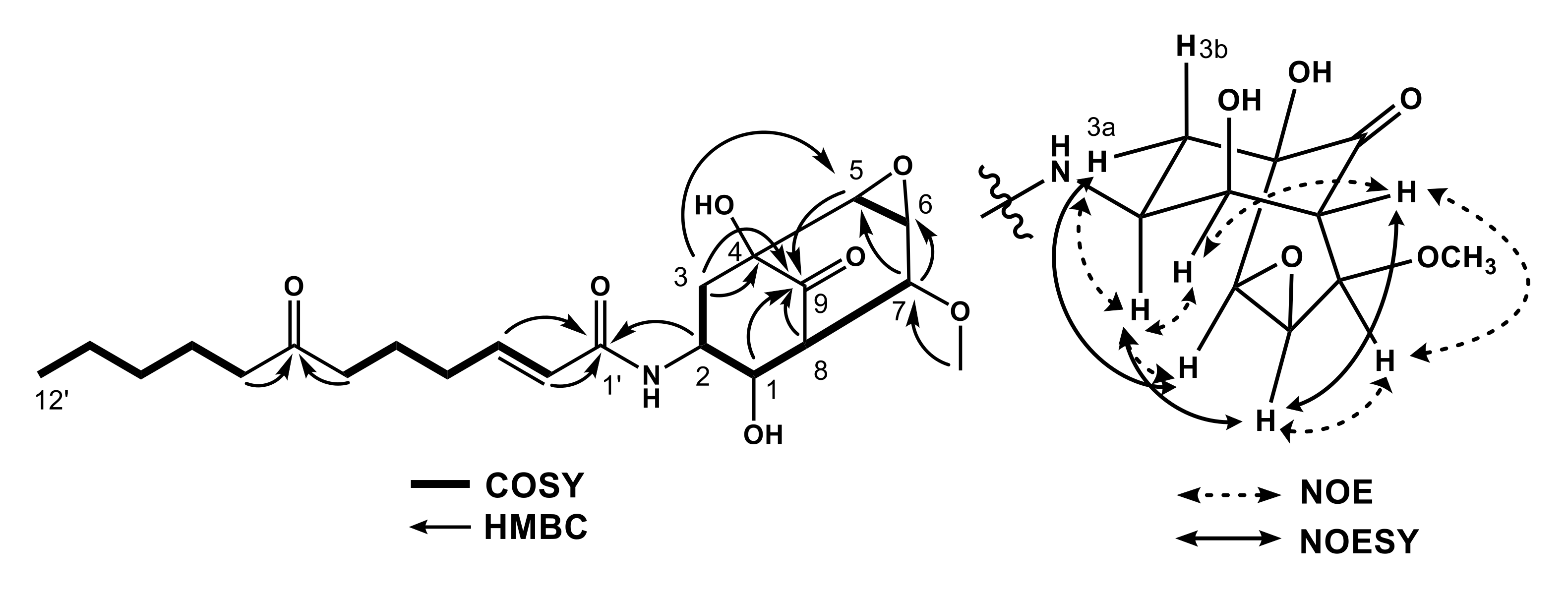
2.1. Structures of 1–4
2.2. Measurement of Cell Viability
2.3. Measurement of Cell Migration by Wound-Healing Assay
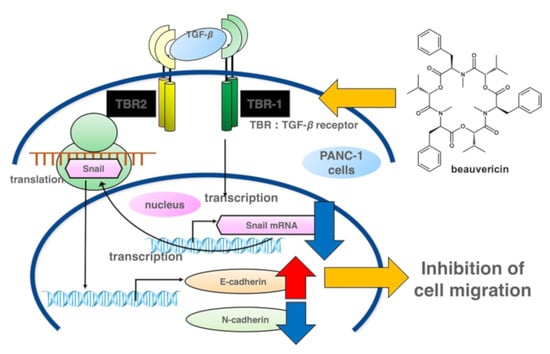
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Cell Culture
4.3. Measurement of Cell Viability by MTT Assay
4.4. Extraction and Isolation of Chemical Compounds from Isaria sp. RD055140
4.4.1. Measurement of Cell Migration by Wound-Healing Assay
4.4.2. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics CA. Cancer. J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Zheng, L. Current standards of chemotherapy for pancreatic cancer. Clin. Ther. 2017, 39, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and treatment resistance in pancreatic cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hirose, R.; Yoneta, M.; Sasaki, S.; Inoue, K.; Kikuchi, M.; Hirase, S.; Chiba, K.; Sakamoto, H.; Arita, M. Potent Immunosuppressants, 2-Alkyl-2-aminopropane-1,3-diols. J. Med. Chem. 1996, 39, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Burns, A.M.; Liu, M.X.; Faeth, S.H.; Gunatilaka, A.A.L. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: Beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J. Nat. Prod. 2007, 70, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Nishikawa, N.; Takahashi, S.; Yamamoto, K. Substances TK-57-164A and TK-57-164B, process for producing the same and agricultural/horticultural bactericides containing the same. J. P. Patent. WO-A1-2004/074269, 2004. [Google Scholar]
- Asai, T.; Chung, Y.M.; Sakurai, H.; Ozeki, T.; Chang, F.R.; Wu, Y.C.; Yamashita, K.; Oshima, Y. Highly oxidized ergosterols and isariotin analogs from an entomopathogenic fungus, Gibellula formosana, cultivated in the presence of epigenetic modifying agents. Tetrahedron 2012, 68, 5817–5823. [Google Scholar] [CrossRef]
- Bozzuto, G.; Ruggieri, P.; Molinari, A. Molecular aspects of tumor cell migration and invasion. Ann. Ist. Super. Sanita. 2010, 46, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. A review on the synthesis and bioactivity aspects of beauvericin, a Fusarium mycotoxin. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.I.; Lee, Y.J.; Chen, B.F.; Tsai, M.C.; Lu, J.L.; Chou, C.J.; Jow, G.M. Involvement of Bcl-2 family, cytochrome c and caspase 3 in induction of apoptosis by beauvericin human non-small cell lung cancer cells. Cancer Lett. 2005, 230, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.W.; Lin, Y.C.; She, Z.G.; Lin, M.T.; Chen, P.X.; Zhang, J.Y. Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med. Chem. 2015, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, V.C.; Mirabelli, V.; Cimmarusti, M.T.; Haidukowski, M.; Leslie, J.F.; Logrieco, A.F.; Caliandro, R.; Fanelli, F.; Mulè, G. Enniatin and beauvericin biosynthesis in Fusarium species: Production profiles and structural determinant prediction. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Q.; Weng, Q. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. RSC Adv. 2019, 1, 172–184. [Google Scholar] [CrossRef]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Intereya, K.; Rachtawee, P.; Hywel-Jones, N.L.; Isaka, M. Isariotins E and F, spirocyclic and bicyclic hemiacetals from the entomopathogenic fungus Isaria tenuipes BCC12625. J. Nat. Prod. 2009, 72, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Srisanoh, U.; Choowong, W.; Tasanathai, K.; Hywel-Jones, N.L.; Luangsa-ard, J.J.; Isaka, M. Isariotins G–J from cultures of the Lepidoptera pathogenic fungus Isaria tenuipes. Phytochem. Lett. 2011, 4, 283–286. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahagi, H.; Yahagi, T.; Furukawa, M.; Matsuzaki, K. Antiproliferative and Antimigration Activities of Beauvericin Isolated from Isaria sp. on Pancreatic Cancer Cells. Molecules 2020, 25, 4586. https://doi.org/10.3390/molecules25194586
Yahagi H, Yahagi T, Furukawa M, Matsuzaki K. Antiproliferative and Antimigration Activities of Beauvericin Isolated from Isaria sp. on Pancreatic Cancer Cells. Molecules. 2020; 25(19):4586. https://doi.org/10.3390/molecules25194586
Chicago/Turabian StyleYahagi, Hiroaki, Tadahiro Yahagi, Megumi Furukawa, and Keiichi Matsuzaki. 2020. "Antiproliferative and Antimigration Activities of Beauvericin Isolated from Isaria sp. on Pancreatic Cancer Cells" Molecules 25, no. 19: 4586. https://doi.org/10.3390/molecules25194586
APA StyleYahagi, H., Yahagi, T., Furukawa, M., & Matsuzaki, K. (2020). Antiproliferative and Antimigration Activities of Beauvericin Isolated from Isaria sp. on Pancreatic Cancer Cells. Molecules, 25(19), 4586. https://doi.org/10.3390/molecules25194586