Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers
Abstract
1. Introduction
2. Results and Discussion
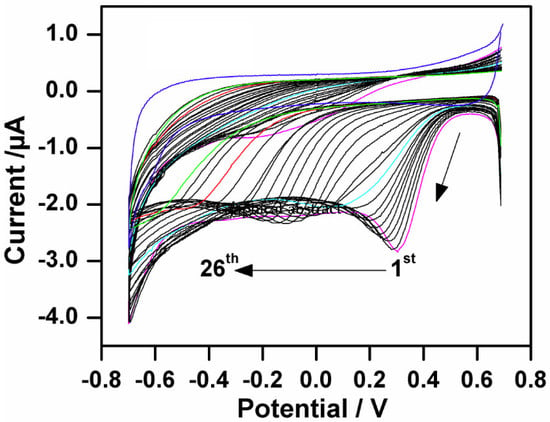
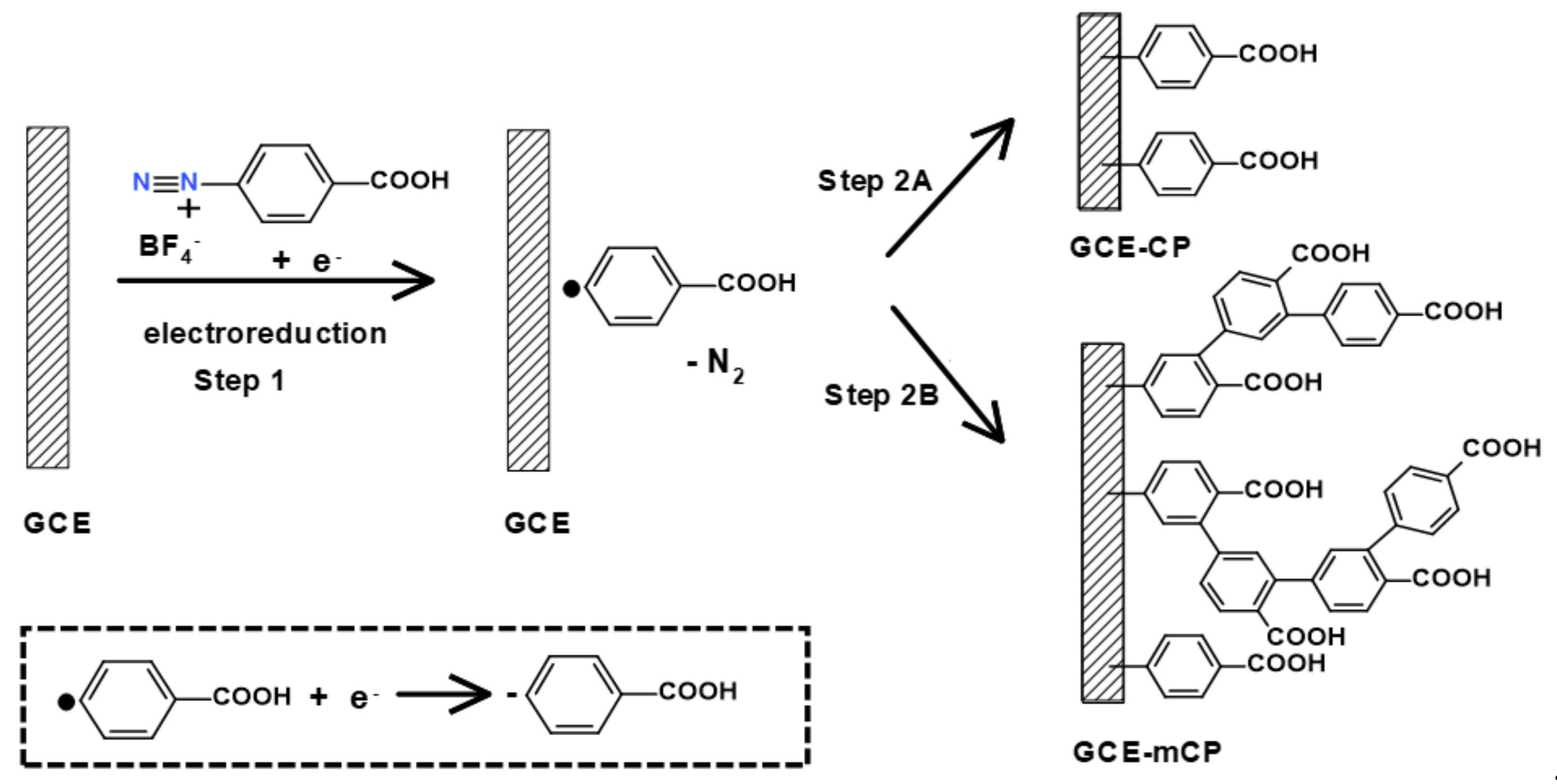
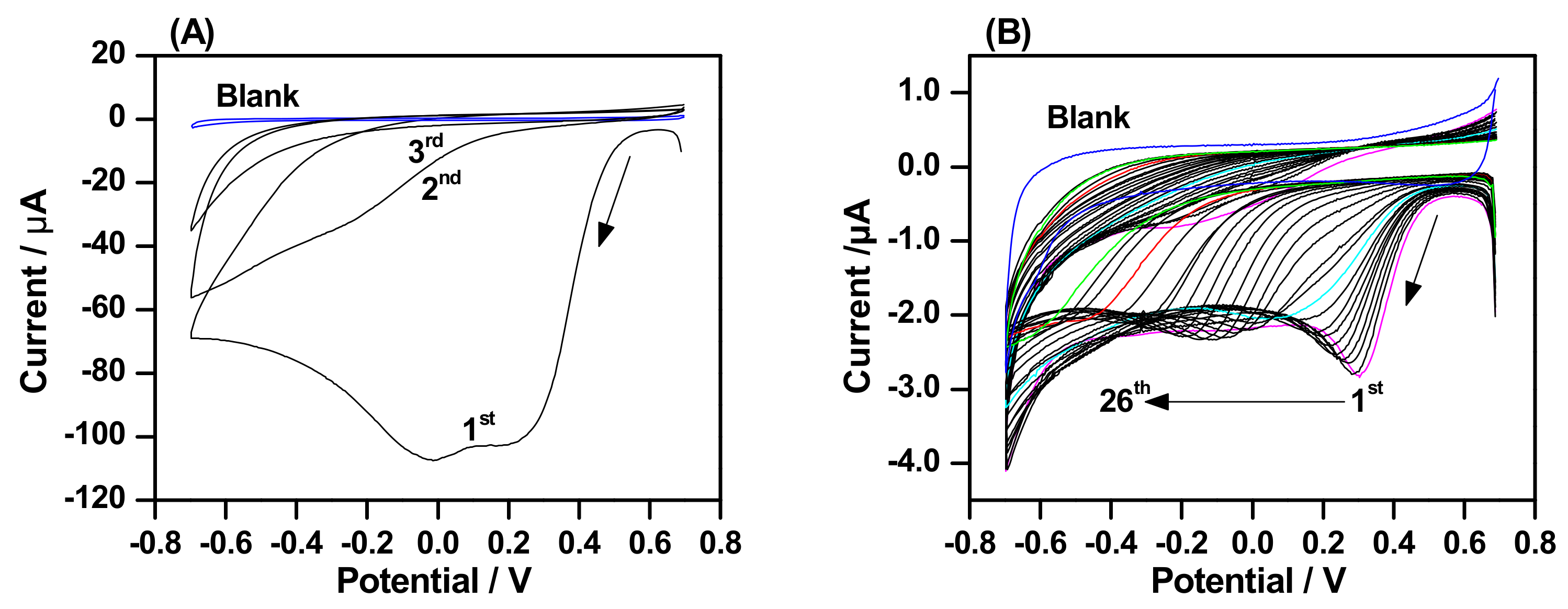
2.1. Electrografting Behavior of 4-CBD
2.2. XPS Characterization
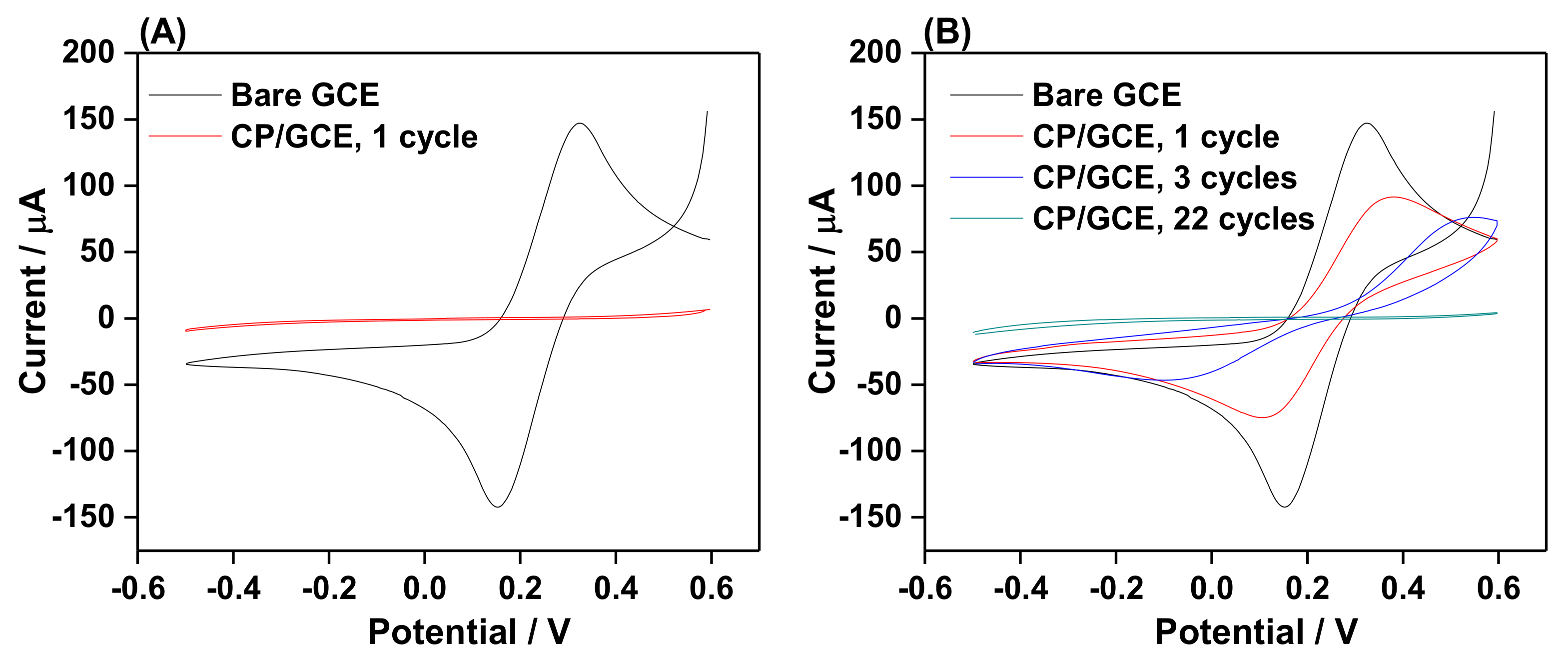
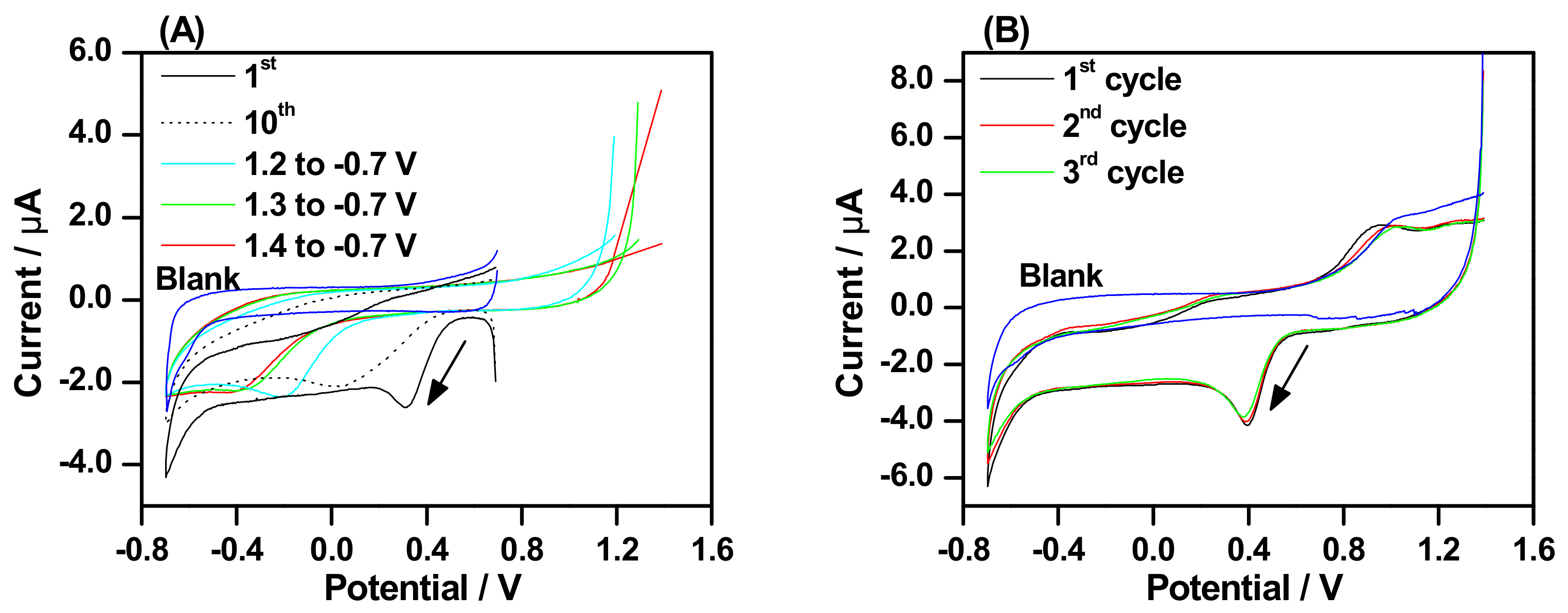
2.3. Electrochemical Stripping of Grafted Layer by Electro-oxidation
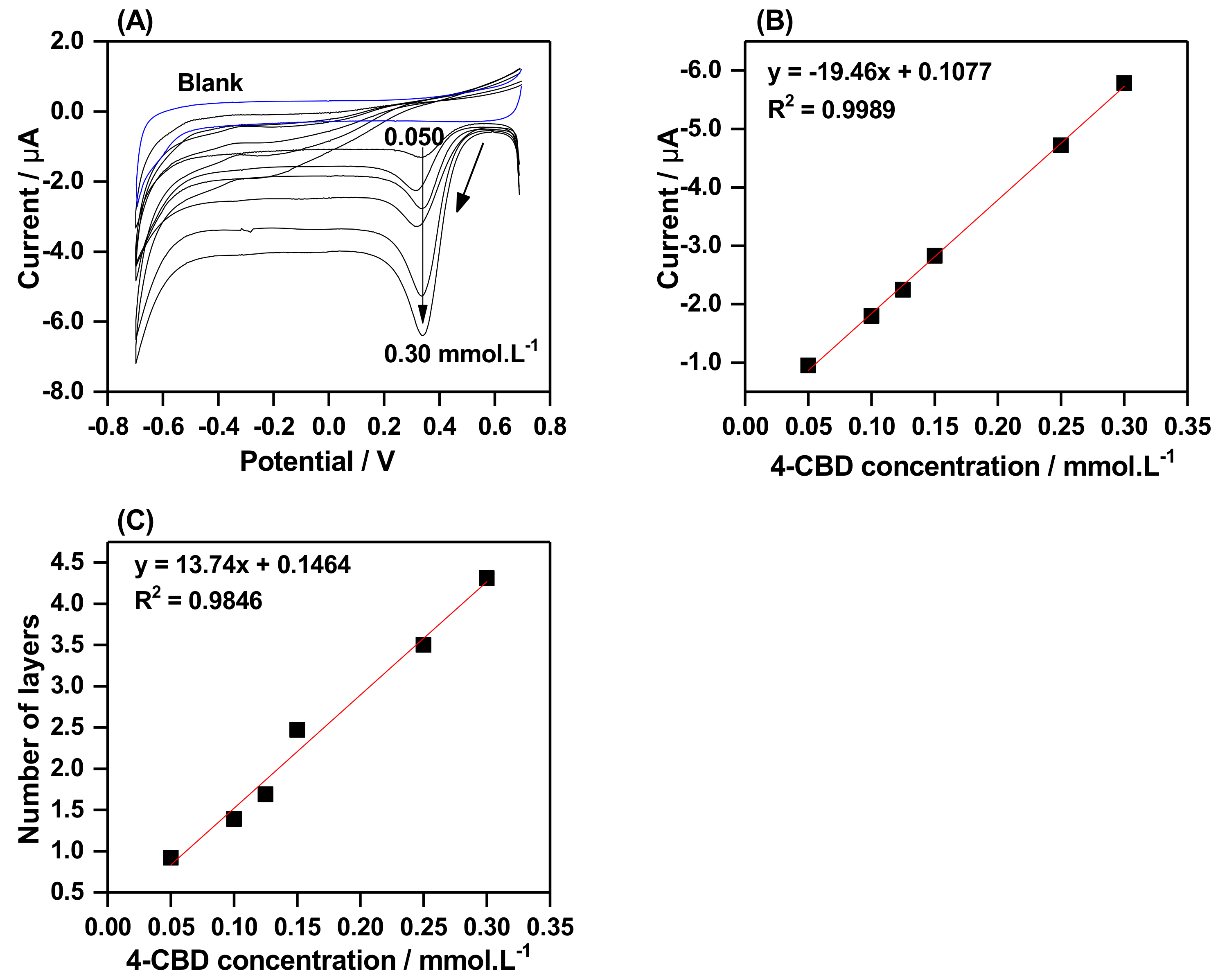
2.4. The Effect of 4-CBD Concentration on Grafting
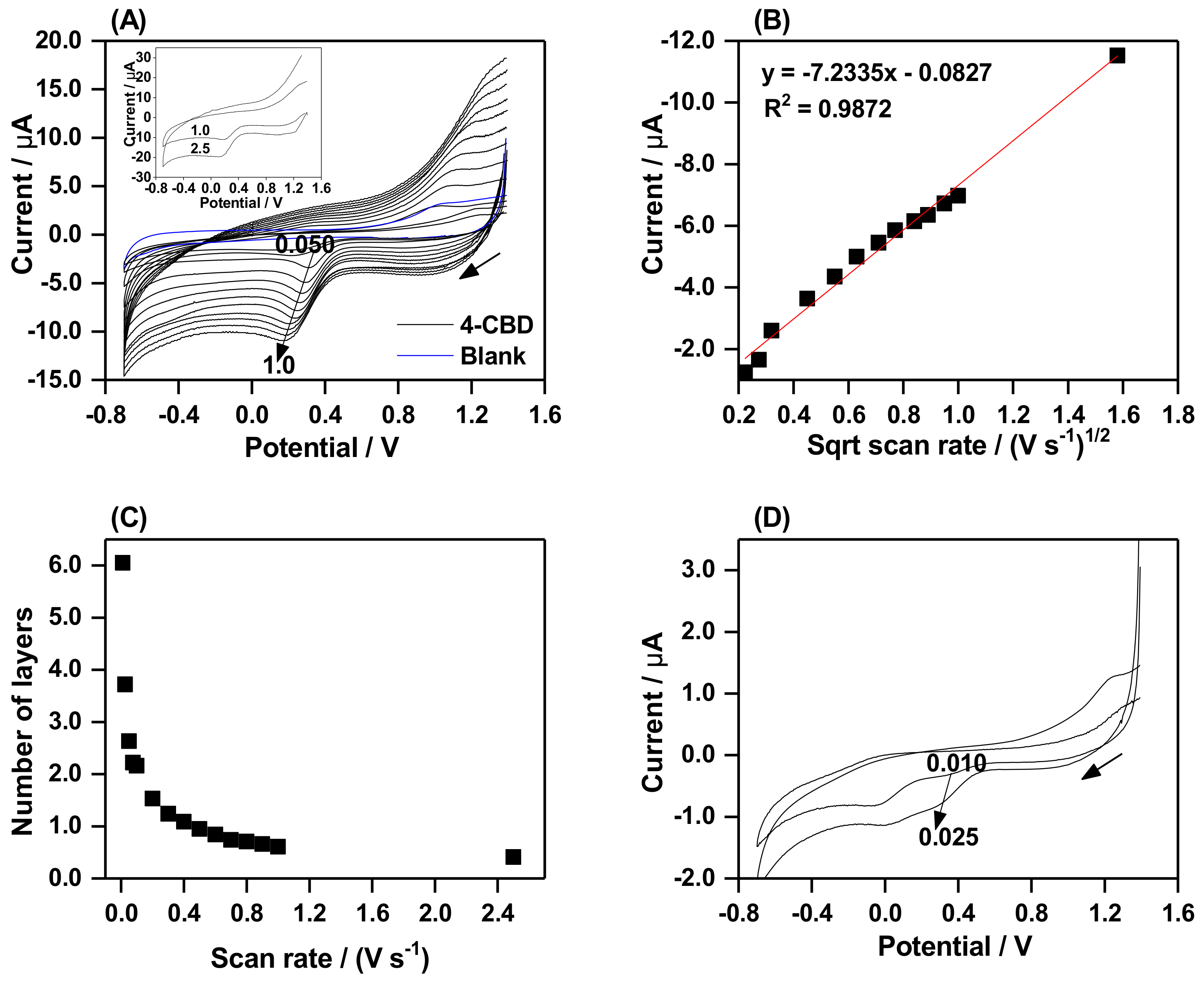
2.5. The Effect of Scan Rate on Grafting
3. Materials and Methods
3.1. Reagents and Materials
3.2. Electrode Preparation
3.3. Electrografting Behavior of 4-CBD
3.4. Electrochemical Stripping of Grafted Layer by Electro-oxidation
3.5. The Effect of 4-CBD Concentration and Scan Rate on Electrografting
3.6. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, G.; Böcking, T.; Gooding, J.J. Diazonium salts: Stable monolayers on gold electrodes for sensing applications. J. Electroanal. Chem. 2007, 600, 335–344. [Google Scholar] [CrossRef]
- Gooding, J.J. Advances in interfacial design for electrochemical biosensors and sensors: Aryl diazonium salts for modifying carbon and metal electrodes. Electroanalysis 2008, 20, 573–582. [Google Scholar] [CrossRef]
- Phal, S.; Shatri, B.; Berisha, A.; Geladi, P.; Lindholm-Sethson, B.; Tesfalidet, S. Covalently electrografted carboxyphenyl layers onto gold surface serving as a platform for the construction of an immunosensor for detection of methotrexate. J. Electroanal. Chem. 2018, 812, 235–243. [Google Scholar] [CrossRef]
- Chung, D.-J.; Oh, S.-H.; Komathi, S.; Gopalan, A.I.; Lee, K.-P.; Choi, S.-H. One-step modification of various electrode surfaces using diazonium salt compounds and the application of this technology to electrochemical DNA (E-DNA) sensors. Electrochim. Acta 2012, 76, 394–403. [Google Scholar] [CrossRef]
- Eissa, S.; L’Hocine, L.; Siaj, M.; Zourob, M. A graphene-based label-free voltammetric immunosensor for sensitive detection of the egg allergen ovalbumin. Analyst 2013, 138, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. A graphene-based electrochemical competitive immunosensor for the sensitive detection of okadaic acid in shellfish. Nanoscale 2012, 4, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Sedeno, P.; Campuzano, S.; Pingarron, J.M. Integrated Affinity Biosensing Platforms on Screen-Printed Electrodes Electrografted with Diazonium Salts. Sensors (Basel) 2018, 18, 1–21. [Google Scholar]
- Chrouda, A.; Braiek, M.; Rokbani, K.B.; Bakhrouf, A.; Maaref, A.; Jaffrezic-Renault, N. An Immunosensor for Pathogenic Staphylococcus aureus Based on Antibody Modified Aminophenyl-Au Electrode. ISRN Electrochem 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311. [Google Scholar] [CrossRef]
- Allongue, P.; Delamar, M.; Desbat, B.; Fagebaume, O.; Hitmi, R.; Pinson, J.; Savéant, J.-M. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc 1997, 119, 201–207. [Google Scholar] [CrossRef]
- Andrieux, C.P.; Pinson, J. The Standard Redox Potential of the Phenyl Radical/Anion Couple. J. Am. Chem. Soc. 2003, 125, 14801–14806. [Google Scholar] [CrossRef] [PubMed]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef]
- Baranton, S.; Bélanger, D. Electrochemical Derivatization of Carbon Surface by Reduction of in Situ Generated Diazonium Cations. J. Phys. Chem. B 2005, 109, 24401–24410. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, M.; Bélanger, D. Stability of Substituted Phenyl Groups Electrochemically Grafted at Carbon Electrode Surface. J. Phys. Chem. B 2003, 107, 4811–4817. [Google Scholar] [CrossRef]
- Menanteau, T.; Dias, M.; Levillain, E.; Downard, A.J.; Breton, T. Electrografting via Diazonium Chemistry: The Key Role of the Aryl Substituent in the Layer Growth Mechanism. J. Phys. Chem. C 2016, 120, 4423–4429. [Google Scholar] [CrossRef]
- Menanteau, T.; Levillain, E.; Downard, A.J.; Breton, T. Evidence of monolayer formation via diazonium grafting with a radical scavenger: Electrochemical, AFM and XPS monitoring. Phys. Chem. Chem. Phys. 2015, 17, 13137–13142. [Google Scholar] [CrossRef]
- Adenier, A.; Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Formation of Polyphenylene Films on Metal Electrodes by Electrochemical Reduction of Benzenediazonium Salts. Chem. Mater. 2006, 18, 2021–2029. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Downard, A.J. Electrochemical and Atomic Force Microscopy Study of Carbon Surface Modification via Diazonium Reduction in Aqueous and Acetonitrile Solutions. Langmuir 2004, 20, 5038–5045. [Google Scholar] [CrossRef]
- Baranton, S.; Bélanger, D. In situ generation of diazonium cations in organic electrolyte for electrochemical modification of electrode surface. Electrochim. Acta 2008, 53, 6961–6967. [Google Scholar] [CrossRef]
- Adenier, A.; Cabet-Deliry, E.; Chaussé, A.; Griveau, S.; Mercier, F.; Pinson, J.; Vautrin-Ul, C. Grafting of Nitrophenyl Groups on Carbon and Metallic Surfaces without Electrochemical Induction. Chem. Mater. 2005, 17, 491–501. [Google Scholar] [CrossRef]
- Haccoun, J.; Vautrin-Ul, C.; Chaussé, A.; Adenier, A. Electrochemical grafting of organic coating onto gold surfaces: Influence of the electrochemical conditions on the grafting of nitrobenzene diazonium salt. Prog. Org. Coat. 2008, 63, 18–24. [Google Scholar] [CrossRef]
- Laforgue, A.; Addou, T.; Bélanger, D. Characterization of the Deposition of Organic Molecules at the Surface of Gold by the Electrochemical Reduction of Aryldiazonium Cations. Langmuir 2005, 21, 6855–6865. [Google Scholar] [CrossRef] [PubMed]
- Pilan, L.; Ungureanu, E.-M.; Bidan, G. Electrochemical Grafting: A Modern Tool to Achieve New Materials Used for Recognition. Nonlinear Optics and Quantum Optics 2009, 39, 41–55. [Google Scholar]
- Kibena, E.; Mäeorg, U.; Matisen, L.; Tammeveski, K. Electrochemical behaviour of ABTS on aryl-modified glassy carbon electrodes. J. Electroanal. Chem. 2011, 661, 343–350. [Google Scholar] [CrossRef]
- Kullapere, M.; Marandi, M.; Matisen, L.; Mirkhalaf, F.; Carvalho, A.E.; Maia, G.; Sammelselg, V.; Tammeveski, K. Blocking properties of gold electrodes modified with 4-nitrophenyl and 4-decylphenyl groups. Solid State Electrochemistry 2011, 16, 569–578. [Google Scholar] [CrossRef]
- Richard, W.; Evrard, D.; Gros, P. New insight into 4-nitrobenzene diazonium reduction process: Evidence for a grafting step distinct from NO2 electrochemical reactivity. J. Electroanal. Chem. 2012, 685, 109–115. [Google Scholar] [CrossRef][Green Version]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Cline, K.K.; Baxter, L.; Lockwood, D.; Saylor, R.; Stalzer, A. Nonaqueous synthesis and reduction of diazonium ions (without isolation) to modify glassy carbon electrodes using mild electrografting conditions. J. Electroanal. Chem. 2009, 633, 283–290. [Google Scholar] [CrossRef]
- Lee, L.; Brooksby, P.A.; Hapiot, P.; Downard, A.J. Electrografting of 4-Nitrobenzenediazonium Ion at Carbon Electrodes: Catalyzed and Uncatalyzed Reduction Processes. Langmuir 2016, 32, 468–476. [Google Scholar] [CrossRef]
- Pinson, J. Attachment of Organic Layers to Materials Surfaces by Reduction of Diazonium Salts; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 2012. [Google Scholar]
- Limbut, W.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Thavarungkul, P. A comparative study of capacitive immunosensors based on self-assembled monolayers formed from thiourea, thioctic acid, and 3-mercaptopropionic acid. Biosens. Bioelectron. 2006, 22, 233–240. [Google Scholar] [CrossRef]
- Liu, Y.-C.; McCreery, R.L. Reactions of Organic Monolayers on Carbon Surfaces Observed with Unenhanced Raman Spectroscopy. J. Am. Chem. Soc. 1995, 117, 11254–11259. [Google Scholar] [CrossRef]
- Charlton, M.R.; Suhr, K.J.; Holliday, B.J.; Stevenson, K.J. Electrochemical modification of indium tin oxide using di(4-nitrophenyl) iodonium tetrafluoroborate. Langmuir 2015, 31, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bouden, S.; Pinson, J.; Vautrin-Ul, C. Electrografting of diazonium salts: A kinetics study. Electrochem. Commun. 2017, 81, 120–123. [Google Scholar] [CrossRef]
- Kam, C.S.; Leung, T.L.; Liu, F.; Djurišić, A.B.; Xie, M.H.; Chan, W.-K.; Zhou, Y.; Shih, K. Lead removal from water—dependence on the form of carbon and surface functionalization. RSC Advances 2018, 8, 18355–18362. [Google Scholar] [CrossRef]
- Fan, L.-Z.; Qiao, S.; Song, W.; Wu, M.; He, X.; Qu, X. Effects of the functional groups on the electrochemical properties of ordered porous carbon for supercapacitors. Electrochim. Acta 2013, 105, 299–304. [Google Scholar] [CrossRef]
- Bhugun, I.; Savéant, J.-M. Derivatization of surfaces and self-inhibition in irreversible electrochemical reactions: Cyclic voltammetry and preparative-scale electrolysis. J. Electroanal. Chem. 1995, 395, 127–131. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Chapter 5: Basic potential Step Methods. In Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Brownson, D.A.C.; Banks, C.E. Chapter 2 Interpreting Electrochemistry; Springer: London, UK, 2014; pp. 23–77. [Google Scholar]
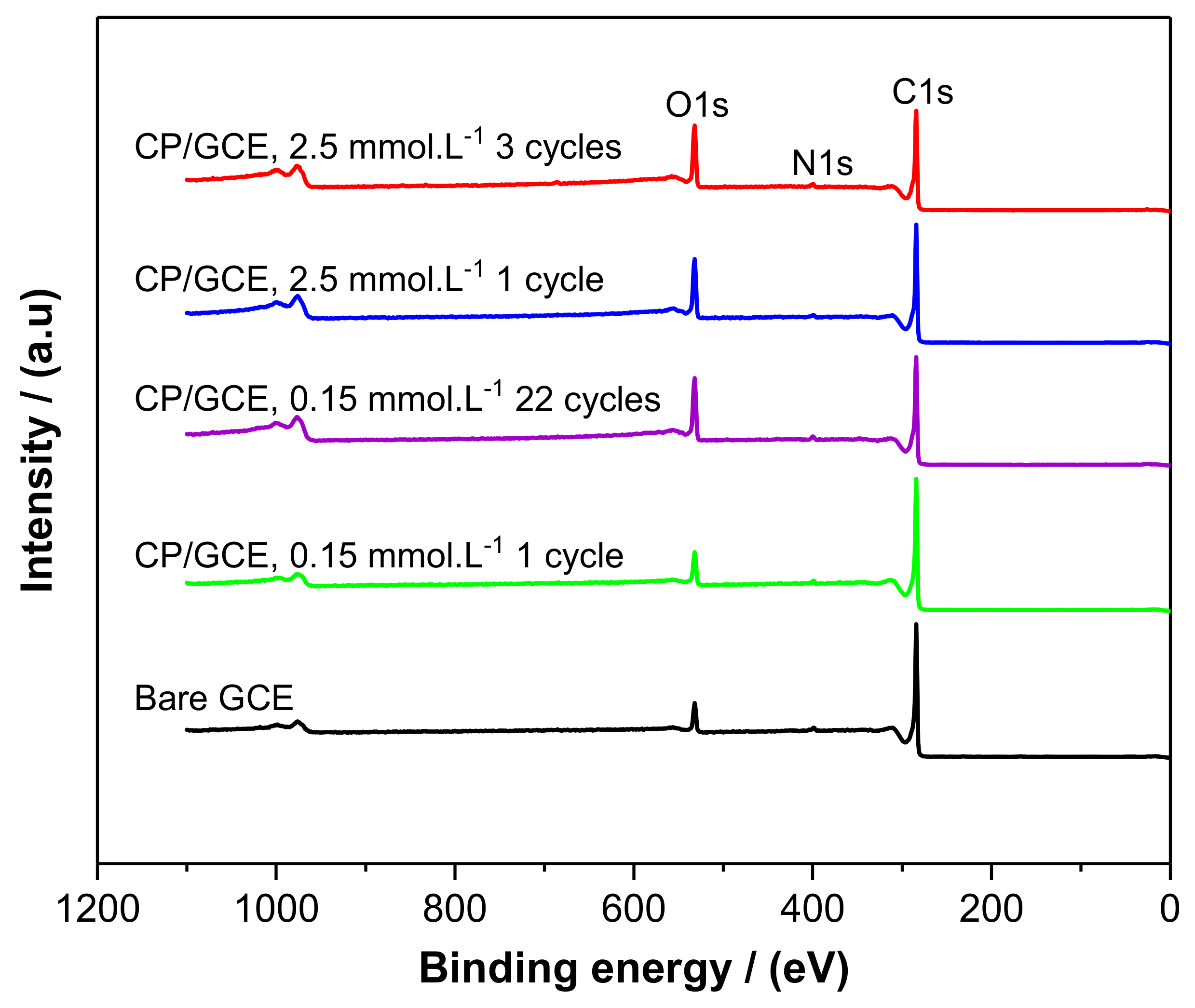
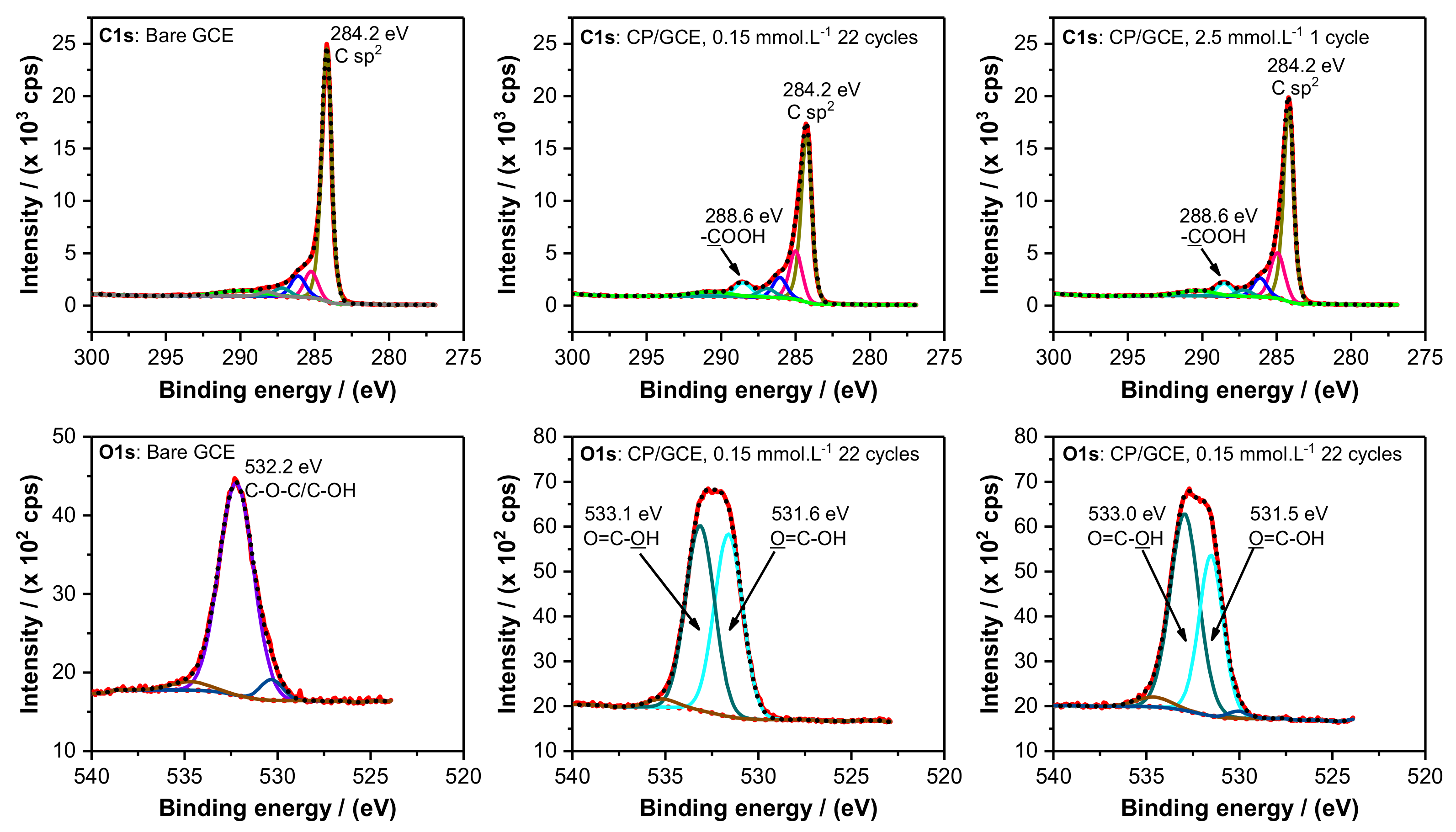
| Electrode | Atomic Concentration/at. % | ||
|---|---|---|---|
| C1s | O1s | N1s | |
| Bare GCE | 91.4 | 7.0 | 1.6 |
| CP/GCE, 0.15 mmol/L 1 cycle | 90.7 | 7.9 | 1.4 |
| CP/GCE, 0.15 mmol/L 22 cycles | 82.6 | 15.6 | 1.7 |
| CP/GCE, 2.5 mmol/L 1 cycle | 83.6 | 15.1 | 1.3 |
| CP/GCE, 2.5 mmol/L 3 cycles | 81.3 | 16.9 | 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phal, S.; Shimizu, K.; Mwanza, D.; Mashazi, P.; Shchukarev, A.; Tesfalidet, S. Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers. Molecules 2020, 25, 4575. https://doi.org/10.3390/molecules25194575
Phal S, Shimizu K, Mwanza D, Mashazi P, Shchukarev A, Tesfalidet S. Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers. Molecules. 2020; 25(19):4575. https://doi.org/10.3390/molecules25194575
Chicago/Turabian StylePhal, Sereilakhena, Kenichi Shimizu, Daniel Mwanza, Philani Mashazi, Andrey Shchukarev, and Solomon Tesfalidet. 2020. "Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers" Molecules 25, no. 19: 4575. https://doi.org/10.3390/molecules25194575
APA StylePhal, S., Shimizu, K., Mwanza, D., Mashazi, P., Shchukarev, A., & Tesfalidet, S. (2020). Electrografting of 4-Carboxybenzenediazonium on Glassy Carbon Electrode: The Effect of Concentration on the Formation of Mono and Multilayers. Molecules, 25(19), 4575. https://doi.org/10.3390/molecules25194575