BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives
Abstract
1. Introduction
2. BODIPY Probes
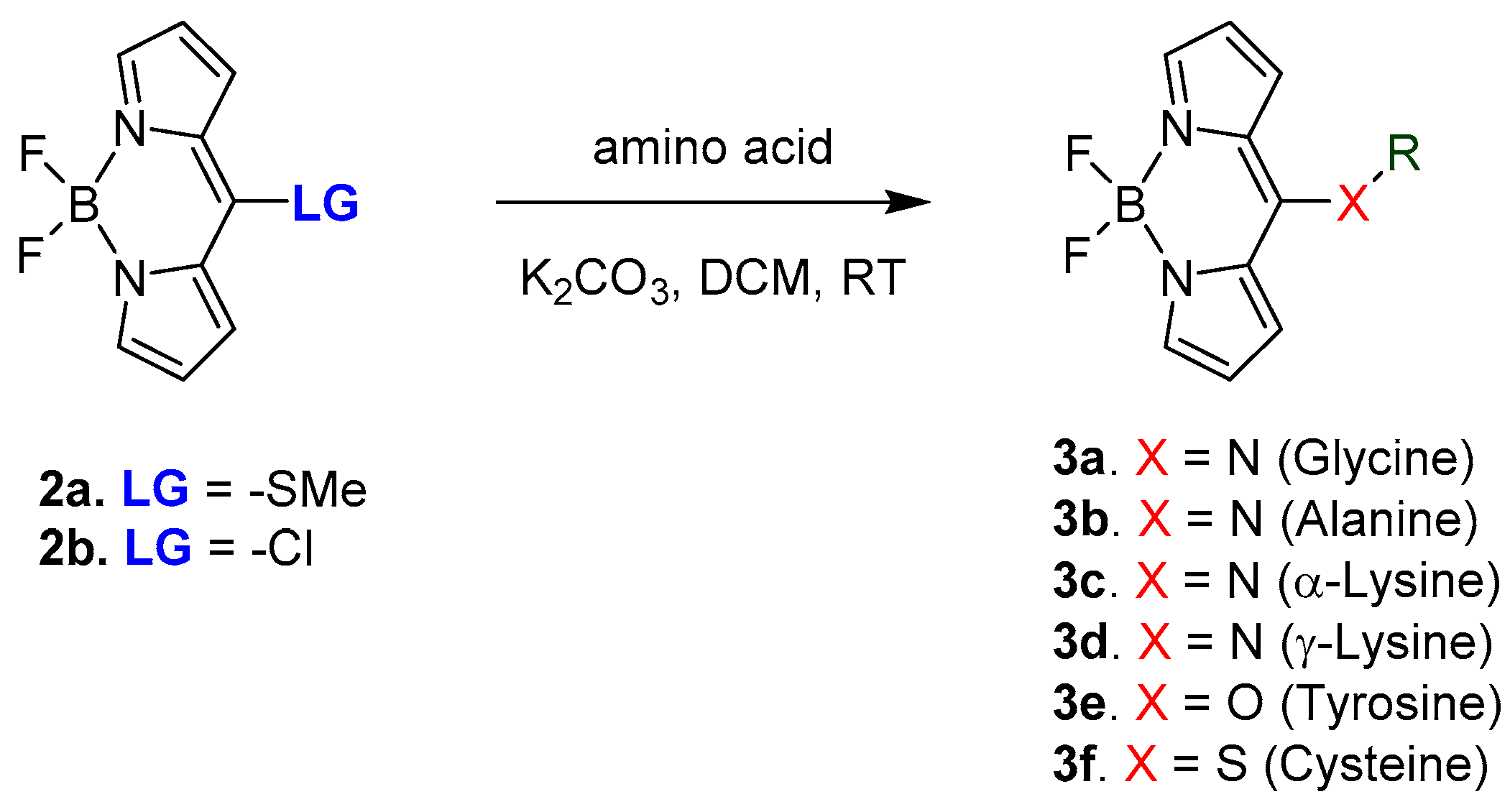
2.1. Covalent Interaction (Recognition)
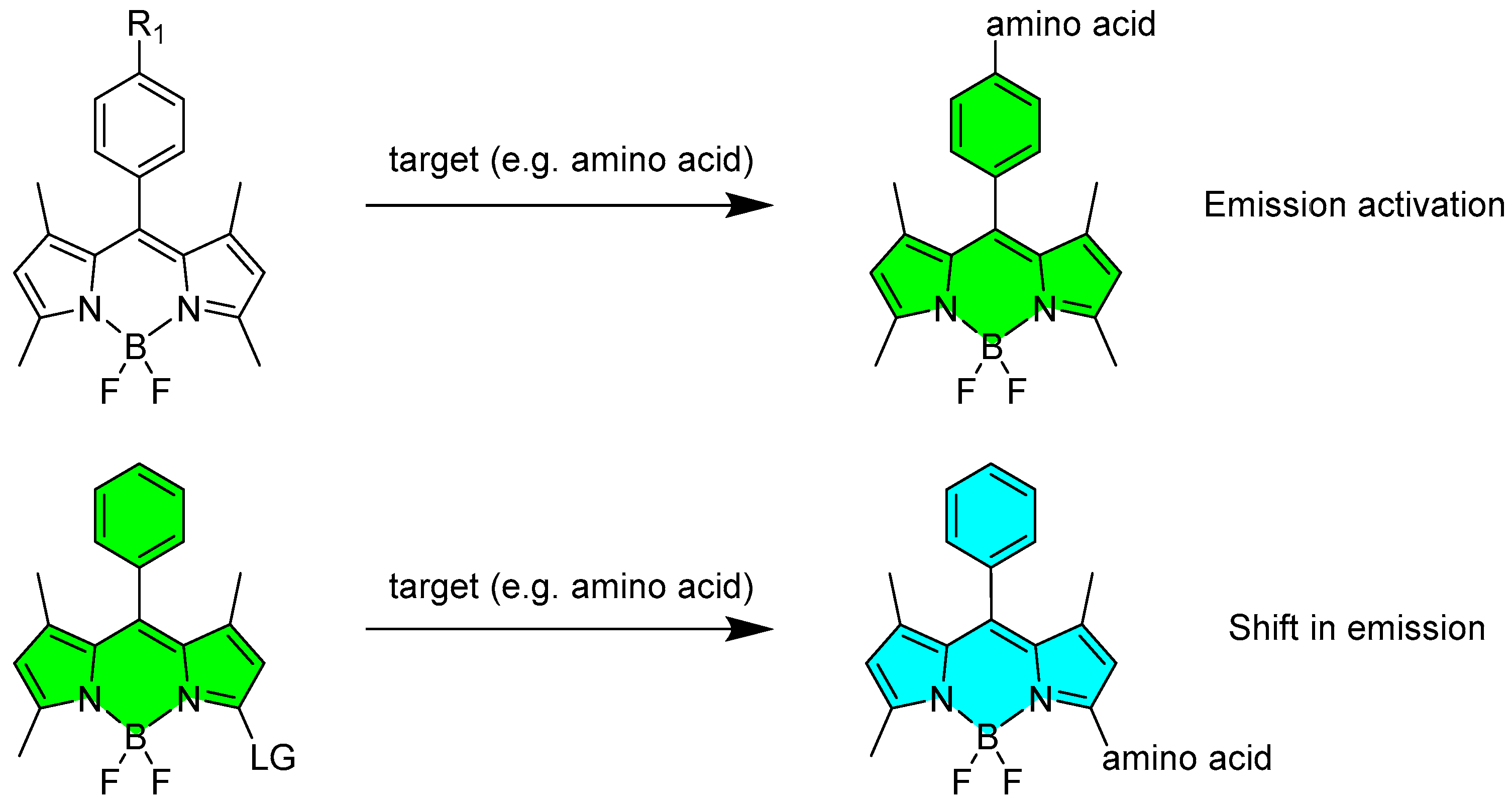
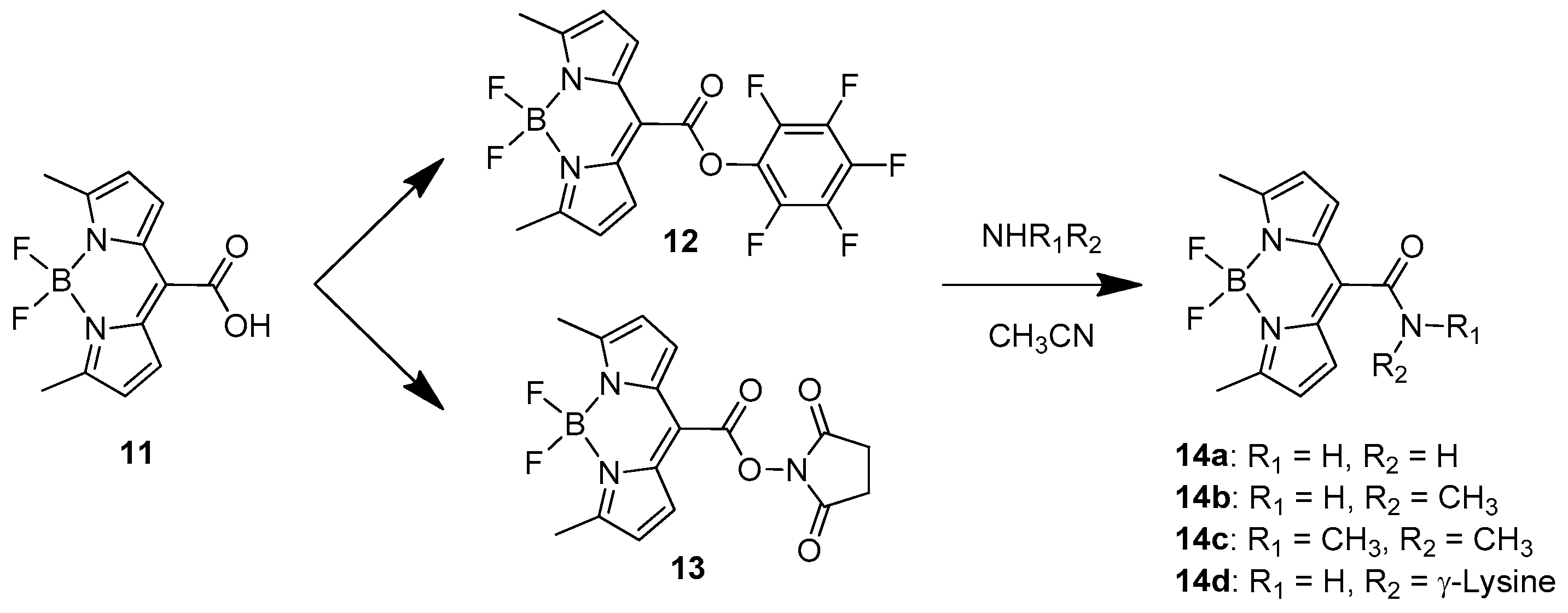
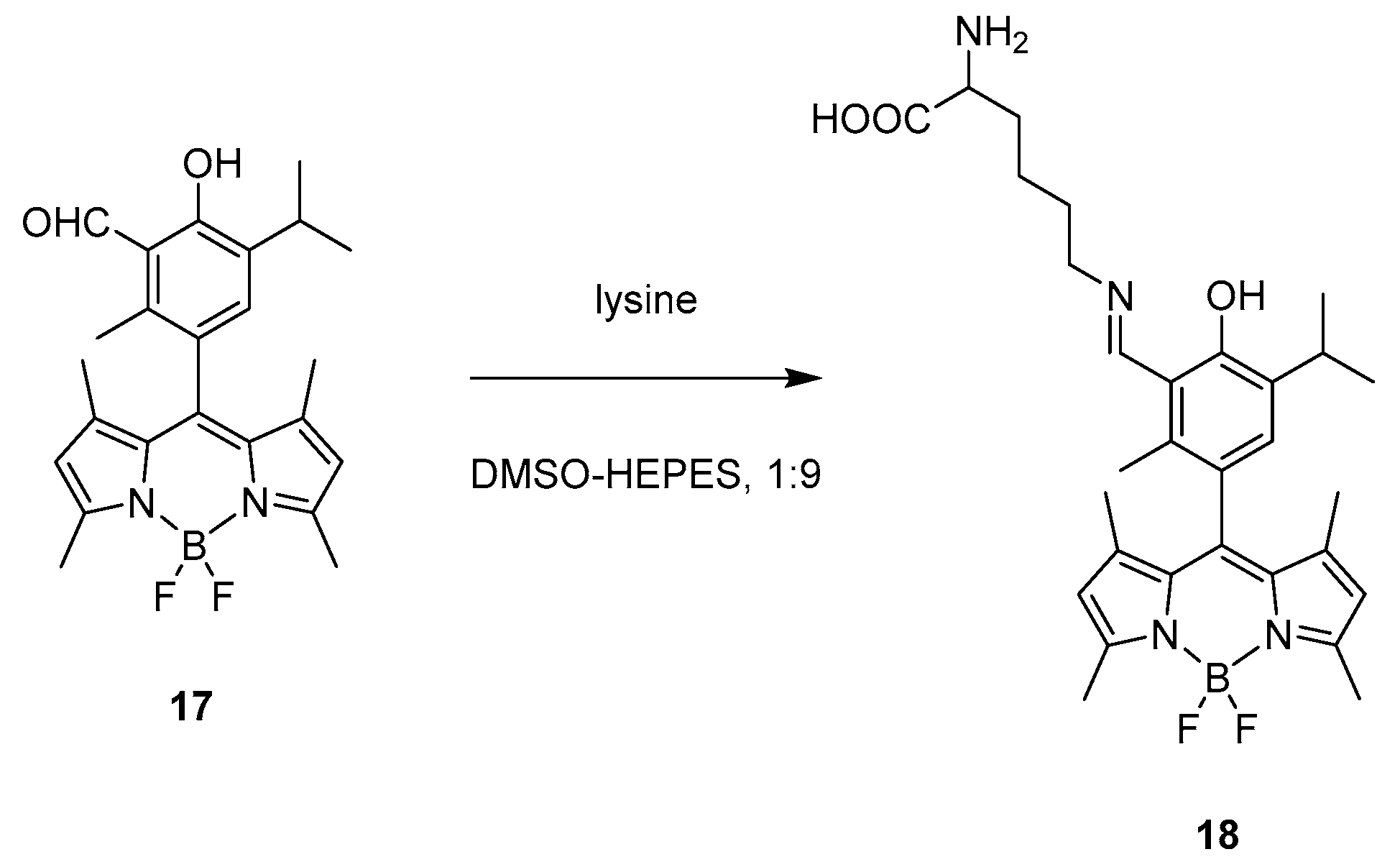
2.1.1. Emission Triggering
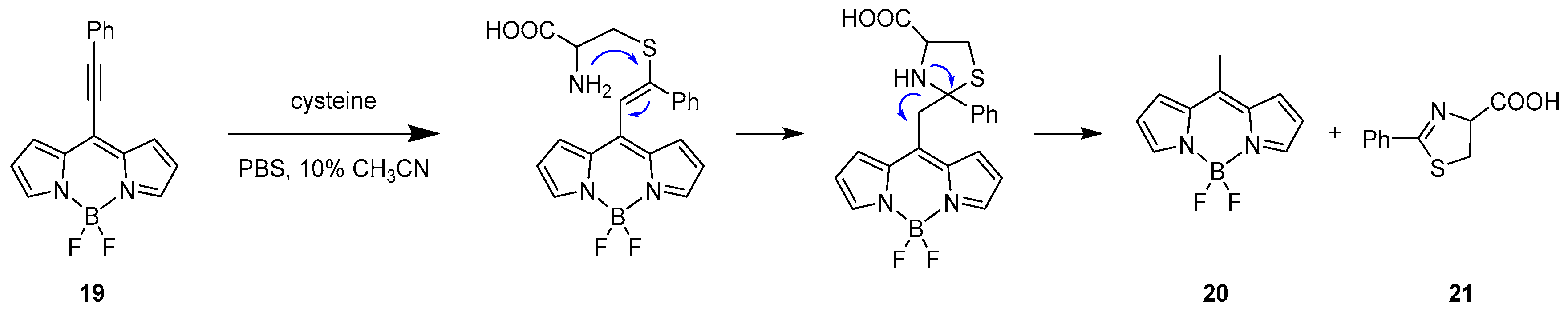
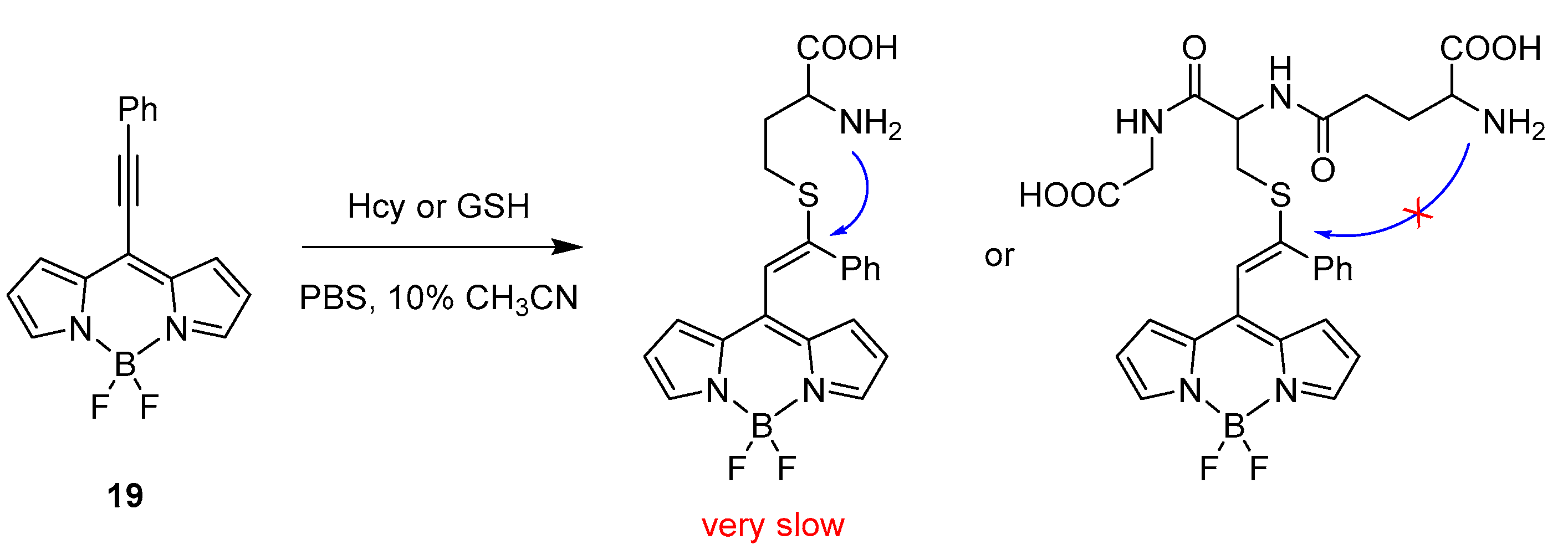
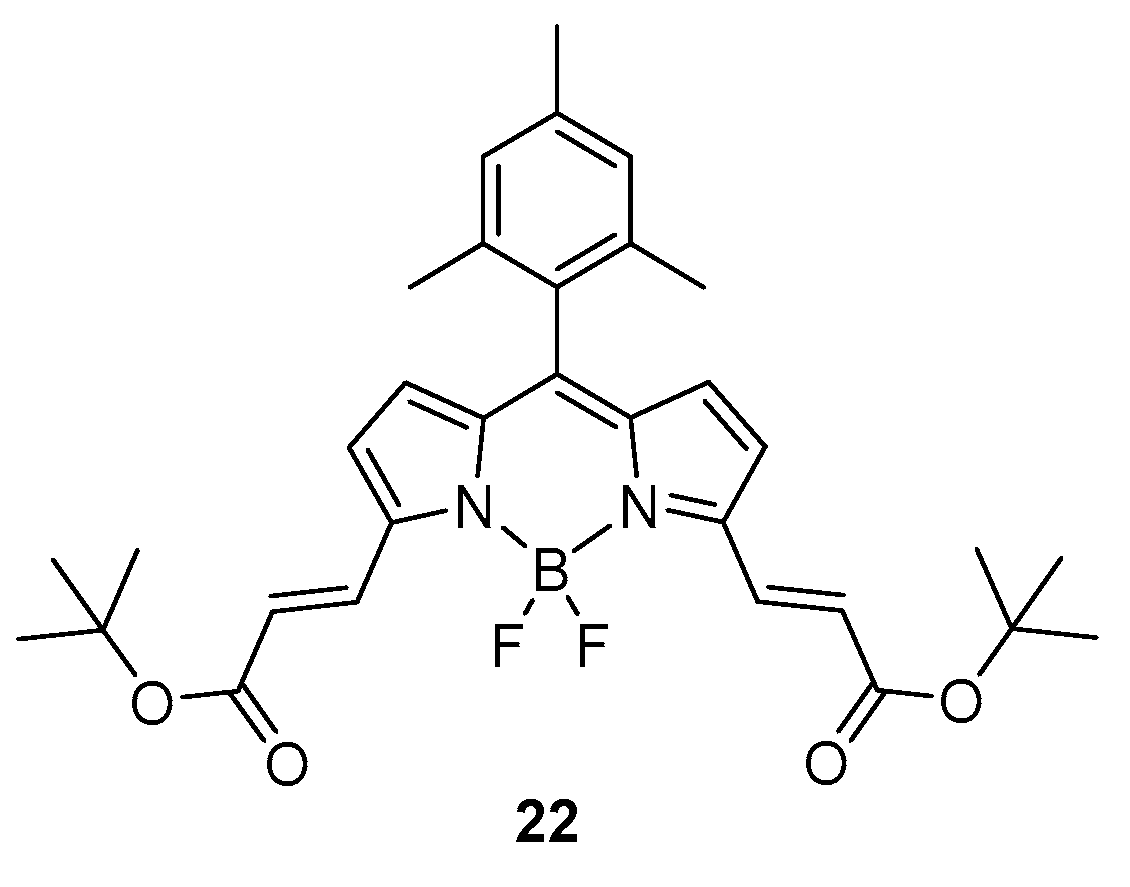
2.1.2. Emission Shift
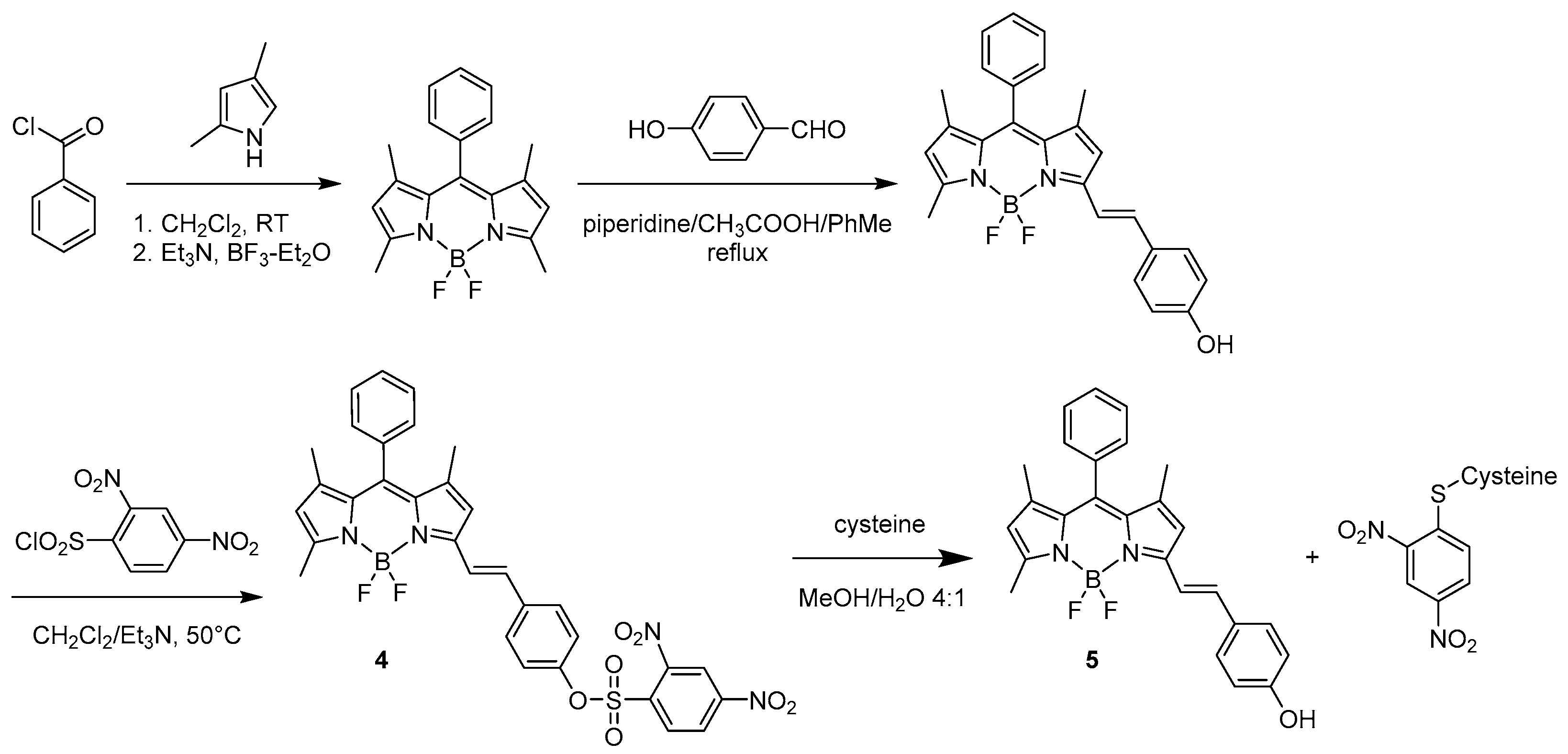
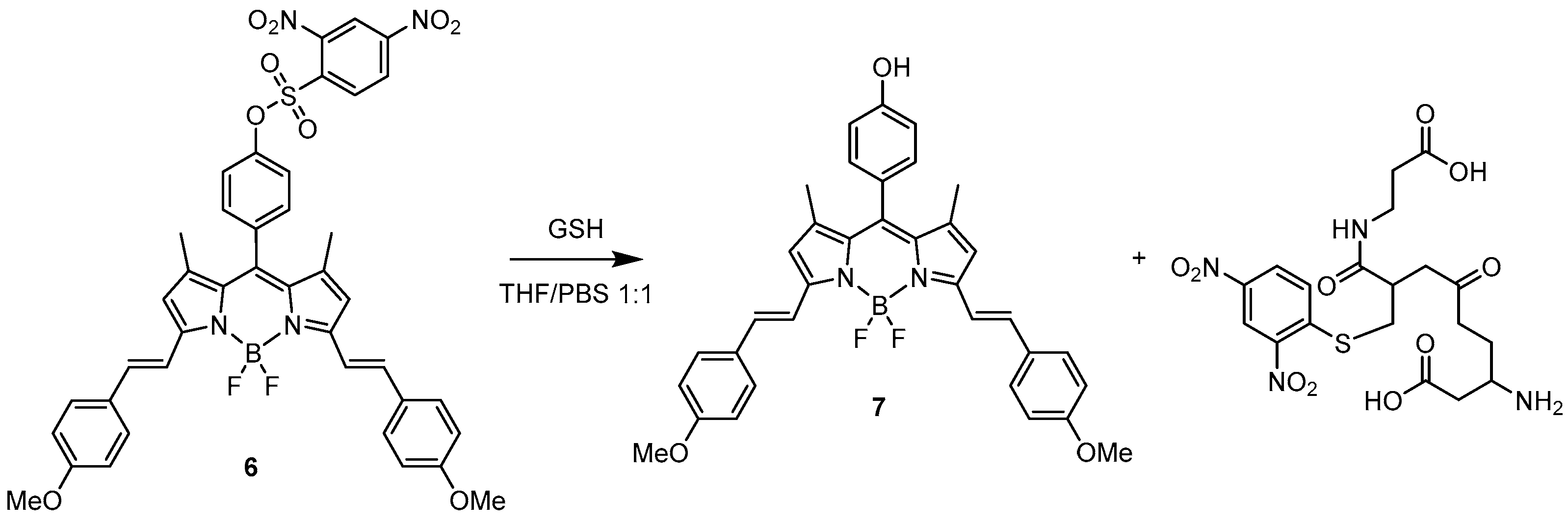
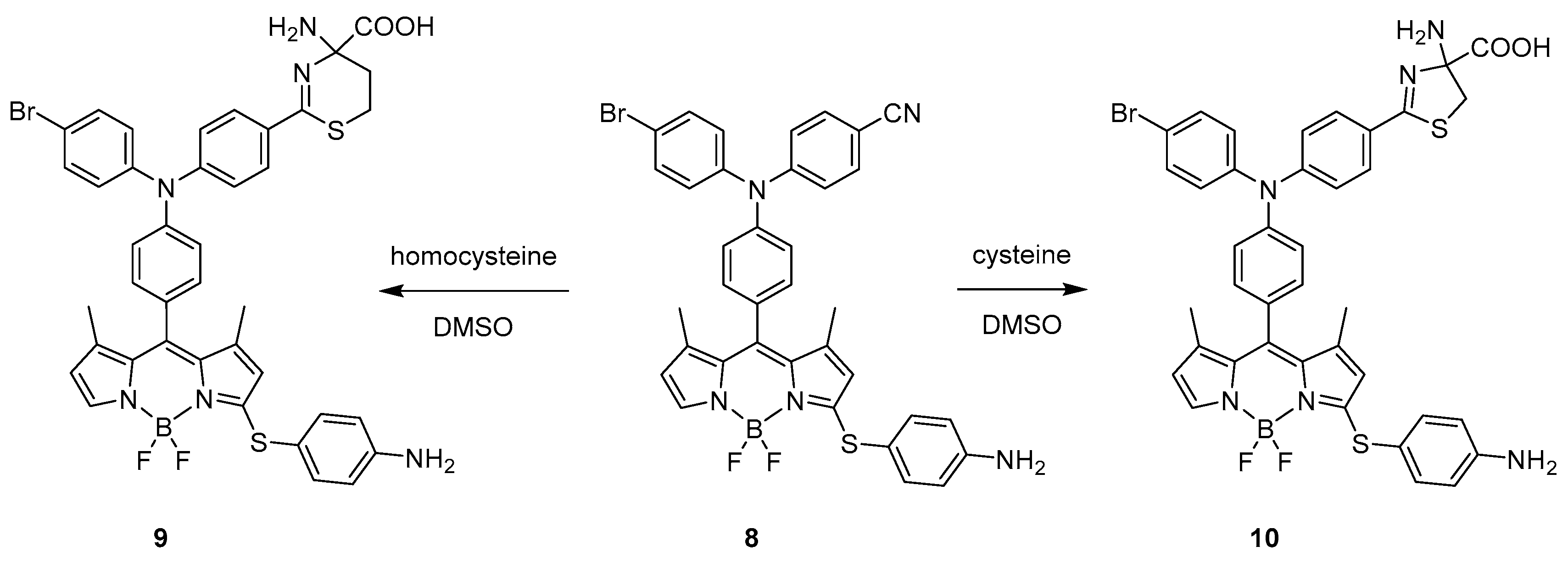
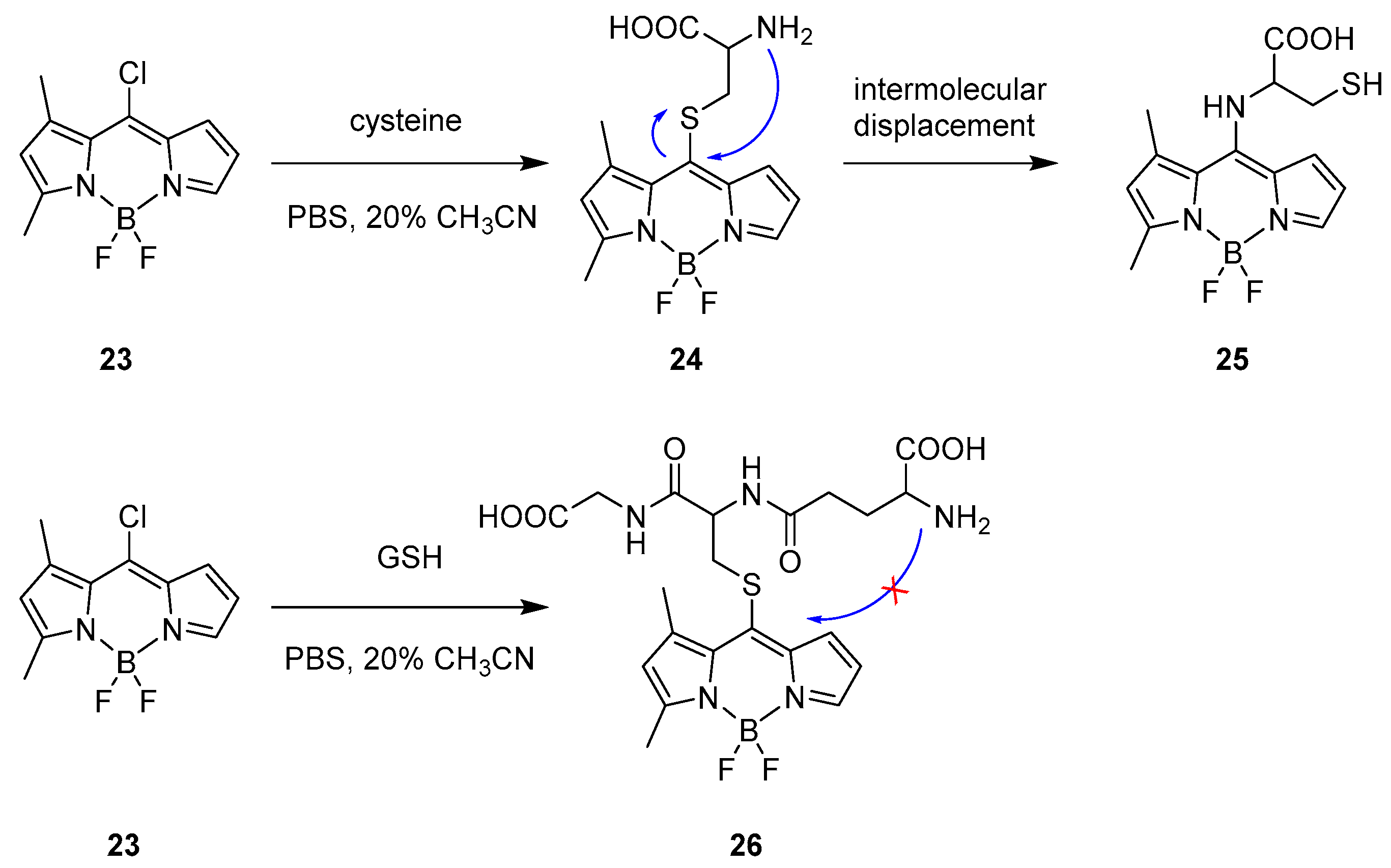
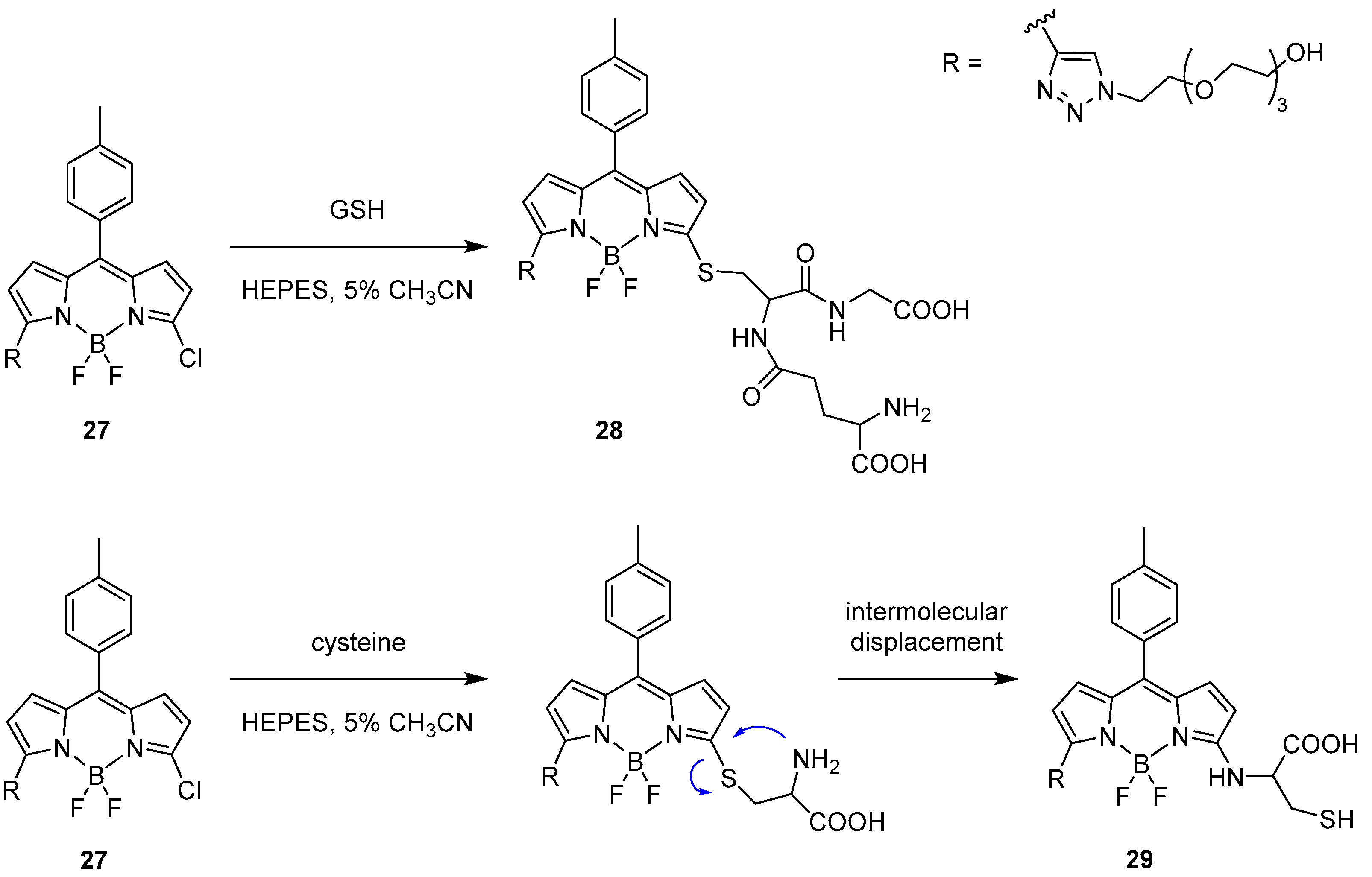
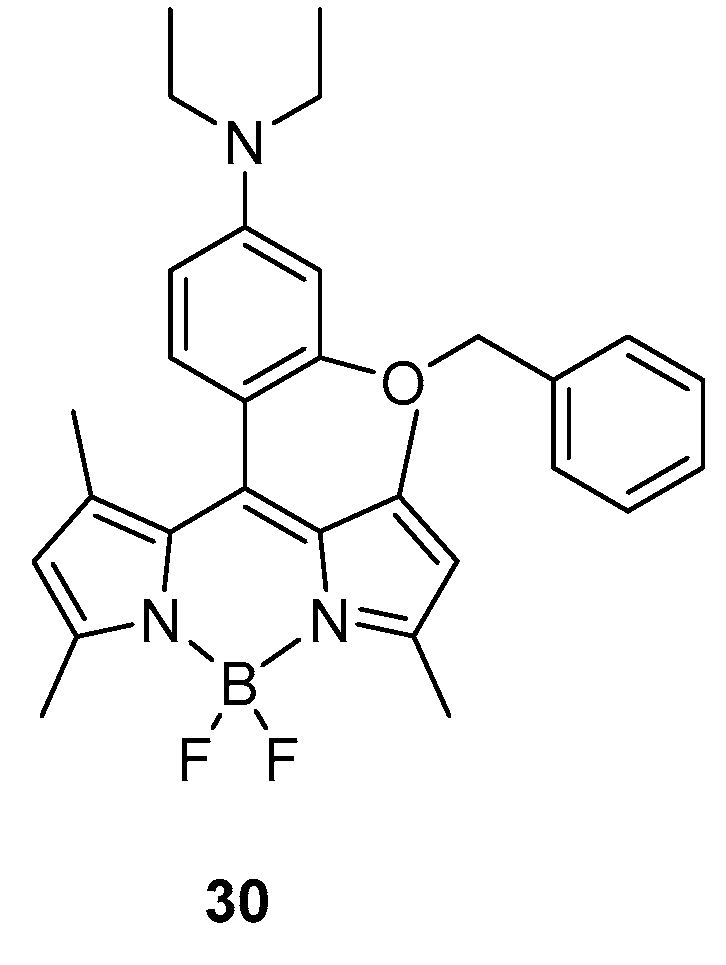
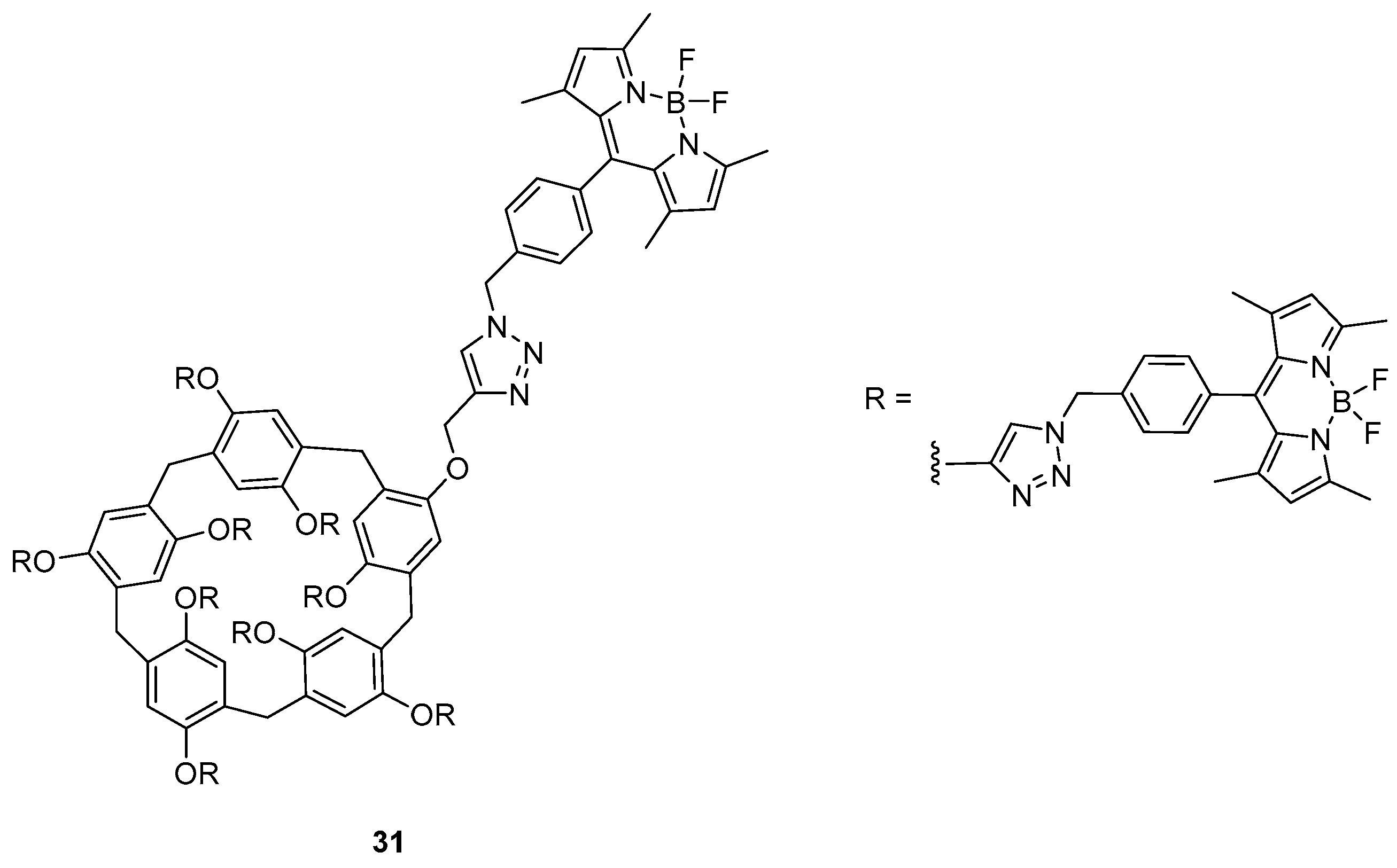
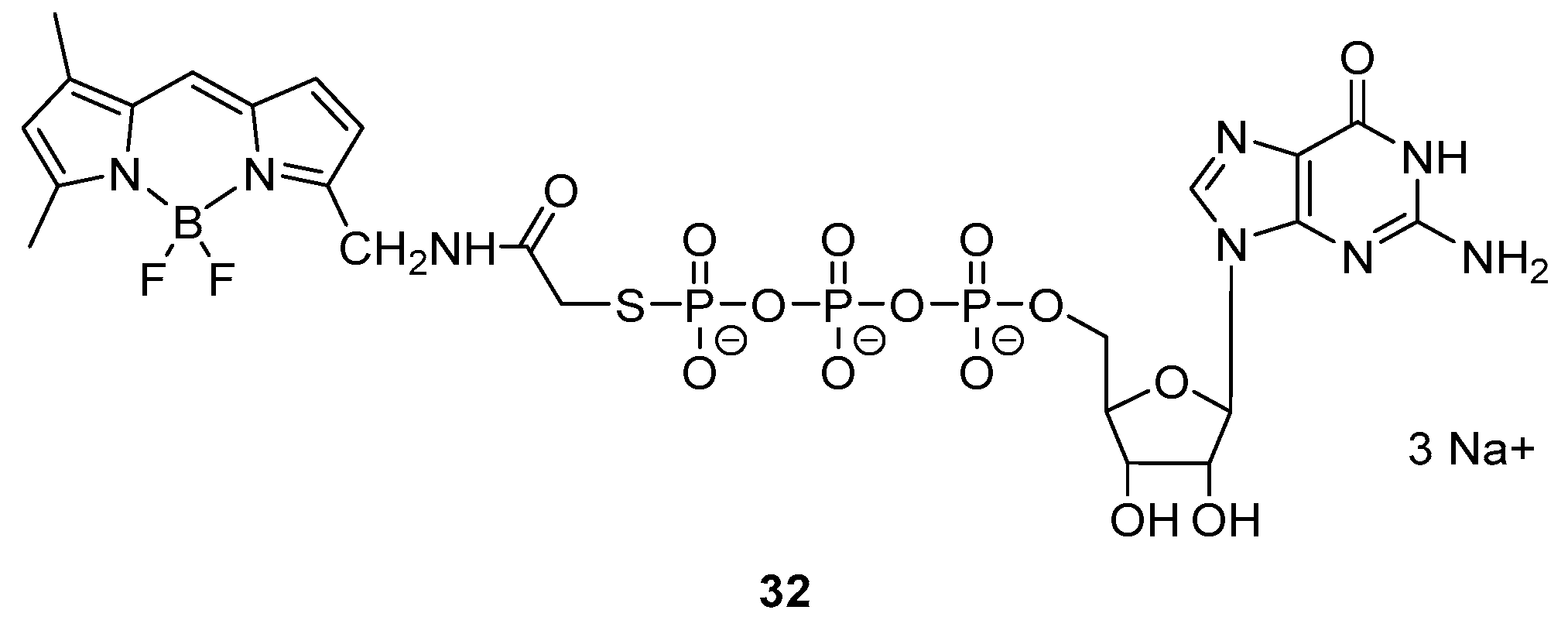
Intermolecular Displacement
2.2. Molecular Recognition (Non-Covalent)
3. Porphyrin Probes
3.1. Monomeric Hosts
3.1.1. Early Reports
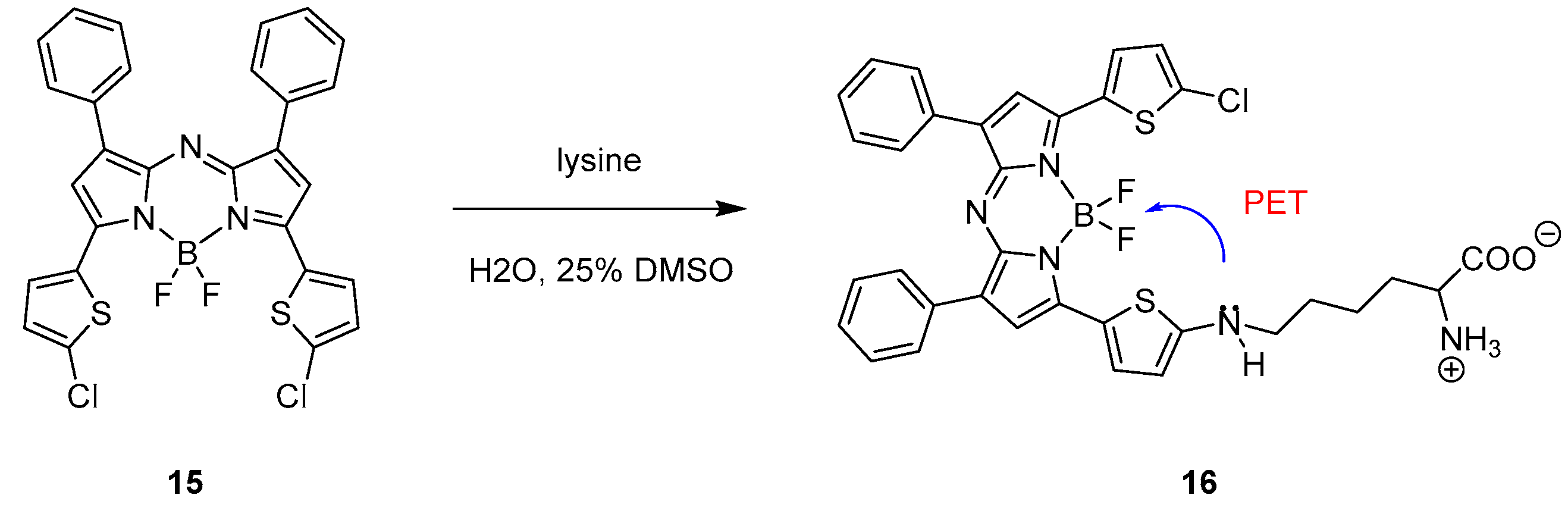
3.1.2. Achiral Hosts
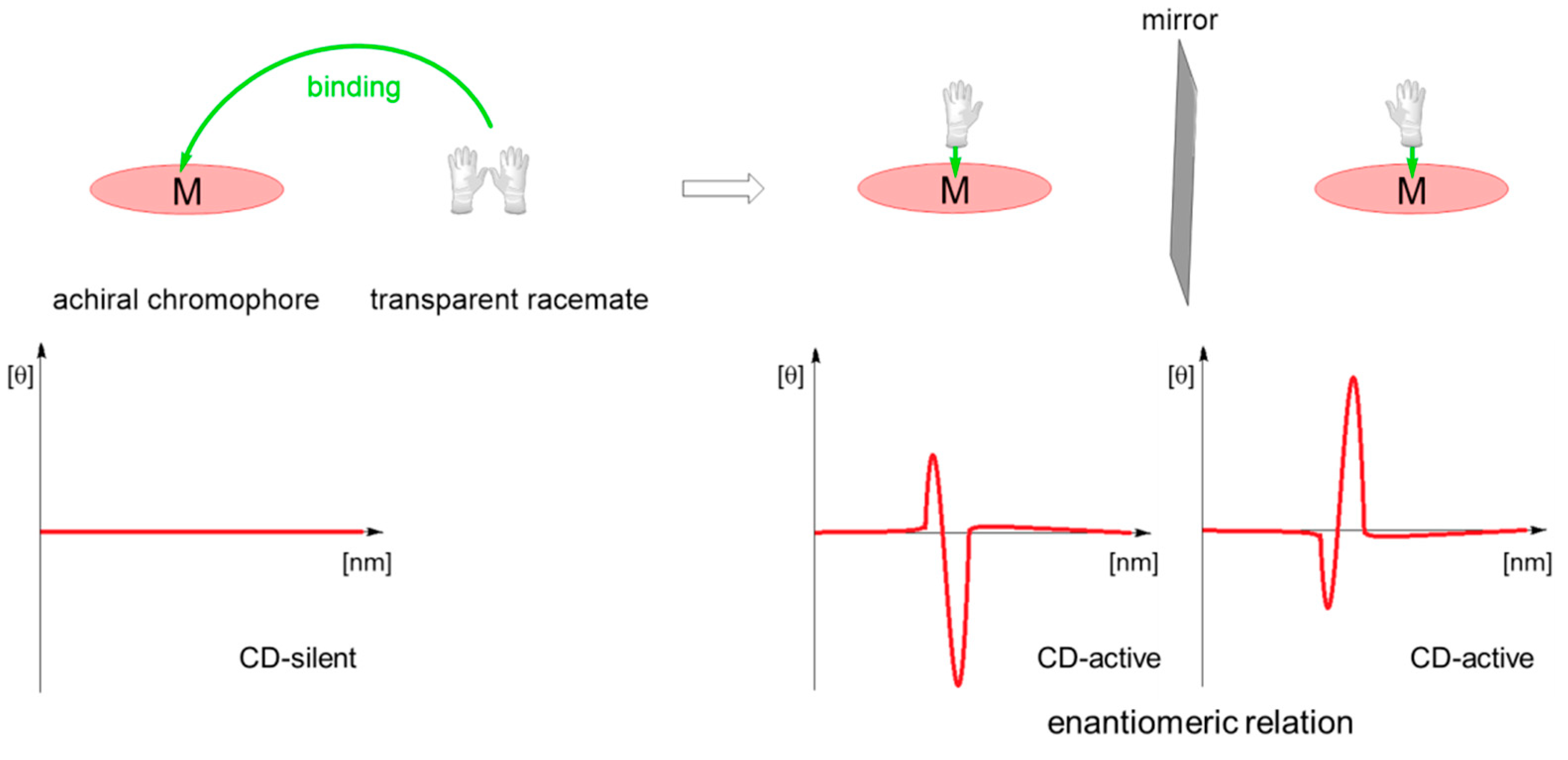
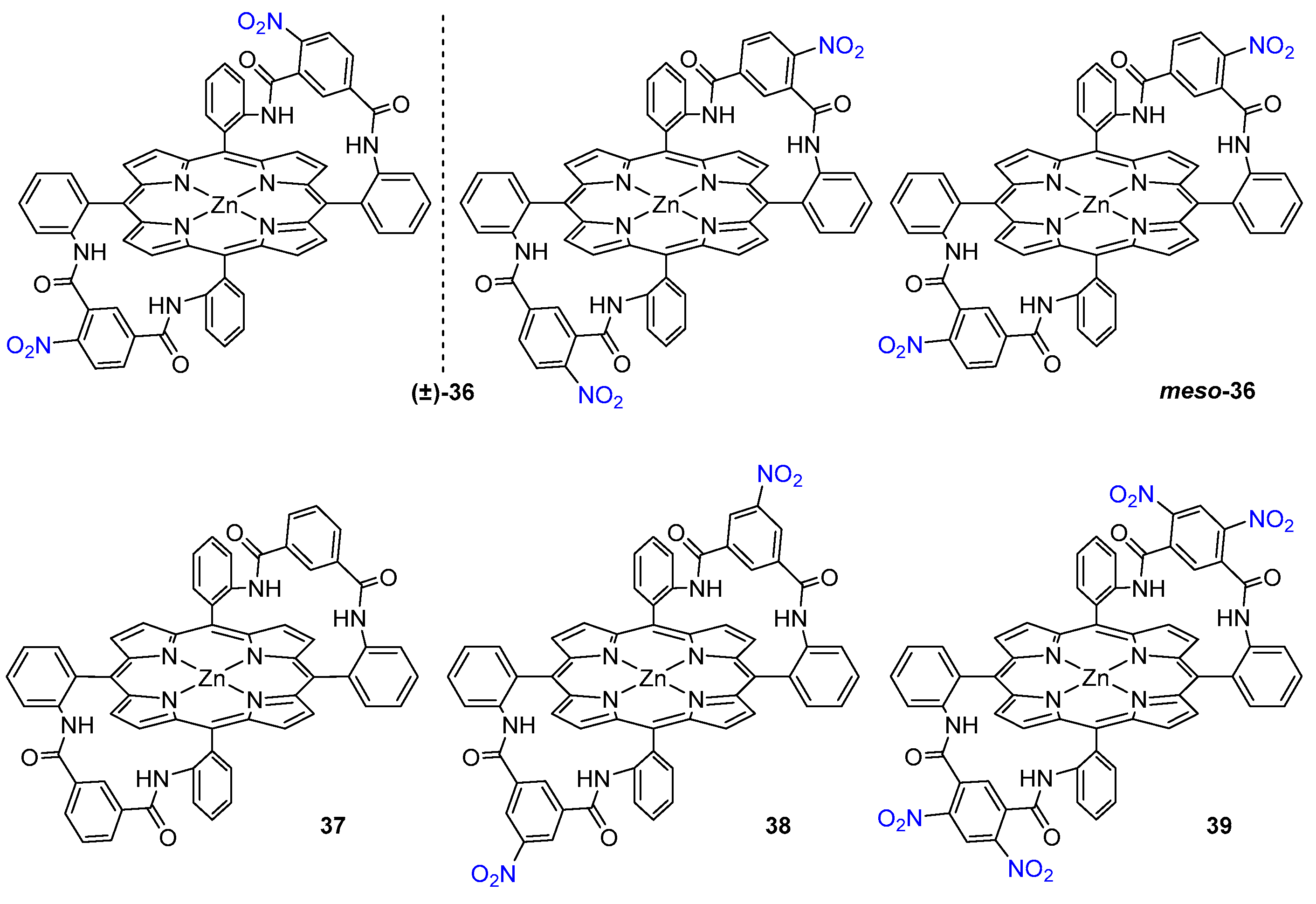
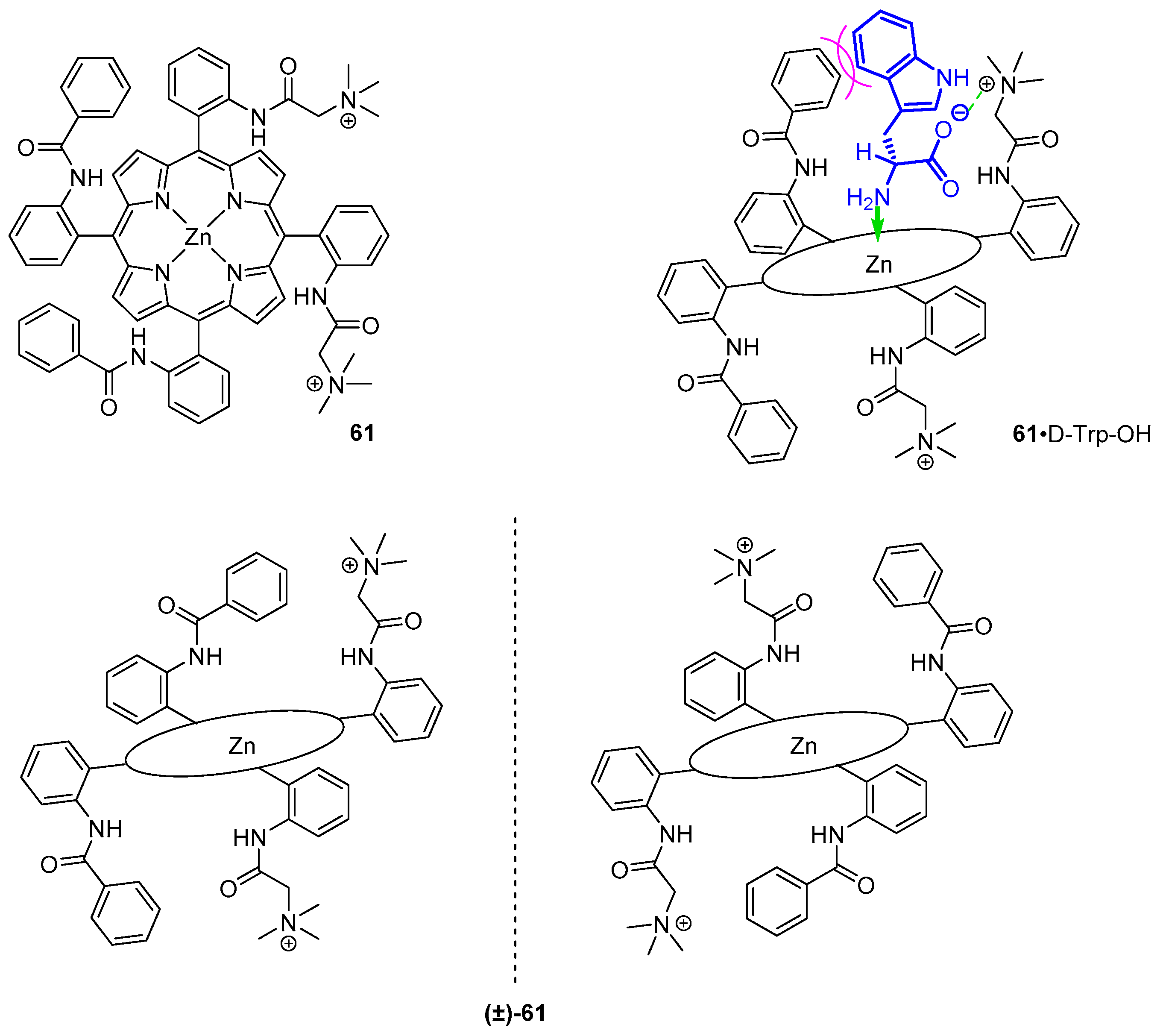
3.1.3. Chiral Hosts
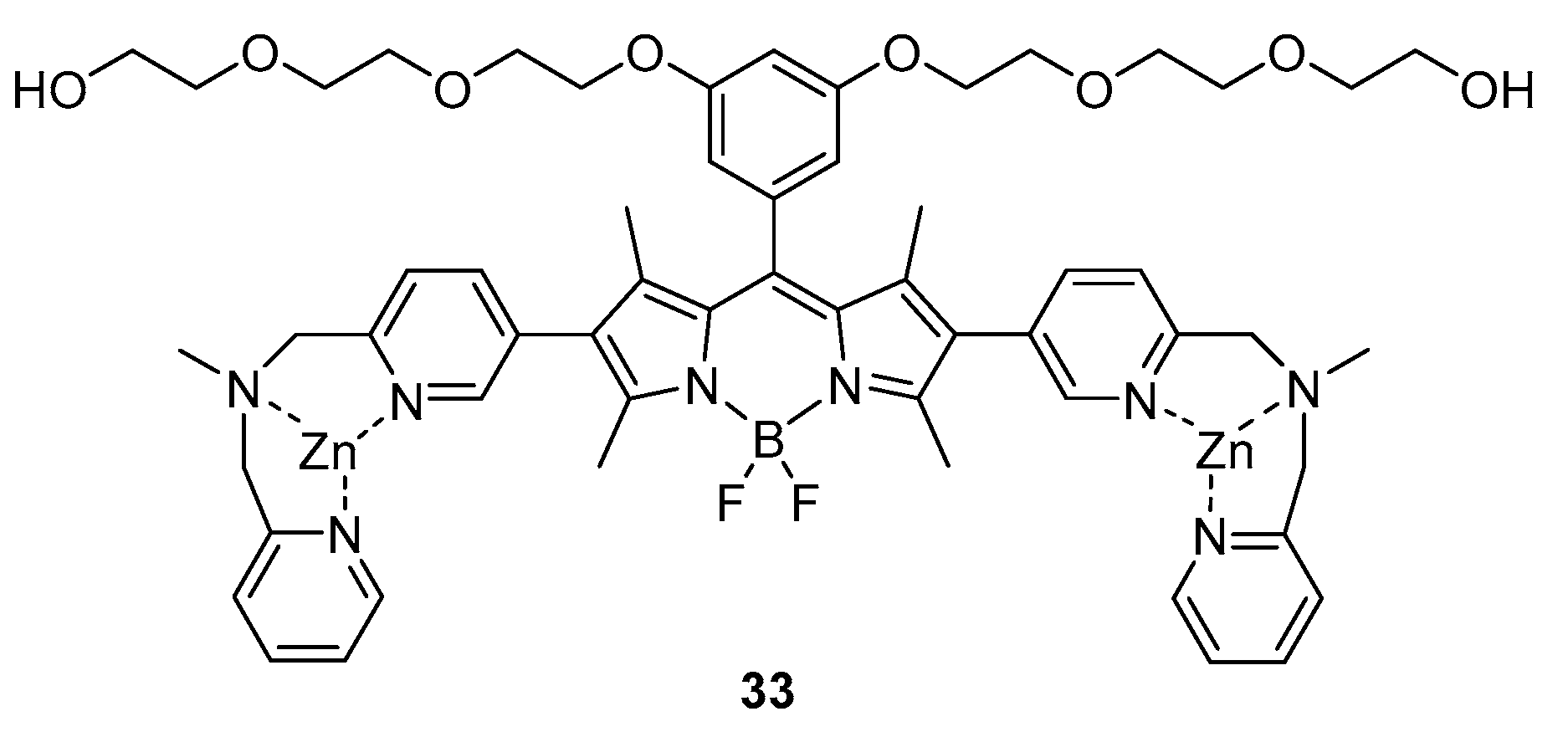
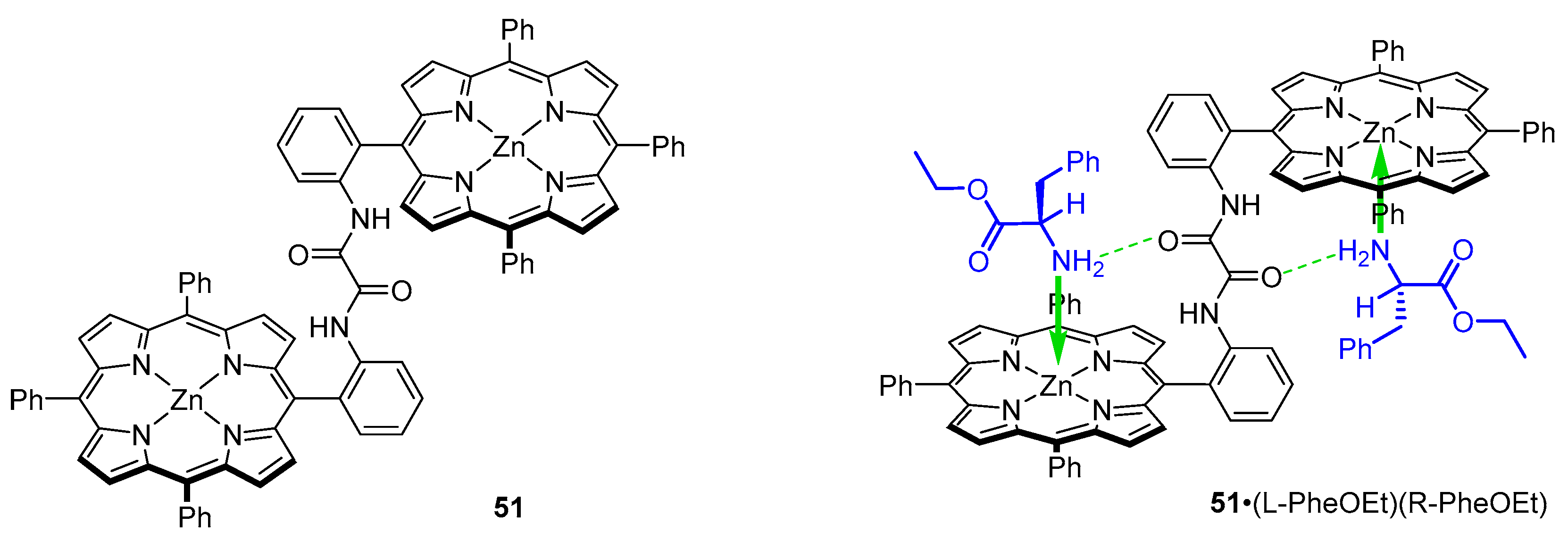
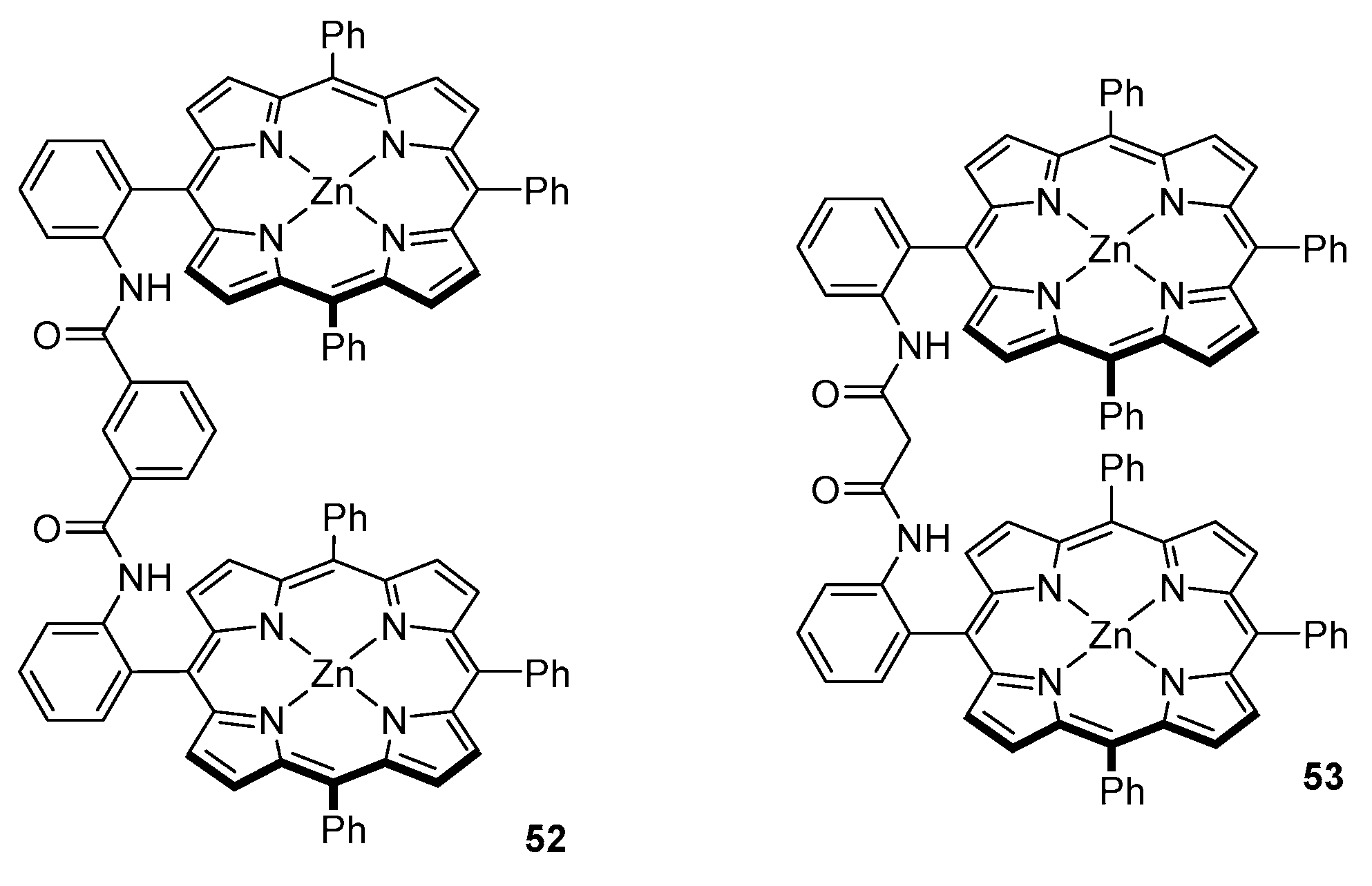
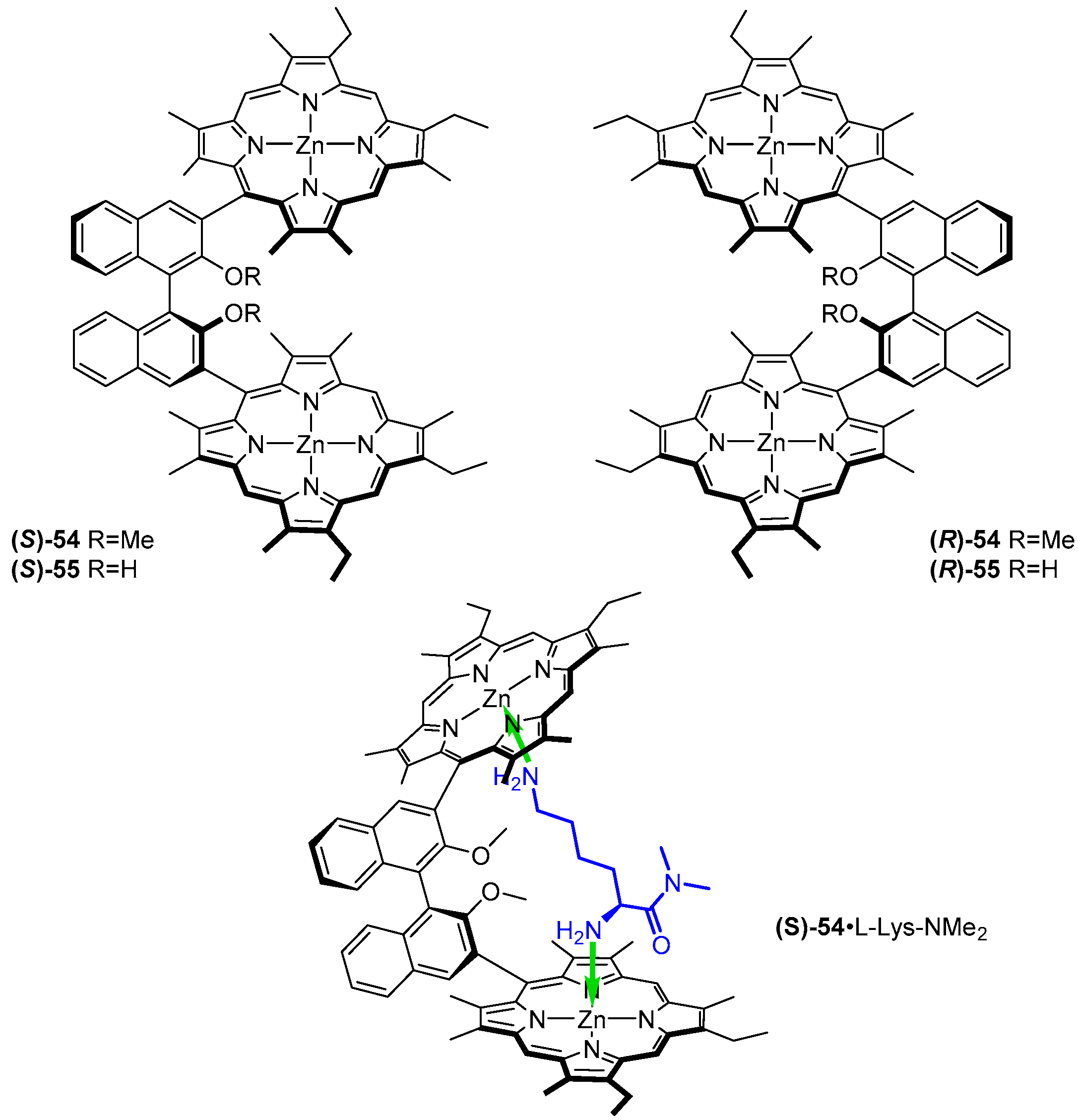
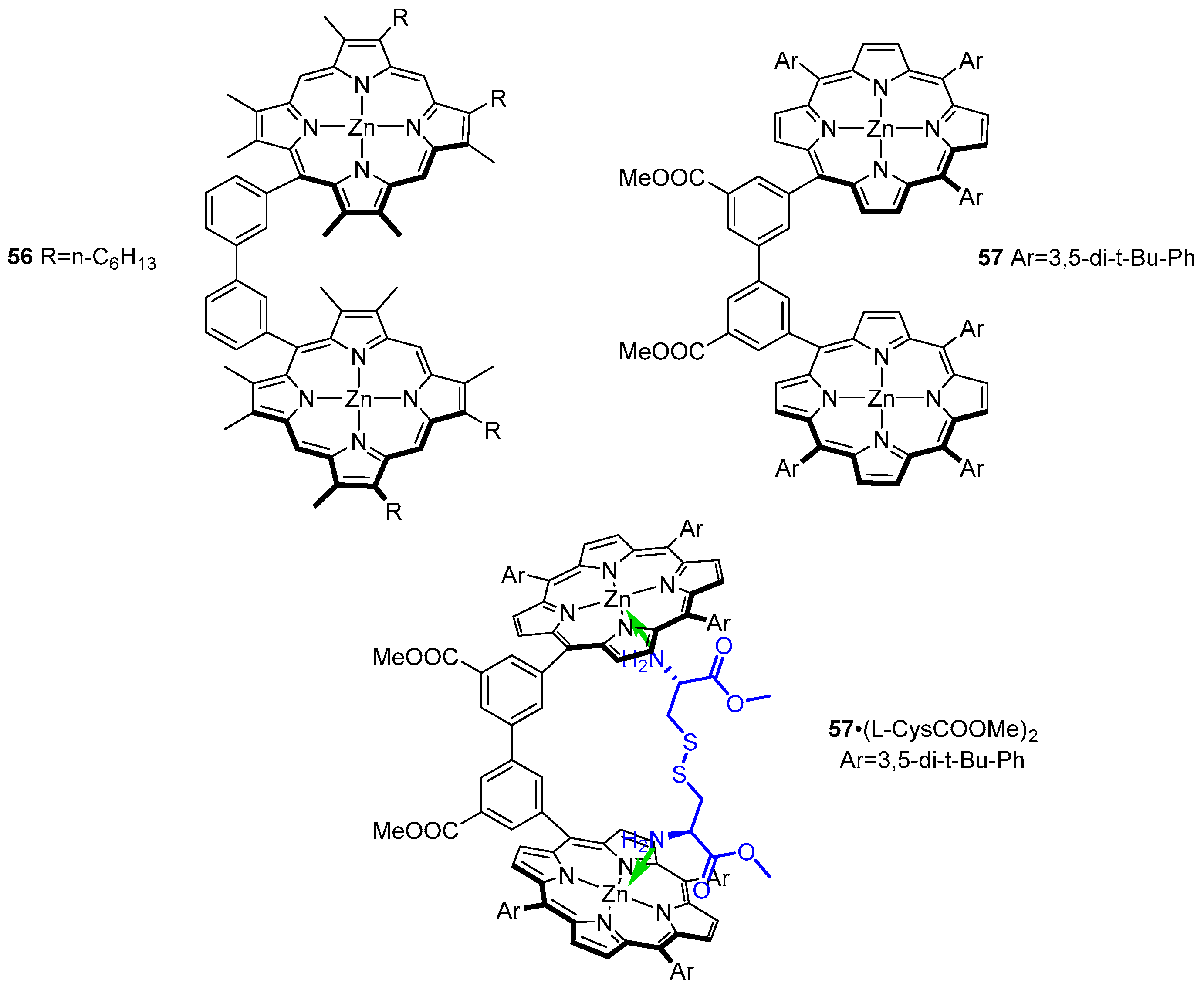
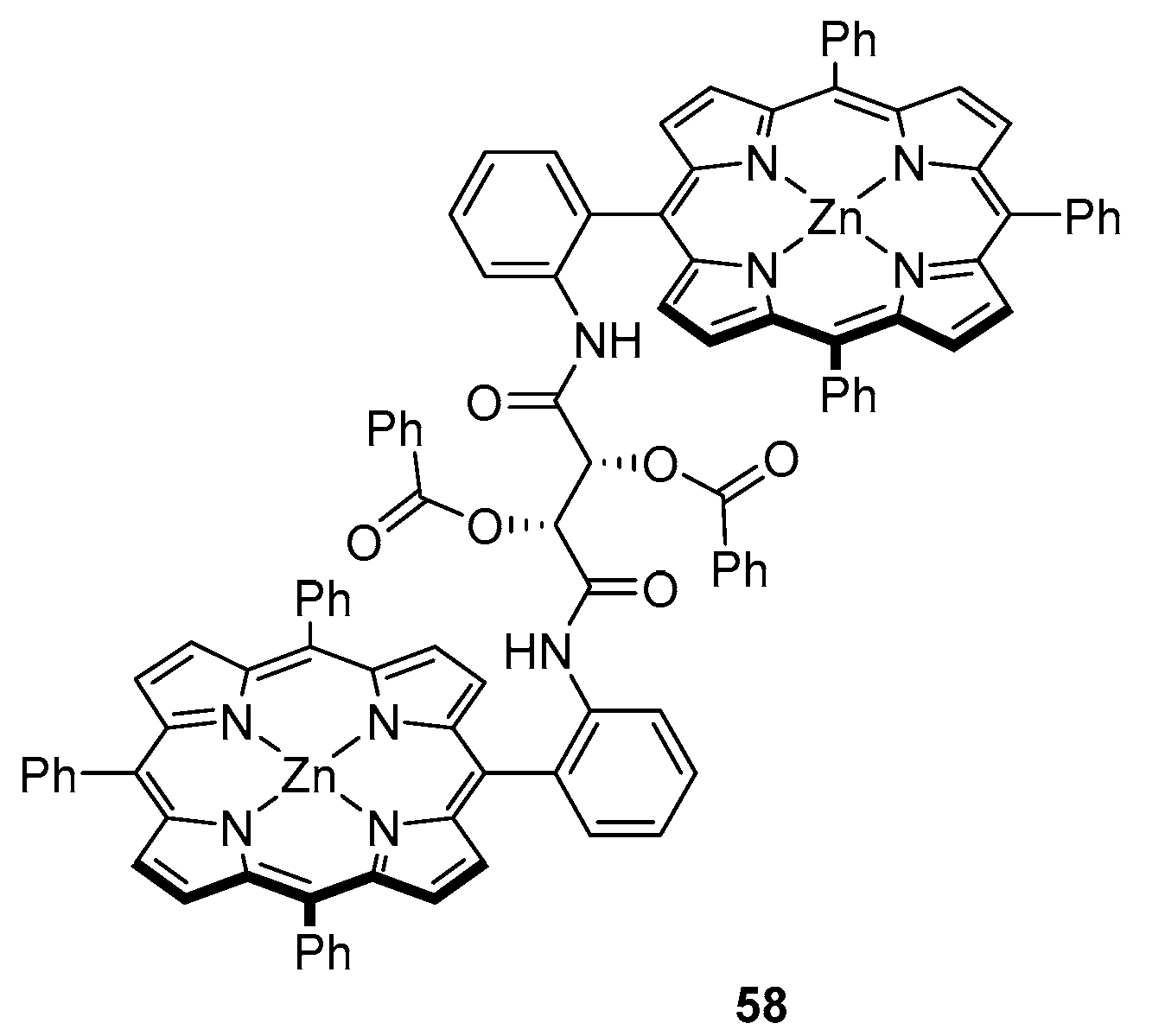
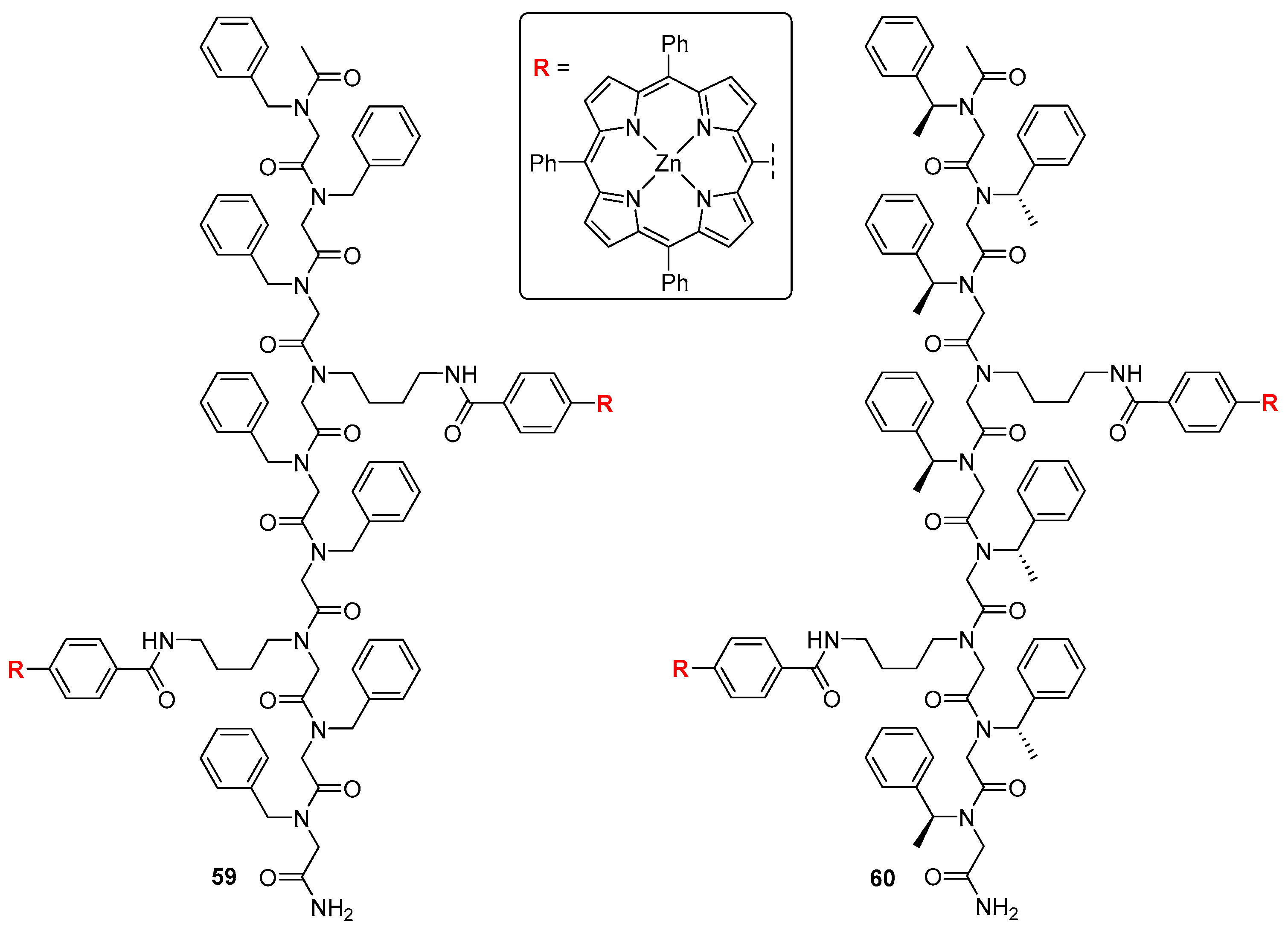
3.2. Dimeric Hosts
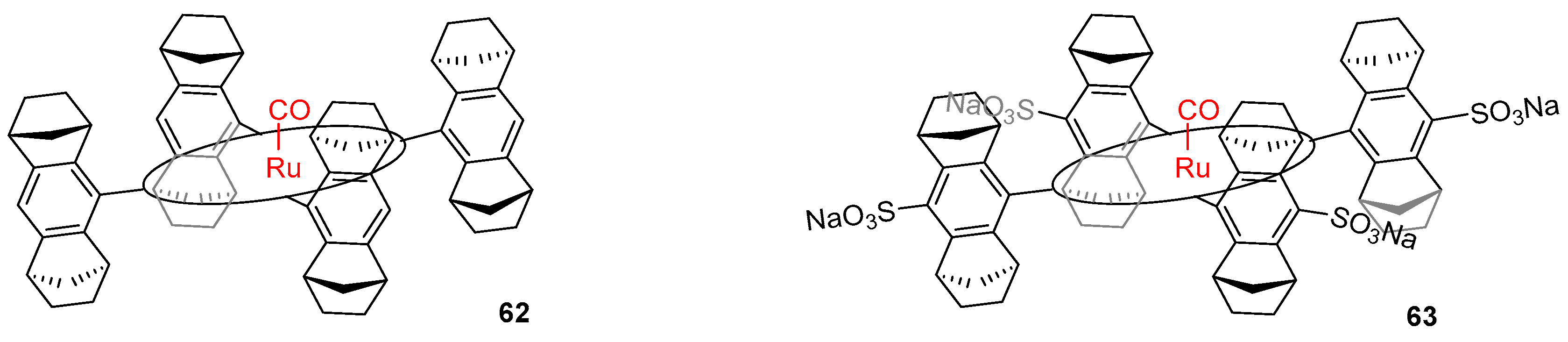
3.3. Aqueous Phase
4. Conclusions
Funding
Conflicts of Interest
References
- Persch, E.; Dumele, O.; Diederich, F. Molecular Recognition in Chemical and Biological Systems. Angew. Chem. Int. Ed. 2015, 54, 3290–3327. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; McCammon, J.A. Molecular Recognition and Ligand Association. Annu. Rev. Phys. Chem. 2013, 64, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ito, H.; Hillab, J.P.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kaneko, Y.; Nishibori, E.; Nabeshima, T. Molecular Recognition by Multiple Metal Coordination inside Wavy-Stacked Macrocycles. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Fan, E.; Van Arman, S.A.; Kincaid, S.; Hamilton, A.D. Molecular Recognition: Hydrogen-Bonding Receptors That Function in Highly Competitive Solvents. J. Am. Chem. Soc. 1993, 115, 369–370. [Google Scholar] [CrossRef]
- Yao, H.; Ke, H.; Zhang, X.; Pan, S.-J.; Li, M.-S.; Yang, L.-P.; Schreckenbach, G.; Jiang, W. Molecular Recognition of Hydrophilic Molecules in Water by Combining the Hydrophobic Effect with Hydrogen Bonding. J. Am. Chem. Soc. 2018, 140, 13466–13477. [Google Scholar] [CrossRef]
- Yang, L.; Adam, C.; Nichol, G.S.; Cockroft, S.L. How Much Do van Der Waals Dispersion Forces Contribute to Molecular Recognition in Solution? Nat. Chem. 2013, 5, 1006–1010. [Google Scholar] [CrossRef]
- Muehldorf, A.V.; Van Engen, D.; Warner, J.C.; Hamilton, A.D. Aromatic-Aromatic Interactions in Molecular Recognition: A Family of Artificial Receptors for Thymine That Shows both Face-to-Face and Edge-to-Face Orientations. J. Am. Chem. Soc. 1988, 110, 6561–6562. [Google Scholar] [CrossRef]
- Crowley, J.D.; Goshe, A.J.; Bosnich, B. Molecular Recognition. Electrostatic Effects in Supramolecular Self-Assembly. Chem. Commun. 2003, 9, 392–393. [Google Scholar] [CrossRef]
- Hosoowi, L.; Hong, K.I.; Jang, W.D. Design and Applications of Molecular Probes Containing Porphyrin Derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Imai, H.; Munakata, H.; Uemori, Y.; Sakura, N. Chiral Recognition of Amino Acids and Dipeptides by a Water-Soluble Zinc Porphyrin. Inorg. Chem. 2004, 43, 1211–1213. [Google Scholar] [CrossRef]
- Randazzo, R.; Gaeta, M.; Gangemi, C.M.A.; Fragalà, M.E.; Purrello, R.; D’Urso, A. Chiral Recognition of l- and d- Amino Acid by Porphyrin Supramolecular Aggregates. Molecules 2019, 24, 84. [Google Scholar] [CrossRef]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and Metalloporphyrins: Versatile Circular Dichroic Reporter Groups for Structural Studies. Chirality 2000, 12, 237–255. [Google Scholar] [CrossRef]
- Davis, S.J.; Ikemizu, S.; Evans, E.J.; Fugger, L.; Bakker, T.R.; van der Merwe, P.A. The Nature of Molecular Recognition by T Cells. Nat. Immunol. 2003, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.D.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteine in proteomes. Nature 2010, 468, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Zhouab, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 2012, 41, 52–67. [Google Scholar] [CrossRef]
- Cheng, Z.; Kuru, E.; Sachdeva, A.; Vendrell, M. Fluorescent amino acids as versatile building blocks for chemical biology. Nat. Rev. Chem. 2020, 4, 275–290. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Jameson, E.E.; Cunliffe, J.M.; Neubig, R.R.; Sunahara, R.K.; Kennedy, R.T. Detection of G Proteins by Affinity Probe Capillary Electrophoresis Using a Fluorescently Labeled GTP Analogue. Anal. Chem. 2003, 75, 4297–4304. [Google Scholar] [CrossRef]
- Ojida, A.; Sakamoto, T.; Inoue, M.; Fujishima, S.; Lippens, G.; Hamachi, I. Fluorescent BODIPY-Based Zn(II) Complex as a Molecular Probe for Selective Detection of Neurofibrillary Tangles in the Brains of Alzheimer’s Disease Patients. J. Am. Chem. Soc. 2009, 131, 6543–6548. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zaho, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef] [PubMed]
- Farinone, M.; Cybińska, J.; Pawlicki, M. A Controlled Blue-Shift in Meso-Nitrogen Aryl Fused DIPY and BODIPY Skeletons. Org. Chem. Front. 2019, 6, 2825–2832. [Google Scholar] [CrossRef]
- Farinone, M.; Cybinska, J.; Pawlicki, M. BODIPY-Amino Acid Conjugates—Tuning the Optical Response with a Meso-Heteroatom. Org. Chem. Front. 2020, 7, 2391–2398. [Google Scholar] [CrossRef]
- Sørensen, M.L.H.; Vosch, T.; Laursen, B.W.; Hansen, T. Spectral Shifts of BODIPY Derivatives: A Simple Continuous Model. Photochem. Photobiol. Sci. 2019, 18, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Prasannan, D.; Arunkumar, C. A “Turn-on-and-off” PH Sensitive BODIPY Fluorescent Probe for Imaging E. Coli Cells. New J. Chem. 2018, 42, 3473–3482. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Bueschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the Development of Sensor Molecules that Display FeIII-amplified Fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar] [CrossRef]
- Zhanga, J.; Pana, F.; Jina, Y.; Wanga, N.; Hea, J.; Zhanga, W.; Zhao, W. A BODIPY-based dual-responsive turn-on fluorescent probe for NO and nitrite. Dyes Pigment. 2018, 155, 276–283. [Google Scholar] [CrossRef]
- Shao, J.; Guo, H.; Ji, S.; Zhao, J. Styryl-BODIPY Based Red-Emitting Fluorescent OFF–ON Molecular Probe for Specific Detection of Cysteine. Biosens. Bioelectron. 2011, 26, 3012–3017. [Google Scholar] [CrossRef]
- Wang, C.; Xia, X.; Luo, J.; Qian, Y. A Novel Near-Infrared Styryl-BODIPY Fluorescent Probe for Discrimination of GSH and Its Application in Living Cells. Dyes Pigment. 2018, 152, 85–92. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X.; Li, C.; Xie, Y. A Novel P-Aminophenylthio- and Cyano- Substituted BODIPY as a Fluorescence Turn-on Probe for Distinguishing Cysteine and Homocysteine from Glutathione. Dyes Pigment. 2018, 148, 212–218. [Google Scholar] [CrossRef]
- Leen, V.; Yuan, P.; Wang, L.; Boens, N.; Dehaen, W. Synthesis of Meso-Halogenated BODIPYs and Access to Meso-Substituted Analogues. Org. Lett. 2012, 14, 6150–6153. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, T.; Jin, H.; Lee, U.; Bae, J.; Bouffard, J.; Kim, Y. Amine-Reactive Activated Esters of Meso-CarboxyBODIPY: Fluorogenic Assays and Labeling of Amines, Amino Acids, and Proteins. J. Am. Chem. Soc. 2020, 142, 9231–9239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yue, S.; Jia, L.; Li, S.; Li, C.; Li, Q.; Xiao, L. NIR Fluorescent AzaBODIPY-Based Probe for the Specific Detection of l-Lysine. Chem. Sel. 2018, 3, 7581–7585. [Google Scholar] [CrossRef]
- Adhikari, S.; Ghosh, A.; Mandal, S.; Guria, S.; Banerjee, P.P.; Chatterjeec, A.; Das, D. Colorimetric and fluorescence probe for the detection of nano-molar lysine in aqueous medium. Org. Biomol. Chem. 2016, 14, 10688–10694. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Hou, M.; Shi, Y.; Guo, W. Selective Fluorescence Detection of Cysteine over Homocysteine and Glutathione Based on a Cysteine-Triggered Dual Michael Addition/Retro-Aza-Aldol Cascade Reaction. Anal. Chem. 2015, 87, 11475–11483. [Google Scholar] [CrossRef]
- Avellanal-Zaballa, E.; Ramos-Torres, Á.; Prieto-Castañeda, A.; García-Garrido, F.; Bañuelos, J.; Agarrabeitia, A.R.; Ortiz, M.J. A BODIPY-Based Fluorescent Sensor for Amino Acids Bearing Thiol. Proceedings 2019, 41, 18. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Gao, J.; Ji, X.; He, J.; Zhang, J.; Zhao, W. A Ratiometric Fluorescent BODIPY-Based Probe for Rapid and Highly Sensitive Detection of Cysteine in Human Plasma. Analyst 2018, 143, 5728–5735. [Google Scholar] [CrossRef]
- Niu, L.; Guan, Y.; Chen, Y.; Wu, L.; Tung, C.; Yang, Q. BODIPY-Based Ratiometric Fluorescent Sensor for Highly Selective Detection of Glutathione over Cysteine and Homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef]
- Guria, S.; Ghosh, A.; Manna, K.; Pal, A.; Adhikary, A.; Adhikari, S. Rapid detection of aspartic acid in water by BODIPY-Based fluorescent probe: Live-cell imaging and DFT studies. Dyes Pigment. 2019, 168, 111–122. [Google Scholar] [CrossRef]
- Bastug, E.; Kursunlu, A.N.; Guler, E. A fluorescent clever macrocycle: Deca-bodipy bearing a pillar [5]arene and its selective binding of asparagine in half-aqueous medium. J. Lumin 2020, 225, 117343. [Google Scholar] [CrossRef]
- Mizutani, T.; Wada, K.; Kitagawa, S. Molecular Recognition of Amines and Amino Esters by Zinc Porphyrin Receptors: Binding Mechanisms and Solvent Effects. J. Org. Chem. 2000, 65, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yotsukura, M.; Noji, M.; Takanami, T. Bis(Zinc Porphyrin) as a CD-Sensitive Bidentate Host Molecule: Direct Determination of Absolute Configuration of Mono-Alcohols. Chem. Commun. 2015, 51, 11068–11071. [Google Scholar] [CrossRef] [PubMed]
- Borovkov, V.V.; Lintuluoto, J.M.; Inoue, Y. Supramolecular Chirogenesis in Zinc Porphyrins: Mechanism, Role of Guest Structure, and Application for the Absolute Configuration Determination. J. Am. Chem. Soc. 2001, 123, 2979–2989. [Google Scholar] [CrossRef]
- Allenmark, S. Induced Circular Dichroism by Chiral Molecular Interaction. Chirality 2003, 15, 409–422. [Google Scholar] [CrossRef]
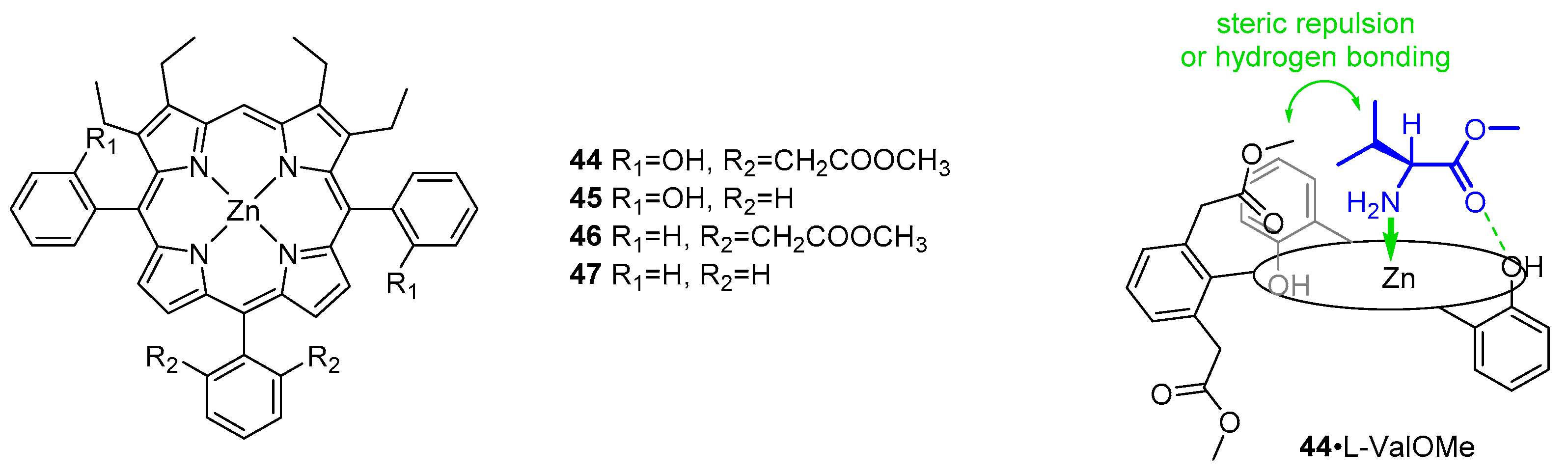
- Mizutani, T.; Ema, T.; Yoshida, T.; Kuroda, Y.; Ogoshi, H. Recognition of.Alpha.-Amino Acid Esters by Zinc Porphyrin Derivatives via Coordination and Hydrogen Bonding Interactions. Evidence for Two-Point Fixation from Thermodynamic and Induced Circular Dichroism Spectroscopic Studies. Inorg. Chem. 1993, 32, 2072–2077. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kato, Y.; Higashioji, T.; Hasegawa, J.; Kawanami, S.; Takahashi, M.; Shiraishi, N.; Tanabe, K.; Ogoshi, H. Chiral Amino Acid Recognition by a Porphyrin-Based Artificial Receptor. J. Am. Chem. Soc. 1995, 117, 10950–10958. [Google Scholar] [CrossRef]
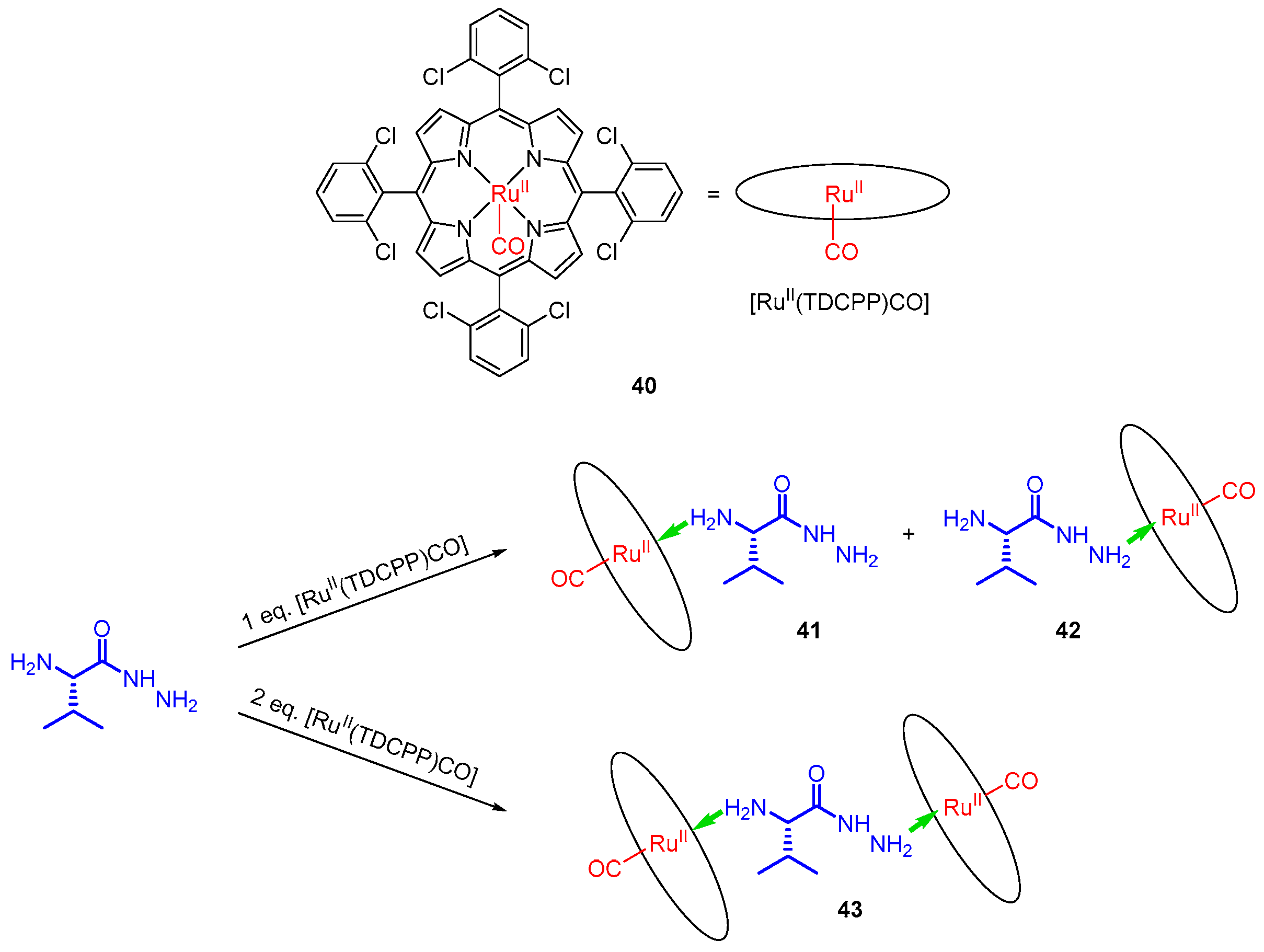
- Liang, Q.-F.; Liu, J.-J.; Chen, J. Sandwich Structure of a Ruthenium Porphyrin and an Amino Acid Hydrazide for Probing Molecular Chirality by Circular Dichroism. Tetrahedron Lett. 2011, 52, 3987–3991. [Google Scholar] [CrossRef]
- Mizutani, T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H. Design and Synthesis of a Trifunctional Chiral Porphyrin with C2 Symmetry as a Chiral Recognition Host for Amino Acid Esters. J. Am. Chem. Soc. 1994, 116, 4240–4250. [Google Scholar] [CrossRef]
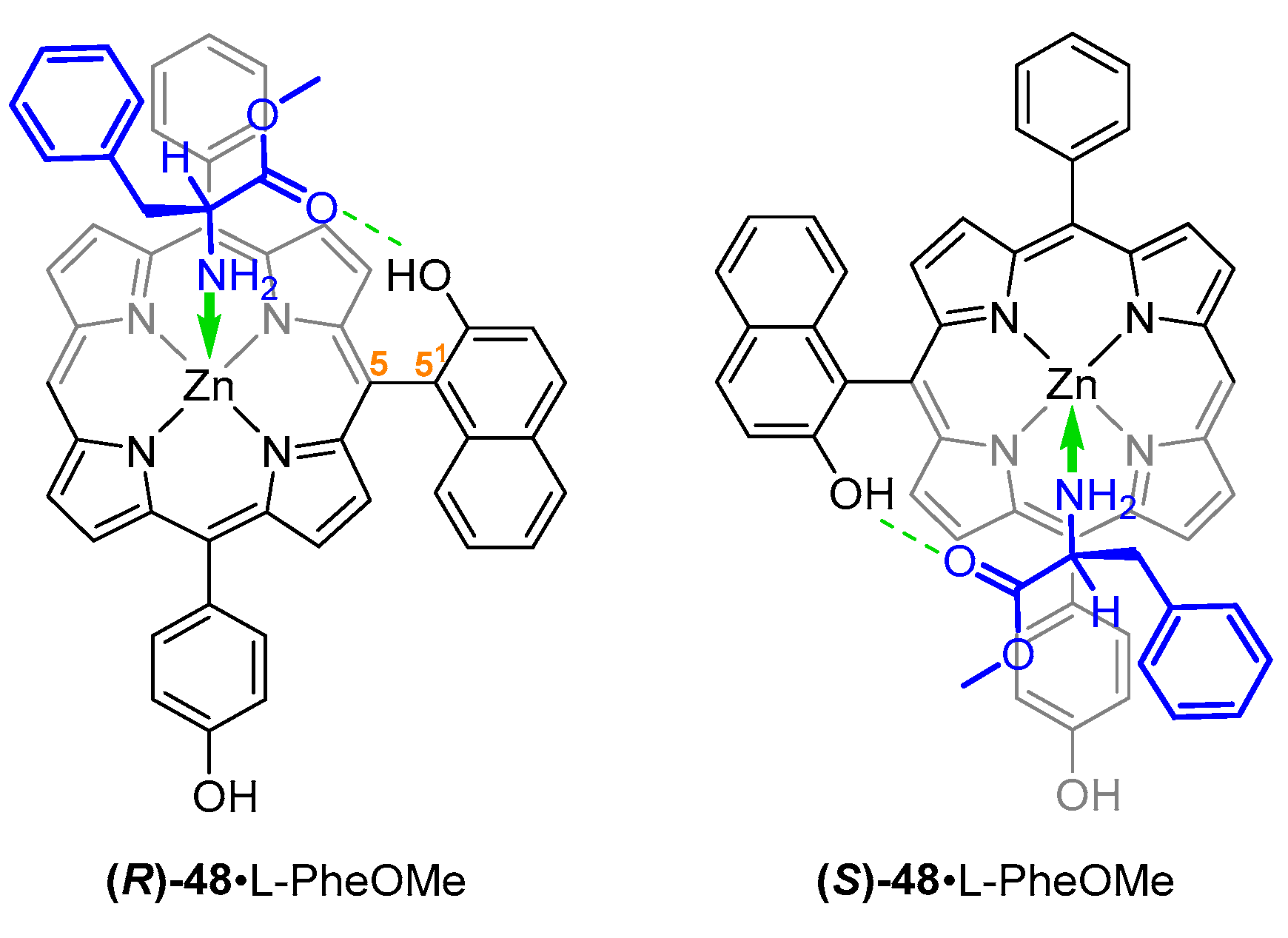
- Yang, L.; Zhou, Y.; Zhu, M.; Zhao, L.; Wei, L.; Bian, Y. Stereochemistry and Solid-State Structure of an Intrinsically Chiral Meso-Patterned Porphyrin: Case Study by NMR and Single-Crystal X-Ray Diffraction Analysis. J. Org. Chem. 2013, 78, 9949–9955. [Google Scholar] [CrossRef]
- Valderrey, V.; Aragay, G.; Ballester, P. Porphyrin Tweezer Receptors: Binding Studies, Conformational Properties and Applications. Coord. Chem. Rev. 2014; 258 259, 137–156. [Google Scholar] [CrossRef]
- Chen, C.W.; Whitlock, H.W. Molecular Tweezers: A Simple Model of Bifunctional Intercalation. J. Am. Chem. Soc. 1978, 100, 4921–4922. [Google Scholar] [CrossRef]
- Sanders, J.K.M.; Bampos, N.; Clyde-Watson, Z.E.; Darling, S.L.; Hawley, J.C.; Kim, H.-J.; Mak, C.C.; Webb, S.J. Axial Coordination Chemistry of Metalloporphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: London, UK, 2000; Volume 3, pp. 30–31. [Google Scholar]
- Borovkov, V.V.; Hembury, G.A.; Yamamoto, N.; Inoue, Y. Supramolecular Chirogenesis in Zinc Porphyrins: Investigation of Zinc-Freebase Bis-Porphyrin, New Mechanistic Insights, Extension of Sensing Abilities, and Solvent Effect. J. Phys. Chem. A 2003, 107, 8677–8686. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, Z.; Liu, B.; Hu, C.; Wang, Y. Chiral Recognition of Amino Acid Esters by a Novel Oxalic Amide-Linked Bisporphyrin. Dalton Trans. 2013, 42, 7651–7659. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fang, X.; Liu, B.; Hu, C. M-Phthalic Diamide-Linked Zinc Bisporphyrinate: Spontaneous Resolution of Its Crystals and Its Application in Chiral Recognition of Amino Acid Esters. Inorg. Chem. 2014, 53, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, J.; Fang, X.; Hu, C. Absolute Configurational Assignments of Amino Acid Esters by a CD-Sensitive Malonamide-Linked Zinc Bisporphyrinate Host. Chin. J. Chem. 2014, 32, 797–802. [Google Scholar] [CrossRef]
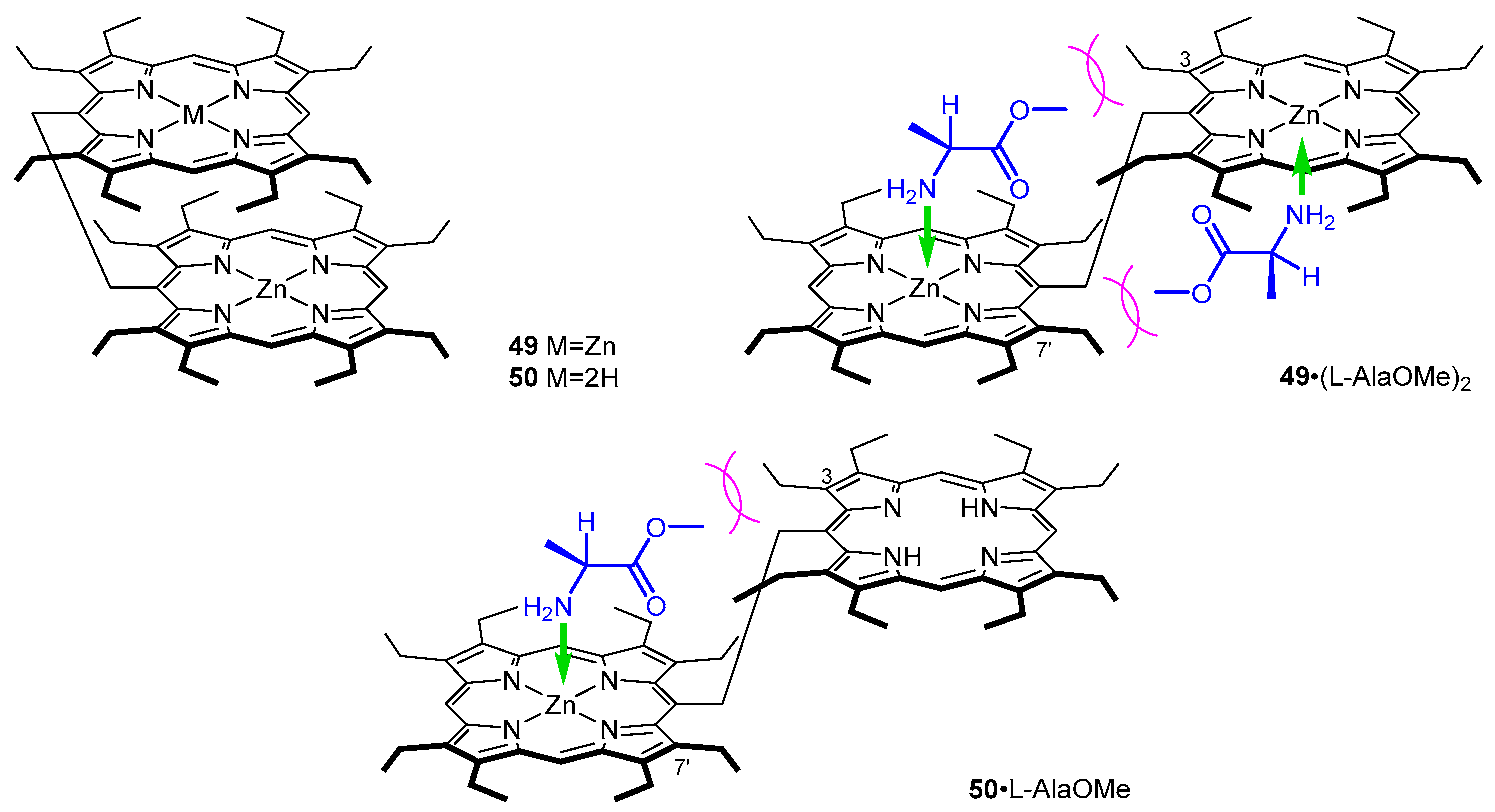
- Hayashi, T.; Aya, T.; Nonoguchi, M.; Mizutani, T.; Hisaeda, Y.; Kitagawa, S.; Ogoshi, H. Chiral Recognition and Chiral Sensing Using Zinc Porphyrin Dimers. Tetrahedron 2002, 58, 2803–2811. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Hu, C.; Wang, Y. Enantioselectivity of a Tartaric Acid Amide Linked Zinc Bisporphyrinate towards Amino Acid Esters. Dyes Pigment. 2020, 176, 108223. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient Method for the Preparation of Peptoids by Submonomer Solid-Phase Synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Kang, B.; Yang, W.; Lee, S.; Mukherjee, S.; Forstater, J.; Kim, H.; Goh, B.; Kim, T.-Y.; Voelz, V.A.; Pang, Y.; et al. Precisely Tuneable Energy Transfer System Using Peptoid Helix-Based Molecular Scaffold. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Kang, B.; Voelz, V.A.; Seo, J. Control of Porphyrin Interactions via Structural Changes of a Peptoid Scaffold. Org. Biomol. Chem. 2017, 15, 9670–9679. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Chung, S.; Ahn, Y.D.; Lee, J.; Seo, J. Porphyrin–Peptoid Conjugates: Face-to-Face Display of Porphyrins on Peptoid Helices. Org. Lett. 2013, 15, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, B.; Seo, J. Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition. Molecules 2018, 23, 2741. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Wada, K.; Kitagawa, S. Porphyrin Receptors for Amines, Amino Acids, and Oligopeptides in Water. J. Am. Chem. Soc. 1999, 121, 11425–11431. [Google Scholar] [CrossRef]
- Imai, H.; Misawa, K.; Munakata, H.; Uemori, Y. Water-Soluble Zinc Porphyrins as Receptors for Amino Carboxylates. Chem. Lett. 2001, 30, 688–689. [Google Scholar] [CrossRef]
- Nicolas, I.; Chevance, S.; Maux, P.L.; Simonneaux, G. Chiral Recognition of Amines and Amino Acid Derivatives by Optically Active Ruthenium Halterman Porphyrins in Organic Solvents and Water. Tetrahedron Asymmetry 2010, 21, 1788–1792. [Google Scholar] [CrossRef]
- Maux, P.L.; Bahri, H.; Simonneaux, G. Molecular Recognition of Racemic Phosphines by a Chiral Ruthenium Porphyrin. J. Chem. Soc. Chem. Commun. 1991, 1350–1352. [Google Scholar] [CrossRef]
- Galardon, E.; Le Maux, P.; Bondon, A.; Simonneaux, G. Chiral Recognition of Amino Esters by a Ruthenium Porphyrin Complex: Kinetics of the Exchange Process Determined by 1H NMR. Tetrahedron Asymmetry 1999, 10, 4203–4210. [Google Scholar] [CrossRef]

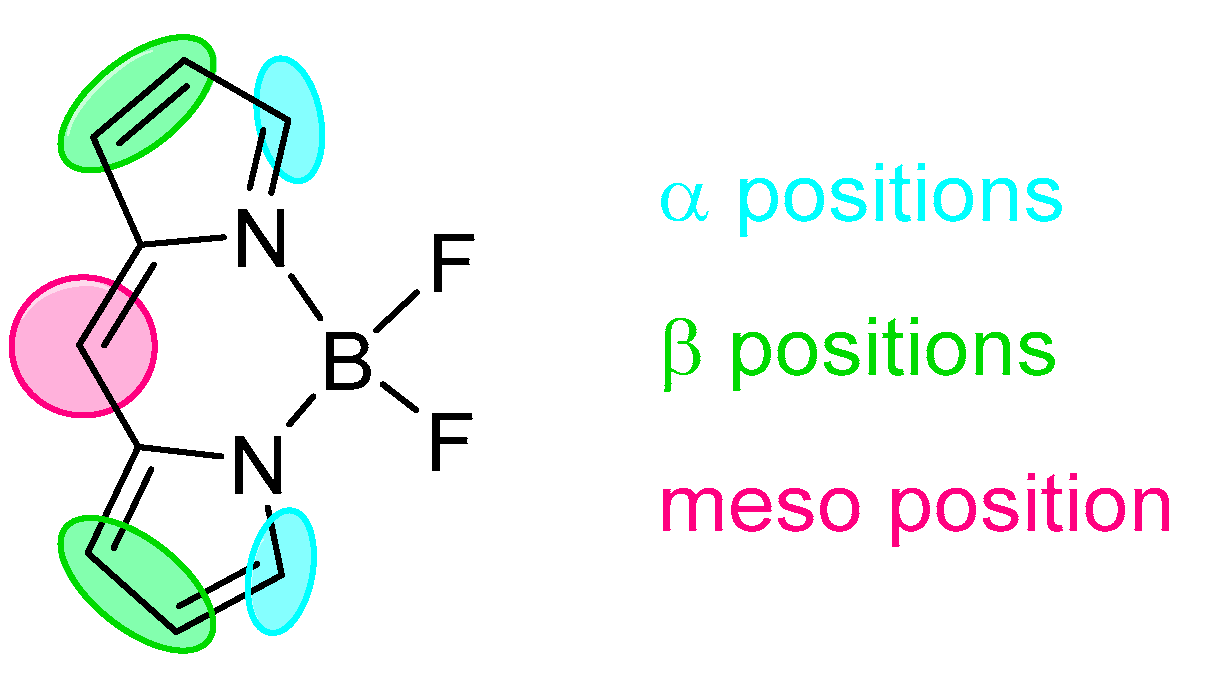


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523. https://doi.org/10.3390/molecules25194523
Farinone M, Urbańska K, Pawlicki M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules. 2020; 25(19):4523. https://doi.org/10.3390/molecules25194523
Chicago/Turabian StyleFarinone, Marco, Karolina Urbańska, and Miłosz Pawlicki. 2020. "BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives" Molecules 25, no. 19: 4523. https://doi.org/10.3390/molecules25194523
APA StyleFarinone, M., Urbańska, K., & Pawlicki, M. (2020). BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules, 25(19), 4523. https://doi.org/10.3390/molecules25194523






