Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review
Abstract
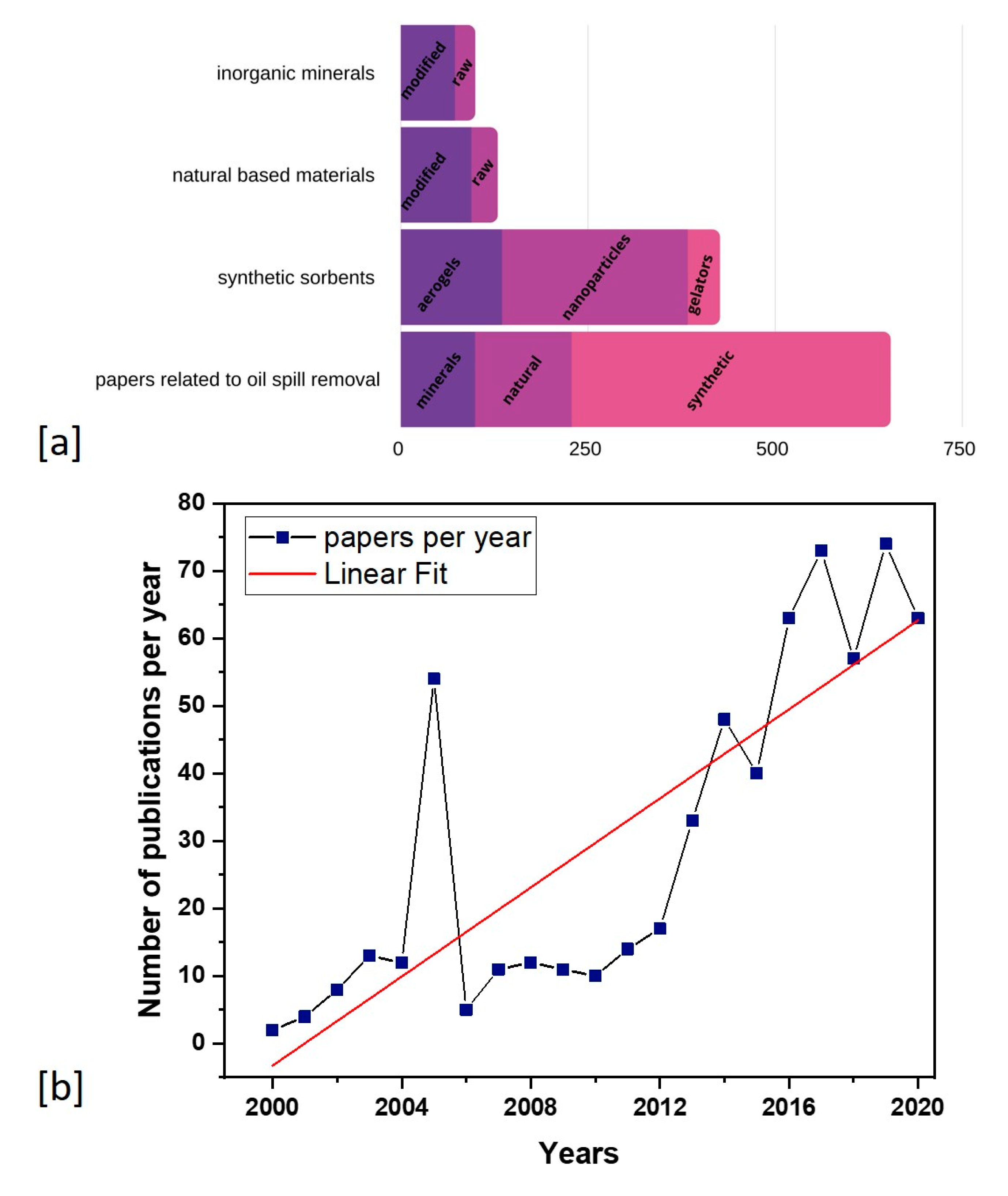
1. Introduction
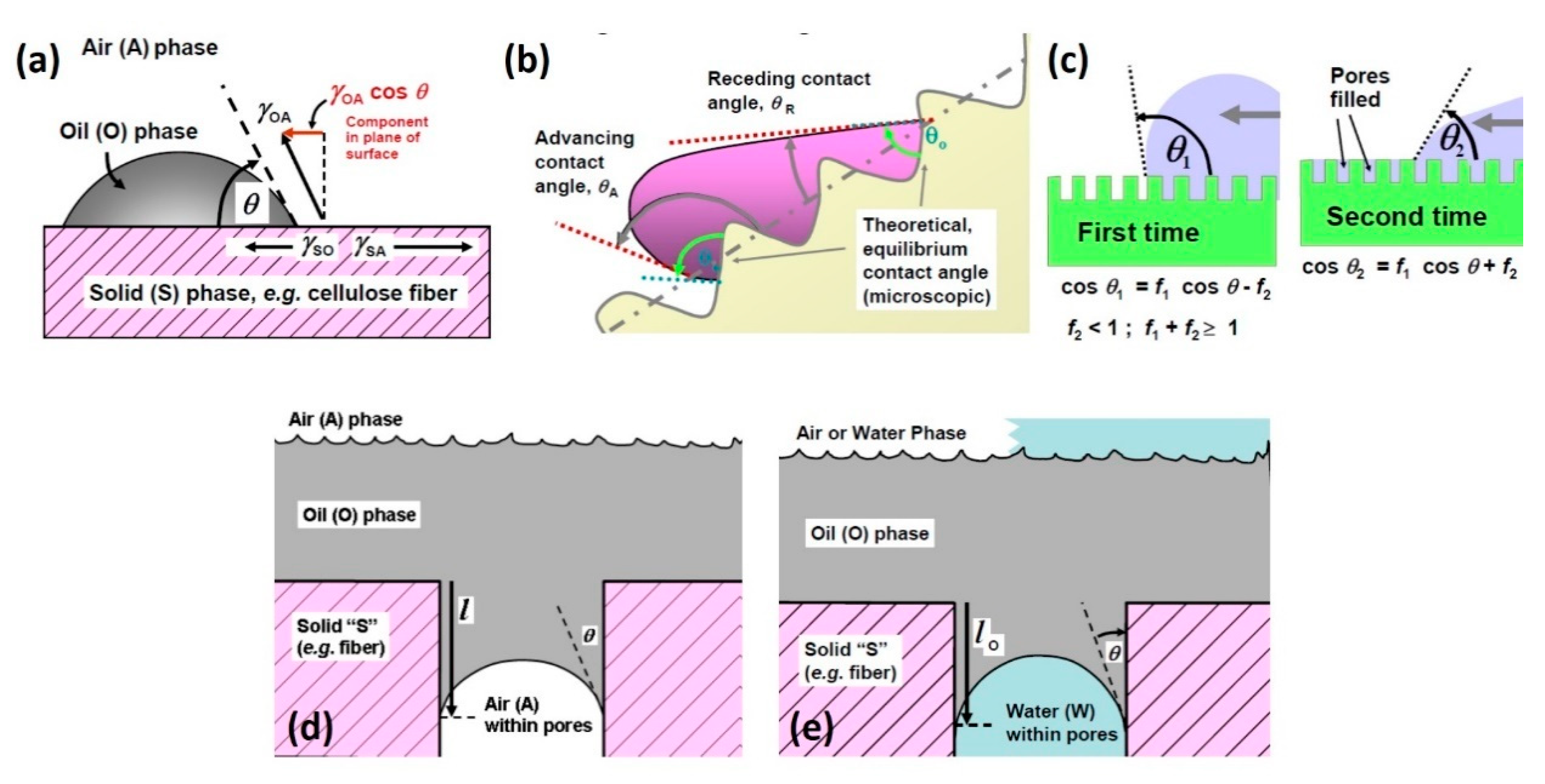
2. The Liquid–Solid Interface between Oil and Sorbent Materials
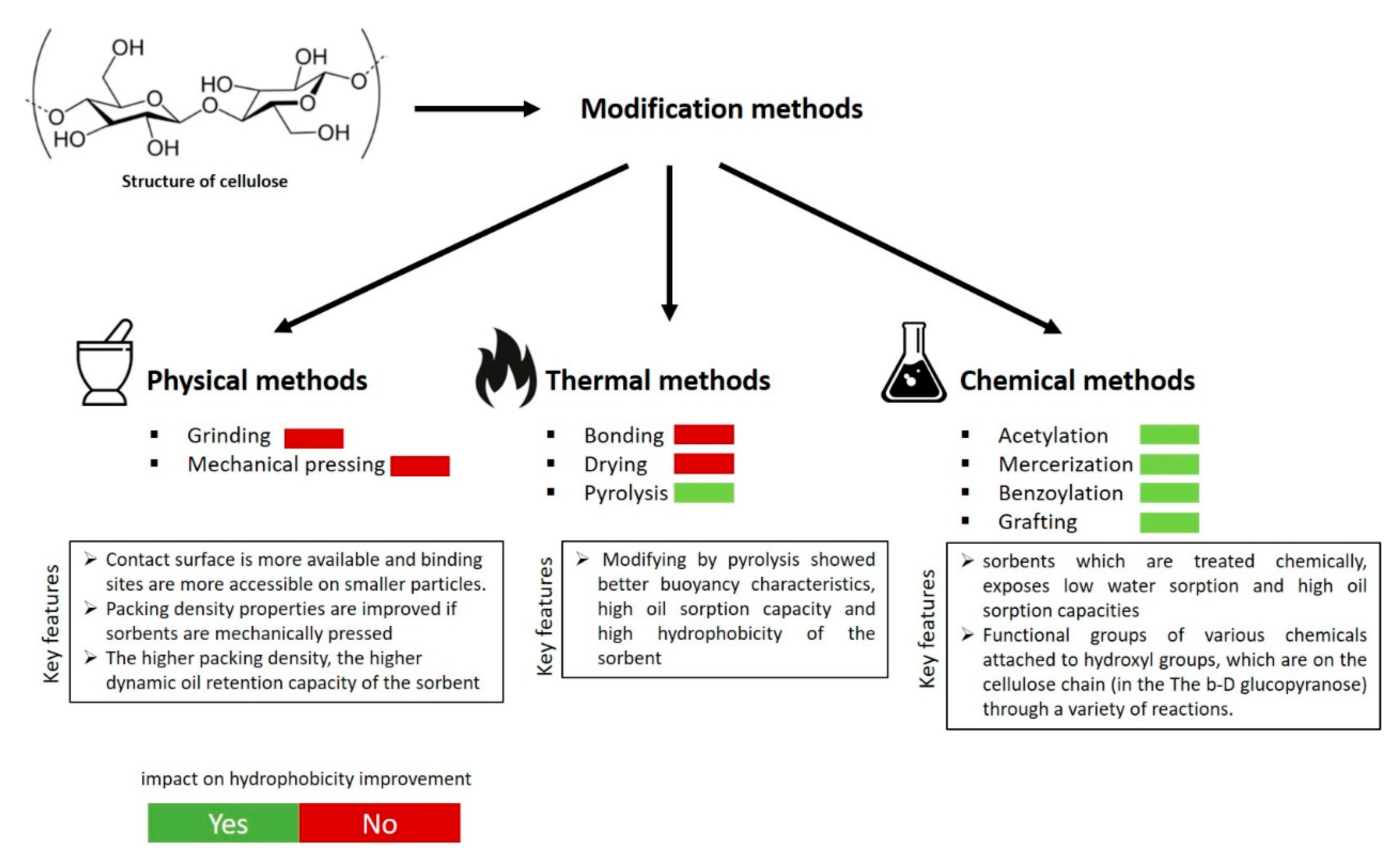
3. Modification Methods for Hydrophobicity Improvement
3.1. Physical and Thermal Treatment
3.2. Hydrothermal Treatment
3.3. Chemical Modification Methods
3.3.1. Mercerization
3.3.2. Acetylation
3.3.3. Grafting
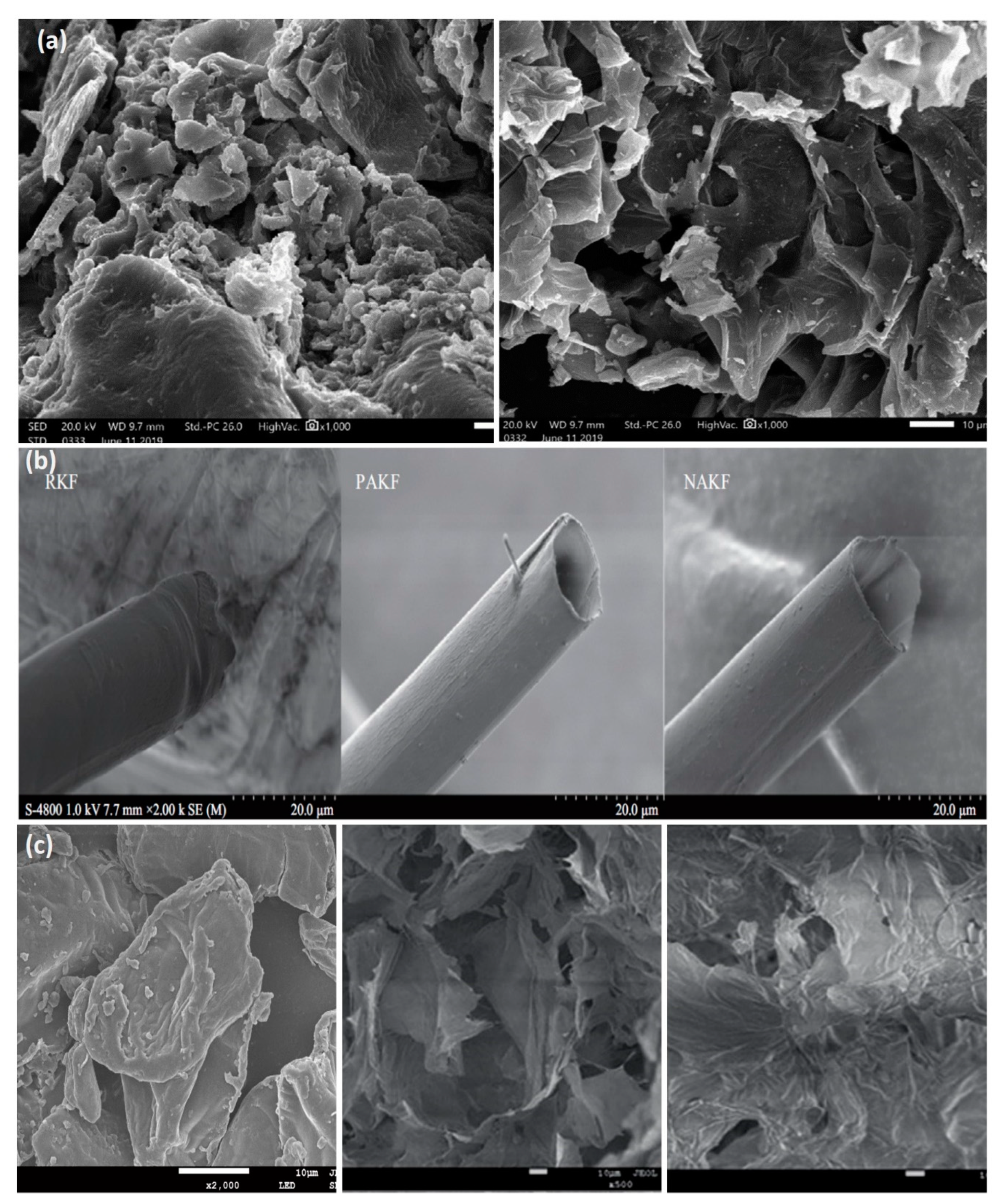
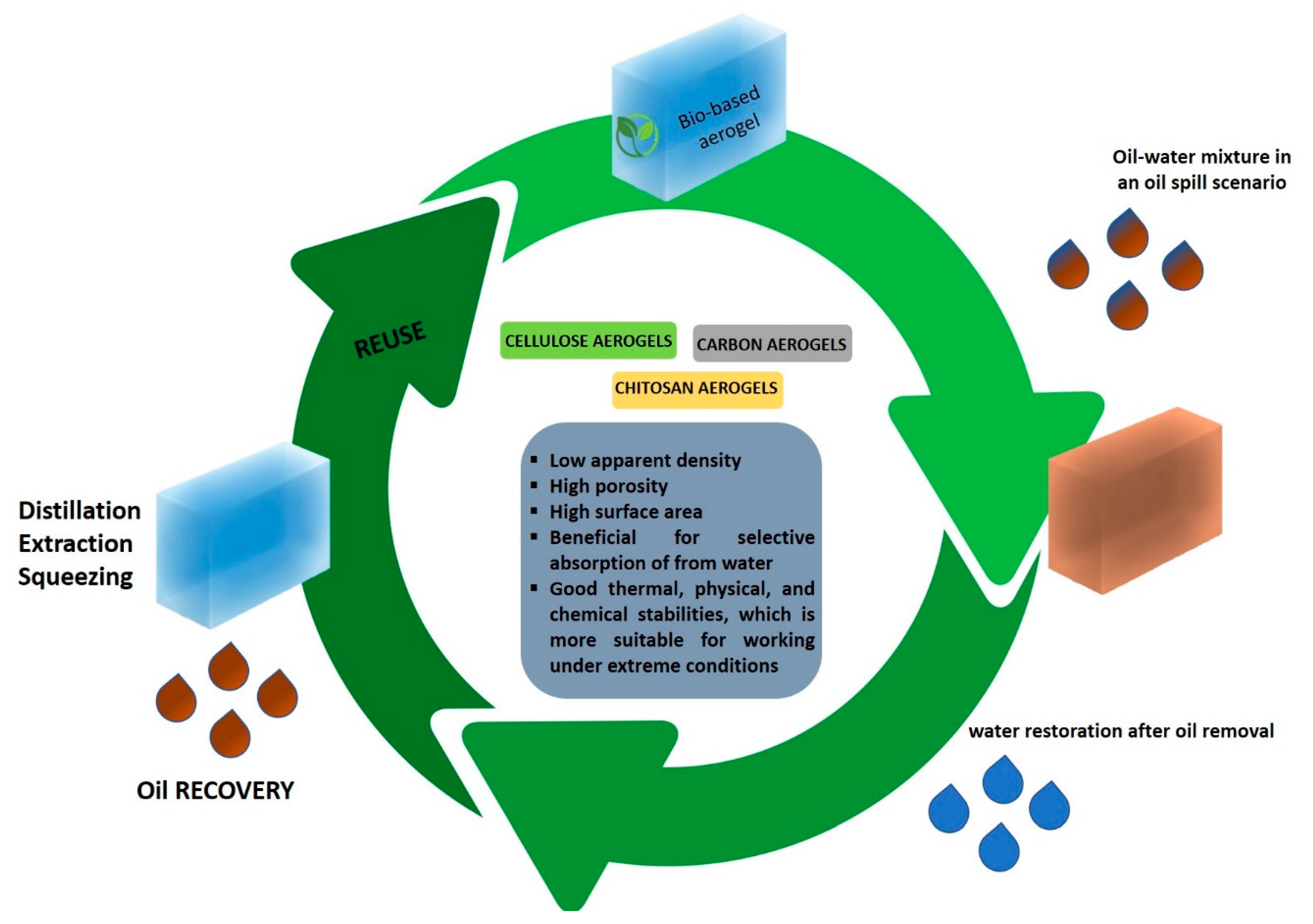
4. Bio-Based Aerogels for Oil Spill Cleanup
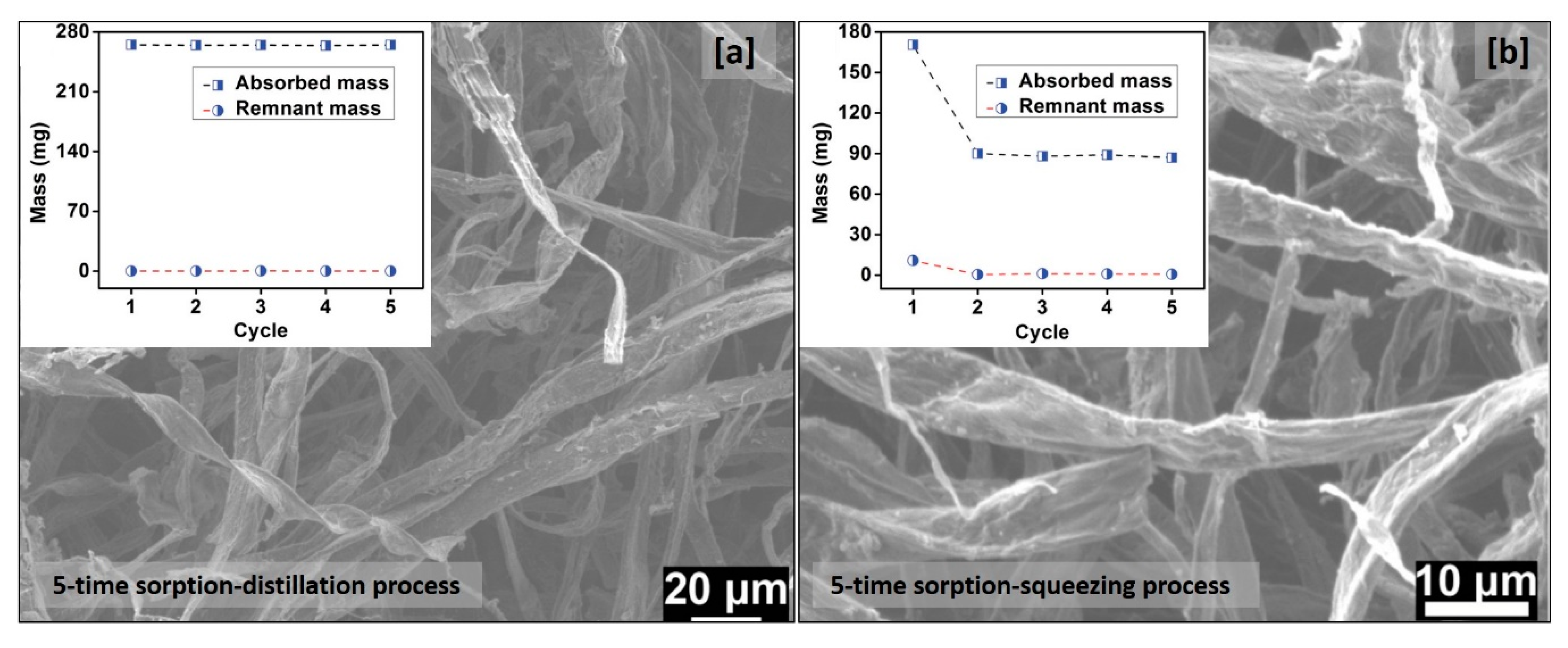
Super-Hydrophobic Bio-Based Aerogels
| Sorbent | Form of Sorbent | Modification Method | Oil Sorption Method | Adsorption Capacity | Type of Oil | Recovery | Refs |
|---|---|---|---|---|---|---|---|
| Banana peel | powder | raw | ASTM * | 5–7 | Crude oil | 20 cycles | [24] |
| Sawdust | fiber | raw | - | 4.1–6.4 | Crude oil | n/a | [32] |
| Coir fiber | fiber | raw | - | 1.8–5.4 | Crude oil | n/a | [32] |
| Luffa | fiber | raw | - | 1.9–4.6 | Crude oil | n/a | [32] |
| Luffa | fiber | raw | - | 14 | Diesel oil | 3 cycles | [107] |
| Orange peel | powder | raw | - | 3–5 | Crude, diesel, and used engine oil | 5 cycles | [34] |
| Barley straw | powder | raw | - | 6.5–12 | Crude oil | 3 cycles | [44] |
| Jute | fiber | raw | ASTM | 2.6 | Engine oil | n/a | [50] |
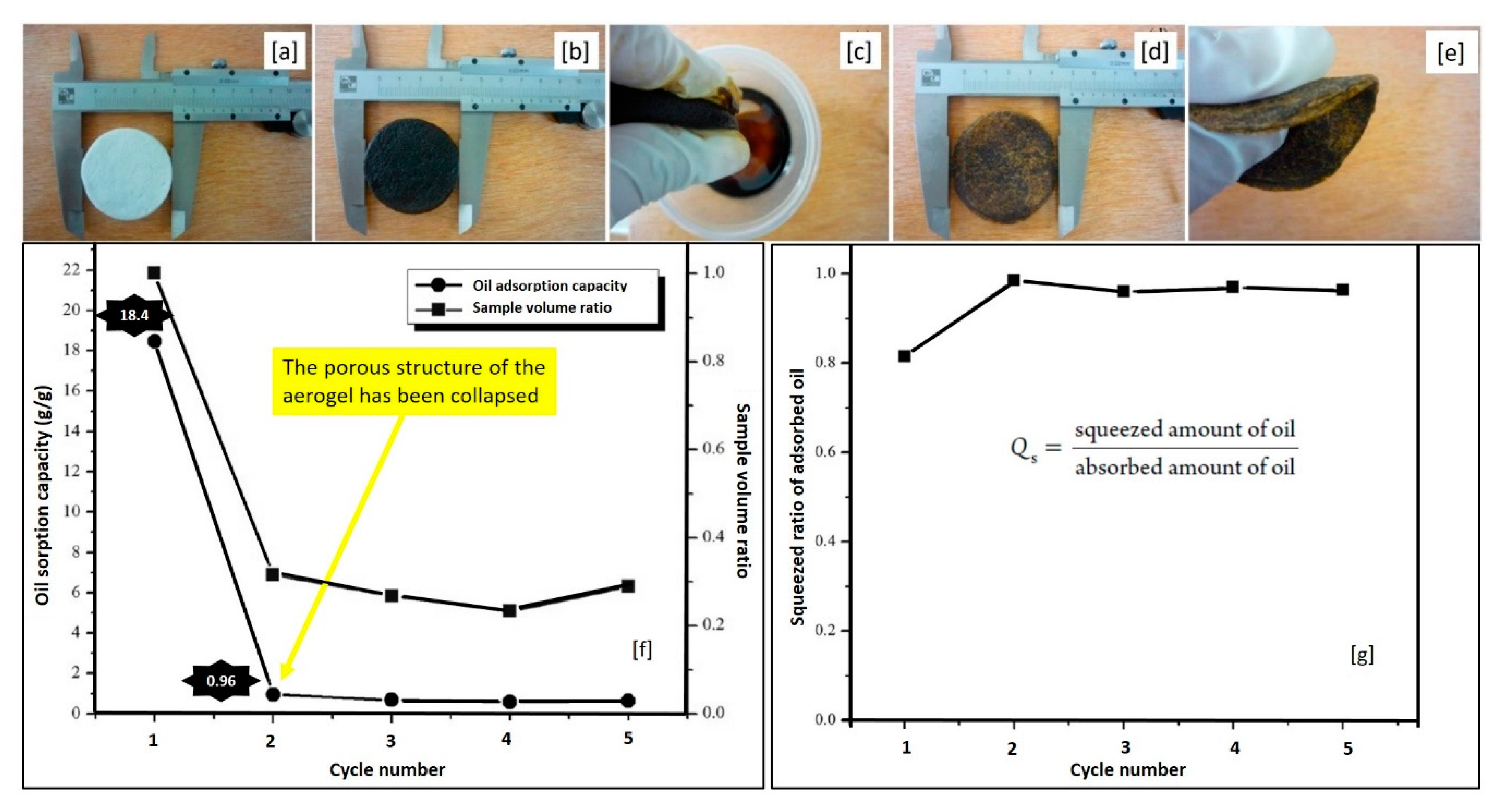
| Cotton | fiber | raw | ASTM | 18.4–22.5 | Used lube oil | 5 cycles | [80] |
| Cotton | fiber | raw | ASTM | 30.5 | Motor oil | n/a | [108] |
| Sugarcane bagasse | raw | - | 10.5–19.9 | Diesel oil | n/a | [103] | |
| Kapok | fiber | raw | - | 19.4–49.9 | Diesel, crude, engine oil, used engine oil | n/a | [103] |
| Kapok | fiber | raw | ASTM | 36–45 | Engine, diesel, and hydraulic oil | 4 cycles | [109] |
| Kapok | fiber | raw | ASTM | 50 | Engine oil | 15 cycles | [110] |
| Kapok | fiber | raw | ASTM | 38 | Diesel oil | 8 cycles | [104] |
| Walnut shell | granular | raw | - | 0.30–0.58 | Mineral oil, vegetable oil and Bright-Edge oil | n/a | [111] |
| Rice husk | powder | raw | 6.2 | n/a | [112] | ||
| Rice husk | powder | pyrolysis | - | 3.7–9.2 | Diesel oil, gasoline, light crude, motor oil, heavy, crude oil | n/a | [58] |
| Rice husk | powder | pyrolysis | - | 15 | Crude oil | [47] | |
| Rice husk | powder | pyrolysis | - | 2.7–6.2 | Diesel oil, crude oil | [59] | |
| Rice husk | powder | pyrolysis | JIS technical standards | 6 | Heavy crude oil | n/a | [112] |
| Barley straw | fiber | pyrolysis | ASTM | 7.6–9.2 | Diesel, heavy crude oil | 2 cycles | [62] |
| Rice husk | powder | mercerization | - | 4–19 | Marine oil | n/a | [75] |
| Soybean | powder | mercerization | ASTM | 5 | Crude oil | n/a | [49] |
| Cattail | fiber | mercerization | ASTM | 4 | Motor oil | n/a | [49] |
| Oil palm empty fruit bunch | granular | acetylation | - | 7 | Crude oil | n/a | [46] |
| Cocoa pods | fiber | acetylation | - | 8 | Crude oil | n/a | [46] |
| Jute | fiber | acetylation | ASTM | 21.8 | Engine oil | 3 cycles | [50] |
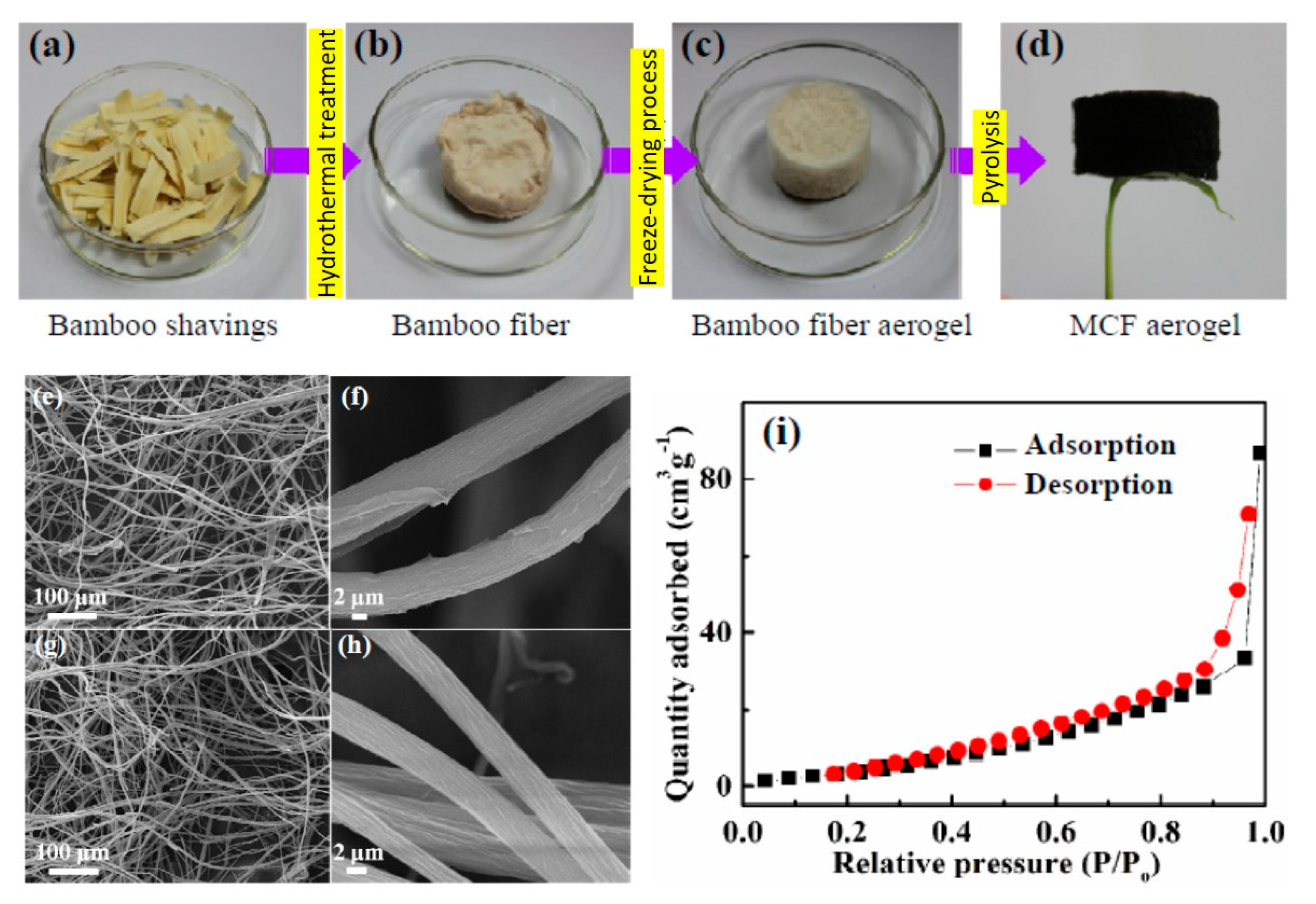
| bamboo | aerogel | aerogel | - | 38 | Engine oil | 5 cycles | [105] |
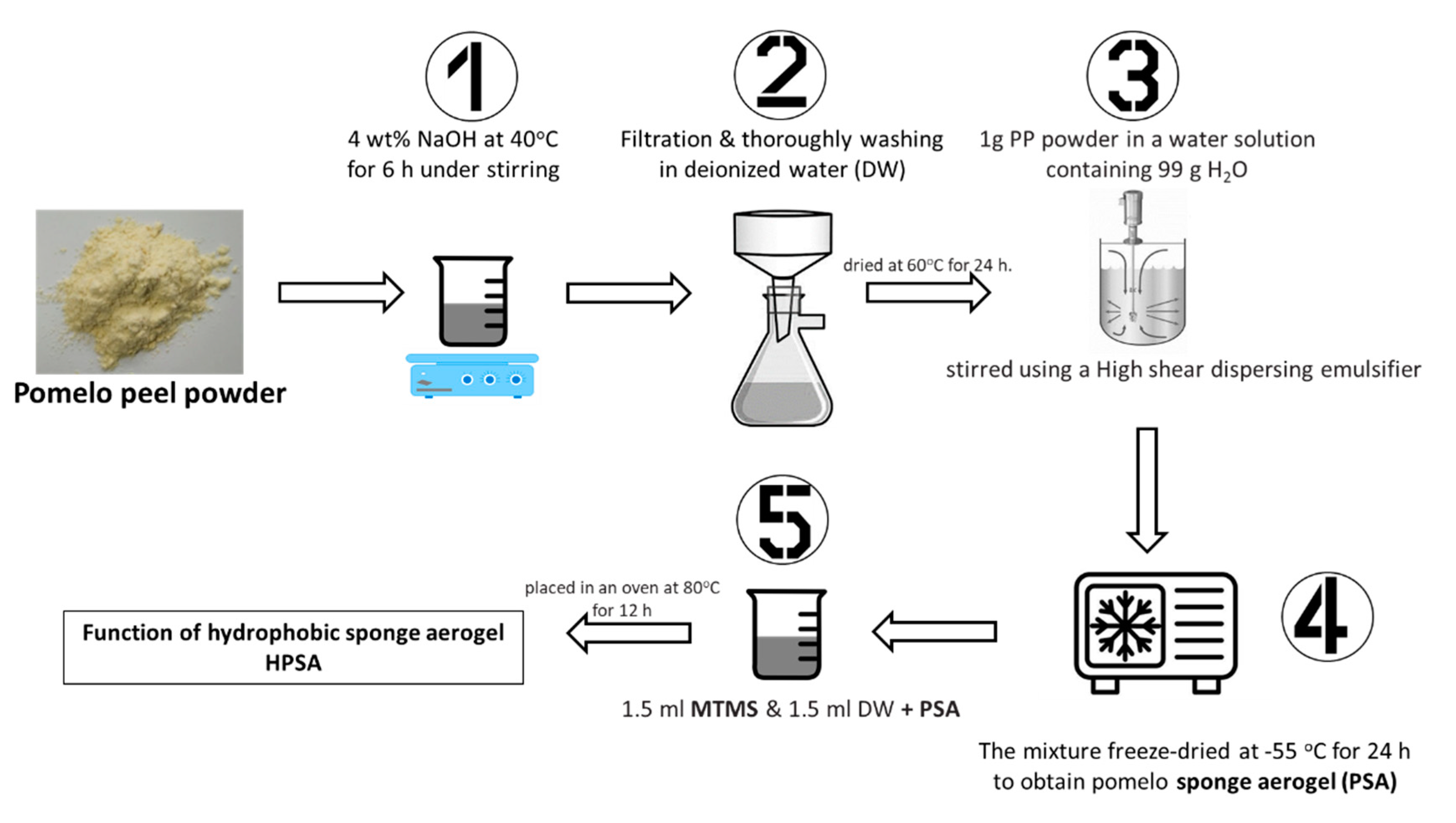
| Pomelo peel | aerogel | aerogel | - | 5 | Castor oil | 5 cycles | [106] |
| Waste paper | aerogel | Aerogel with MTMS | - | 24.4 | Crude oil | 5 cycles | [95] |
| Pomelo peel | aerogel | Aerogel with MTMS | - | 49.8 | Crude oil | 10 cycles | [86] |
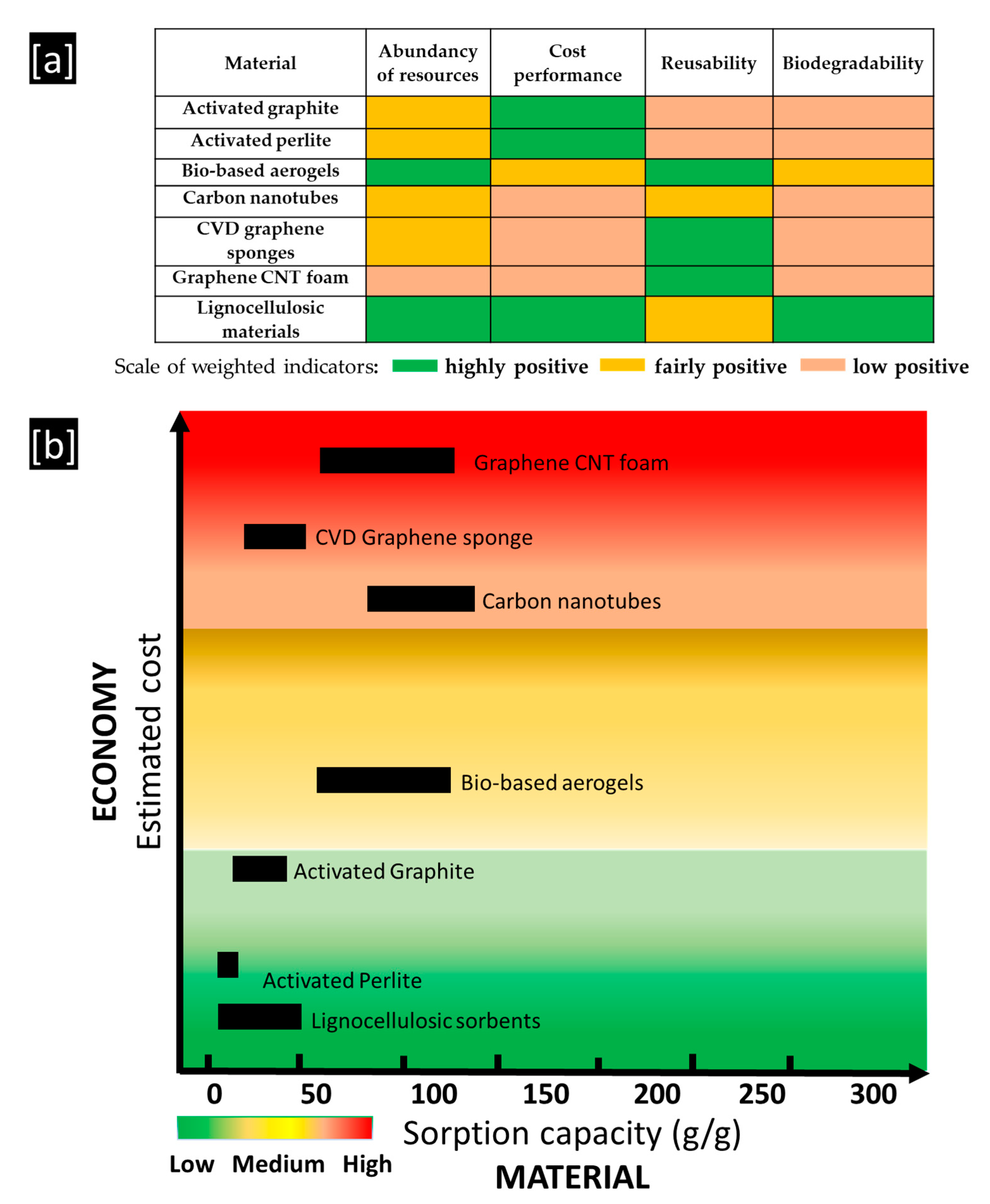
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, S.E.; Stone, J.; Demes, K.; Piscitelli, M. Consequences of oil spills: A review and framework for informing planning. Ecol. Soc. 2014, 19. [Google Scholar] [CrossRef]
- Michel, J.; Fingas, M. Oil Spills: Causes, Consequences, Prevention, and Countermeasures. In Fossil Fuels: Current Status and Future Directions; WSPC: Singapore, 2016; pp. 159–201. ISBN 9789814699983. [Google Scholar]
- Brody, T.M.; Bianca, P.D.; Krysa, J. Analysis of Inland Crude Oil Spill Threats, Vulnerabilities, and Emergency Response in the Midwest United States. Risk Anal. 2012, 32, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Kolokoussis, P.; Karathanassi, V. Oil Spill Detection and Mapping Using Sentinel 2 Imagery. J. Mar. Sci. Eng. 2018, 6, 4. [Google Scholar] [CrossRef]
- Varela, M.; Bode, A.; Lorenzo, J.; Álvarez-Ossorio, M.T.; Miranda, A.; Patrocinio, T.; Anadón, R.; Viesca, L.; Rodríguez, N.; Valdés, L.; et al. The effect of the “Prestige” oil spill on the plankton of the N–NW Spanish coast. Mar. Pollut. Bull. 2006, 53, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakakis, E.; Hahladakis, J.; Gidarakos, E. The “Sea Diamond” shipwreck: Environmental impact assessment in the water column and sediments of the wreck area. Int. J. Environ. Sci. Technol. 2014, 11, 1421–1432. [Google Scholar] [CrossRef]
- Zodiatis, G.; Coppini, G.; Perivoliotis, L.; Lardner, R.; Alves, T.; Pinardi, N.; Liubartseva, S.; De Dominicis, M.; Bourma, E.; Sepp Neves, A.A. Numerical Modeling of Oil Pollution in the Eastern Mediterranean Sea. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–254. [Google Scholar]
- Fodrie, F.J.; Heck, K.L. Response of Coastal Fishes to the Gulf of Mexico Oil Disaster. PLoS ONE 2011, 6, e21609. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Health Consequences among Subjects Involved in Gulf Oil Spill Clean-up Activities. Am. J. Med. 2013, 126, 966–974. [Google Scholar] [CrossRef]
- Fingas, M. Oil Spill Dispersants: A Technical Summary. In Oil Spill Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 435–582. ISBN 9781856179430. [Google Scholar]
- Zekri, A.Y.; Chaalal, O. Effect of Temperature on Biodegradation of Crude Oil. Energy Sources 2005, 27, 233–244. [Google Scholar] [CrossRef]
- Warnock, A.M.; Hagen, S.C.; Passeri, D.L. Marine Tar Residues: A Review. Water Air Soil Pollut. 2015, 226, 68. [Google Scholar] [CrossRef]
- Teas, C.; Kalligeros, S.; Zanikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G. Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination 2001, 140, 259–264. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K. How kaolinite plays an essential role in remediating oil-polluted seawater. Clay Min. 2005, 40, 481–491. [Google Scholar] [CrossRef]
- Qi, P.; Lin, N.; Liu, Y.; Zhao, J. Improvement of oil/water selectivity by stearic acid modified expanded perlite for oil spill cleanup. J. Shanghai Jiaotong Univ. 2013, 18, 500–507. [Google Scholar] [CrossRef]
- Zadaka-Amir, D.; Bleiman, N.; Mishael, Y.G. Sepiolite as an effective natural porous adsorbent for surface oil-spill. Microporous Mesoporous Mater. 2013, 169, 153–159. [Google Scholar] [CrossRef]
- Roulia, M.; Chassapis, K.; Fotinopoulos, C.; Savvidis, T.; Katakis, D. Dispersion and Sorption of Oil Spills by Emulsifier-Modified Expanded Perlite. Spill Sci. Technol. Bull. 2003, 8, 425–431. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Geng, G. Highly efficient oil-in-water emulsion and oil layer/water mixture separation based on durably superhydrophobic sponge prepared via a facile route. Mar. Pollut. Bull. 2018, 127, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Narayanan, P.; Ravirajan, A.; Umasankaran, A.; Prakash, D.G.; Kumar, P.S. Theoretical and experimental investigation on the removal of oil spill by selective sorbents. J. Ind. Eng. Chem. 2018, 63, 1–11. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Khaskhoussi, A.; Proverbio, E.; Milone, C. Thermo-Physical Characterization of Carbon Nanotube Composite Foam for Oil Recovery Applications. Nanomaterials 2020, 10, 86. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of Electrospun Polyvinyl Chloride/Polystyrene Fibers As Sorbent Materials for Oil Spill Cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Teli, M.D.; Valia, S.P. Acetylation of banana fibre to improve oil absorbency. Carbohydr. Polym. 2013, 92, 328–333. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Hamedallah, N. Oil spill removal from water by using corn stalk: Factors affecting sorption process. Int. J. Environ. Waste Manag. 2015, 16, 281. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Adebayo, A.R.; Hossain, M.E. A sustainable approach to controlling oil spills. J. Environ. Manag. 2012, 113, 213–227. [Google Scholar] [CrossRef] [PubMed]
- SciDev Net, S.D. Study on Banana Fibre Oil Spill Absorbency. Available online: https://officerofthewatch.com/2012/11/02/study-on-banana-fibre-oil-spill-absorbency/ (accessed on 22 May 2019).
- Cao, S.; Dong, T.; Xu, G.; Wang, F. Oil Spill Cleanup by Hydrophobic Natural Fibers. J. Nat. Fibers 2017, 14, 727–735. [Google Scholar] [CrossRef]
- Panahi, S.; Moghaddam, M.K.; Moezzi, M. Assessment of milkweed floss as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. J. Environ. Manag. 2020, 268, 110688. [Google Scholar] [CrossRef]
- Elias, E.; Costa, R.; Marques, F.; Oliveira, G.; Guo, Q.; Thomas, S.; Souza, F.G., Jr. Oil-spill cleanup: The influence of acetylated curaua fibers on the oil-removal capability of magnetic composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Wang, F. Oil spill cleanup by structured natural sorbents made from cattail fibers. Ind. Crop. Prod. 2015, 76, 25–33. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Viju, S.; Thilagavathi, G.; Vignesh, B.; Brindha, R. Oil sorption behavior of acetylated nettle fiber. J. Text. Inst. 2019, 110, 1415–1423. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; Ahmad El Saqa, F.; Abd El Razek Amer, A.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Nasr, S.M.; Thabet, W.M. Palm fibers and modified palm fibers adsorbents for different oils. Alex. Eng. J. 2017, 56, 749–755. [Google Scholar] [CrossRef]
- Cheu, S.C.; Kong, H.; Song, S.T.; Saman, N.; Johari, K.; Mat, H. High removal performance of dissolved oil from aqueous solution by sorption using fatty acid esterified pineapple leaves as novel sorbents. RSC Adv. 2016, 6, 13710–13722. [Google Scholar] [CrossRef]
- Sánchez-Galván, G.; Mercado, F.J.; Olguín, E.J. Leaves and Roots of Pistia stratiotes as Sorbent Materials for the Removal of Crude Oil from Saline Solutions. Water Air Soil Pollut. 2013, 224, 1421. [Google Scholar] [CrossRef]
- Chai, W.; Liu, X.; Zou, J.; Zhang, X.; Li, B.; Yin, T. Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution. Carbohydr. Polym. 2015, 132, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Mojžiš, M.; Bubeníková, T.; Zachar, M.; Kačíková, D.; Štefková, J. Comparison of natural and synthetic sorbents’ efficiency at oil spill removal. BioResources 2019, 14, 8738–8752. [Google Scholar] [CrossRef]
- Lv, E.; Xia, W.; Tang, M.; Pu, Y. Preparation of an Efficient Oil-Spill Adsorbent Based on Wheat Straw. BioResources 2016, 12, 296–315. [Google Scholar] [CrossRef]
- Behnood, R.; Anvaripour, B.; Jaafarzadeh, N.; Farasati, M. Oil spill sorption using raw and acetylated sugarcane bagasse. J. Cent. South Univ. 2016, 23, 1618–1625. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Removal of oil by walnut shell media. Bioresour. Technol. 2008, 99, 8217–8220. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Taha, N.A. Availability of barley straw application on oil spill clean up. Int. J. Environ. Sci. Technol. 2009, 6, 123–130. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Hamedallah, N. A comprehensive characterization of corn stalk and study of carbonized corn stalk in dye and gas oil sorption. J. Anal. Appl. Pyrolysis 2009, 86, 360–363. [Google Scholar] [CrossRef]
- Onwuka, J.C.; Agbaji, E.B.; Ajibola, V.O.; Okibe, F.G. Treatment of crude oil-contaminated water with chemically modified natural fiber. Appl. Water Sci. 2018, 8, 86. [Google Scholar] [CrossRef]
- Kudaybergenov, K.K.; Ongarbayev, E.K.; Mansurov, Z.A. Petroleum Sorption by Thermally Treated Rice Husks Derived from Agricultural Byproducts. Eurasian Chem. J. 2012, 15, 57. [Google Scholar] [CrossRef]
- Wang, J.; Han, F.; Liang, B.; Geng, G. Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J. Ind. Eng. Chem. 2017, 54, 174–183. [Google Scholar] [CrossRef]
- Wong, C.; McGowan, T.; Bajwa, S.G.; Bajwa, D.S. Impact of Fiber Treatment on the Oil Absorption Characteristics of Plant Fibers. BioResources 2016, 11, 6452–6463. [Google Scholar] [CrossRef][Green Version]
- Teli, M.D.; Valia, S.P. Acetylation of Jute fiber to improve oil absorbency. Fibers Polym. 2013, 14, 915–919. [Google Scholar] [CrossRef]
- Bhatia, J.K.; Kaith, B.S.; Singla, R.; Mehta, P.; Yadav, V.; Dhiman, J.; Bhatti, M.S. RSM optimized soy protein fibre as a sorbent material for treatment of water contaminated with petroleum products. Desalin. Water Treat. 2016, 57, 4245–4254. [Google Scholar] [CrossRef]
- Teli, M.D.; Valia, S.P. Grafting of Butyl Acrylate on to Banana Fibers for Improved Oil Absorption. J. Nat. Fibers 2016, 13, 470–476. [Google Scholar] [CrossRef]
- Wenzel, R.N. RESISTANCE OF SOLID SURFACES TO WETTING BY WATER. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Fingas, M.; Gupta, B.S. Cellulosic Substrates for Removal of Pollutants from Aqueous Systems: A Review. 3. Spilled Oil and Emulsified Organic Liquids. BioResources 2013, 8, 3038–3097. [Google Scholar] [CrossRef]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.-H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.; Uzunov, I.; Uzunova, S.; Gigova, A.; Minchev, L. Kinetics of oil and oil products adsorption by carbonized rice husks. Chem. Eng. J. 2011, 172, 306–311. [Google Scholar] [CrossRef]
- Vlaev, L.; Petkov, P.; Dimitrov, A.; Genieva, S. Cleanup of water polluted with crude oil or diesel fuel using rice husks ash. J. Taiwan Inst. Chem. Eng. 2011, 42, 957–964. [Google Scholar] [CrossRef]
- Kudaybergenov, K.K.; Ongarbayev, E.K.; Mansurov, Z.A. Carbonaceous Composites from Agricultural Wastes for Adsorption of Hydrocarbon Contamination in Water. Eurasian Chem. J. 2010, 12, 151. [Google Scholar] [CrossRef]
- Uzunov, I.; Uzunova, S.; Angelova, D.; Gigova, A. Effects of the pyrolysis process on the oil sorption capacity of rice husk. J. Anal. Appl. Pyrolysis 2012, 98, 166–176. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Taha, N.A. Experimental Investigation of Thermal Modification Influence on Sorption Qualities of Barley Straw. J. Appl. Sci. Res. 2008, 4, 652–657. [Google Scholar]
- Hrnčič, M.K.; Kravanja, G.; Knez, Ž. Hydrothermal treatment of biomass for energy and chemicals. Energy 2016, 116, 1312–1322. [Google Scholar] [CrossRef]
- Karakasi, O.K.; Moutsatsou, A. Surface modification of high calcium fly ash for its application in oil spill clean up. Fuel 2010, 89, 3966–3970. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Hauer, E.; Galla, U.; Dinjus, E.; Hitzmann, B.; Larsen, T.; Nygaard, S.D. Catalytic conversion of waste biomass by hydrothermal treatment. Fuel 2011, 90, 555–562. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Mu, L.; Hao, B.; Ma, P.-C. Low cost carbon fiber aerogel derived from bamboo for the adsorption of oils and organic solvents with excellent performances. RSC Adv. 2015, 5, 38470–38478. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; Wang, W. Robustly superhydrophobic/superoleophilic kapok fiber with ZnO nanoneedles coating: Highly efficient separation of oil layer in water and capture of oil droplets in oil-in-water emulsions. Ind. Crop. Prod. 2017, 108, 303–311. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Gert, E.V.; Shishonok, M.V.; Torgashov, V.I.; Kaputskii, F.N. Crystallization of amorphous cellulose in the form of inclusion compounds. J. Polym. Sci. Part C Polym. Lett. 1990, 28, 163–166. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Satar, M.A.H.; Hameed, B.H. Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem. Eng. J. 2012, 203, 9–18. [Google Scholar] [CrossRef]
- Hassan, M.A.; Onyekwere, O.S.; Yami, A.M.; Raji, A. Effects of Chemical Modification on Physical And Mechanical Properties of Rice Husk—Stripped Oil Palm Fruit Bunch Fiber Polypropylene Hybrid Composite. IOSR J. Mech. Civ. Eng. 2014, 11, 01–05. [Google Scholar] [CrossRef]
- Gulati, I.; Park, J.; Maken, S.; Lee, M.-G. Production of carboxymethylcellulose fibers from waste lignocellulosic sawdust using NaOH/NaClO2 pretreatment. Fibers Polym. 2014, 15, 680–686. [Google Scholar] [CrossRef]
- Bazargan, A.; Tan, J.; Hui, C.W.; McKay, G. Utilization of rice husks for the production of oil sorbent materials. Cellulose 2014, 21, 1679–1688. [Google Scholar] [CrossRef]
- Asadpour, R.; Sapari, N.B.; Isa, M.H.; Kakooei, S. Acetylation of oil palm empty fruit bunch fiber as an adsorbent for removal of crude oil. Environ. Sci. Pollut. Res. 2016, 23, 11740–11750. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Sun, R.; Sun, J.-X. Acetylation of Rice Straw with or without Catalysts and Its Characterization as a Natural Sorbent in Oil Spill Cleanup. J. Agric. Food Chem. 2002, 50, 6428–6433. [Google Scholar] [CrossRef]
- Nwadiogbu, J.O.; Okoye, P.A.C.; Ajiwe, V.I.; Nnaji, N.J.N. Hydrophobic treatment of corn cob by acetylation: Kinetics and thermodynamics studies. J. Environ. Chem. Eng. 2014, 2, 1699–1704. [Google Scholar] [CrossRef]
- Deschamps, G.; Caruel, H.; Borredon, M.-E.; Bonnin, C.; Vignoles, C. Oil Removal from Water by Selective Sorption on Hydrophobic Cotton Fibers. 1. Study of Sorption Properties and Comparison with Other Cotton Fiber-Based Sorbents. Environ. Sci. Technol. 2003, 37, 1013–1015. [Google Scholar] [CrossRef]
- Hussein, M.; Amer, A.A.; Sawsan, I.I. Heavy oil spill cleanup using law grade raw cotton fibers: Trial for practical application. J. Pet. Technol. Altern. Fuels 2011, 2, 132–140. [Google Scholar]
- Li, D.; Zhu, F.Z.; Li, J.Y.; Na, P.; Wang, N. Preparation and Characterization of Cellulose Fibers from Corn Straw as Natural Oil Sorbents. Ind. Eng. Chem. Res. 2013, 52, 516–524. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Investigation of acetylated kapok fibers on the sorption of oil in water. J. Environ. Sci. 2013, 25, 246–253. [Google Scholar] [CrossRef]
- Viju, S.; Brindha, R.; Thilagavathi, G. Surface modification of nettle fibers by grafting to improve oil sorption capacity. J. Ind. Text. 2019. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Ji, Y.; Chen, W.; Wei, J. Fabrication of Hydrophilic and Hydrophobic Sites on Polypropylene Nonwoven for Oil Spill Cleanup: Two Dilemmas Affecting Oil Sorption. Environ. Sci. Technol. 2016, 50, 3860–3865. [Google Scholar] [CrossRef]
- Teli, M.D.; Valia, S.P.; Mifta, J. Application of functionalized coir fibre as eco-friendly oil sorbent. J. Text. Inst. 2017, 108, 1106–1111. [Google Scholar] [CrossRef]
- Shi, G.; Qian, Y.; Tan, F.; Cai, W.; Li, Y.; Cao, Y. Controllable synthesis of pomelo peel-based aerogel and its application in adsorption of oil/organic pollutants. R. Soc. Open Sci. 2019, 6, 181823. [Google Scholar] [CrossRef] [PubMed]
- Ratcha, A.; Yoosuk, B.; Kongparakul, S. Grafted Methyl Methacrylate and Butyl Methacrylate onto Natural Rubber Foam for Oil Sorbent. Adv. Mater. Res. 2013, 844, 385–390. [Google Scholar] [CrossRef]
- Said, A.; Ludwick, A.; Aglan, H. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components. Bioresour. Technol. 2009, 100, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Young, T.M.; Liu, P.; Contescu, C.I.; Huang, B.; Wang, S. Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 2015, 22, 435–447. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Jin, C.; Han, S.; Li, J.; Sun, Q. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.-H. Carbon materials as oil sorbents: A review on the synthesis and performance. J. Mater. Chem. A 2016, 4, 1550–1565. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Bi, H.; Huang, X.; Wu, X.; Cao, X.; Tan, C.; Yin, Z.; Lu, X.; Sun, L.; Zhang, H. Carbon Microbelt Aerogel Prepared by Waste Paper: An Efficient and Recyclable Sorbent for Oils and Organic Solvents. Small 2014, 10, 3544–3550. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose Aerogel from Paper Waste for Crude Oil Spill Cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Tang, Z.; Hess, D.W.; Breedveld, V. Fabrication of oleophobic paper with tunable hydrophilicity by treatment with non-fluorinated chemicals. J. Mater. Chem. A 2015, 3, 14651–14660. [Google Scholar] [CrossRef]
- Do, N.H.N.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and application of pineapple aerogels from agricultural waste. Mater. Technol. 2020, 35, 807–814. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Bastani, D.; Safekordi, A.A.; Alihosseini, A.; Taghikhani, V. Study of oil sorption by expanded perlite at 298.15K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Li, A.; Sun, H.-X.; Tan, D.-Z.; Fan, W.-J.; Wen, S.-H.; Qing, X.-J.; Li, G.-X.; Li, S.-Y.; Deng, W.-Q. Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ. Sci. 2011, 4, 2062–2065. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Dong, X.; Chen, J.; Ma, Y.; Wang, J.; Chan-Park, M.B.; Liu, X.; Wang, L.; Huang, W.; Chen, P. Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem. Commun. 2012, 48, 10660. [Google Scholar] [CrossRef]
- Ali, N.; El-Harbawi, M.; Jabal, A.A.; Yin, C.-Y. Characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: Oil removal suitability matrix. Environ. Technol. 2012, 33, 481–486. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Superhydrophobic kapok fiber oil-absorbent: Preparation and high oil absorbency. Chem. Eng. J. 2012, 213, 1–7. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Li, J. Synthesis of carbon fiber aerogel from natural bamboo fiber and its application as a green high-efficiency and recyclable adsorbent. Mater. Des. 2016, 107, 26–32. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Abdelwahab, O. Assessment of raw luffa as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. Alex. Eng. J. 2014, 53, 213–218. [Google Scholar] [CrossRef]
- Singh, V.; Jinka, S.; Hake, K.; Parameswaran, S.; Kendall, R.J.; Ramkumar, S. Novel Natural Sorbent for Oil Spill Cleanup. Ind. Eng. Chem. Res. 2014, 53, 11954–11961. [Google Scholar] [CrossRef]
- Lim, T.-T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic–oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.A.; Rahmah, A.U.; Man, Z. Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. J. Hazard. Mater. 2010, 177, 683–691. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Oil removal from water using biomaterials. Bioresour. Technol. 2010, 101, 6594–6600. [Google Scholar] [CrossRef]
- Wang, Z.; Barford, J.P.; Hui, C.W.; McKay, G. Kinetic and equilibrium studies of hydrophilic and hydrophobic rice husk cellulosic fibers used as oil spill sorbents. Chem. Eng. J. 2015, 281, 961–969. [Google Scholar] [CrossRef]
- Kumagai, S.; Noguchi, Y.; Kurimoto, Y.; Takeda, K. Oil adsorbent produced by the carbonization of rice husks. Waste Manag. 2007, 27, 554–561. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review. Molecules 2020, 25, 4522. https://doi.org/10.3390/molecules25194522
Zamparas M, Tzivras D, Dracopoulos V, Ioannides T. Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review. Molecules. 2020; 25(19):4522. https://doi.org/10.3390/molecules25194522
Chicago/Turabian StyleZamparas, Miltiadis, Dimitrios Tzivras, Vassilios Dracopoulos, and Theophilos Ioannides. 2020. "Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review" Molecules 25, no. 19: 4522. https://doi.org/10.3390/molecules25194522
APA StyleZamparas, M., Tzivras, D., Dracopoulos, V., & Ioannides, T. (2020). Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review. Molecules, 25(19), 4522. https://doi.org/10.3390/molecules25194522








