Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of TM Phytochemicals
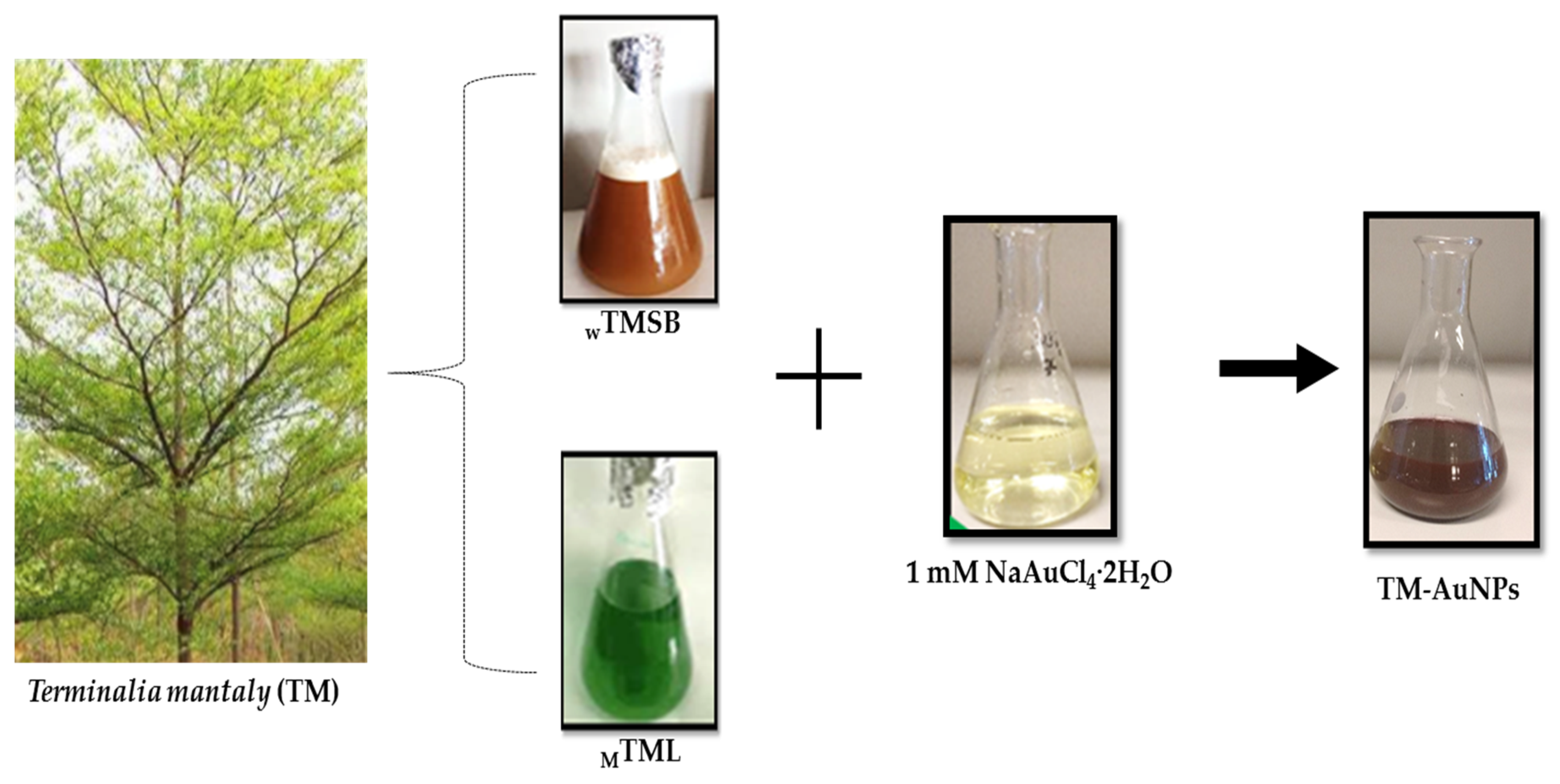
2.2. Green Synthesis of AuNPs
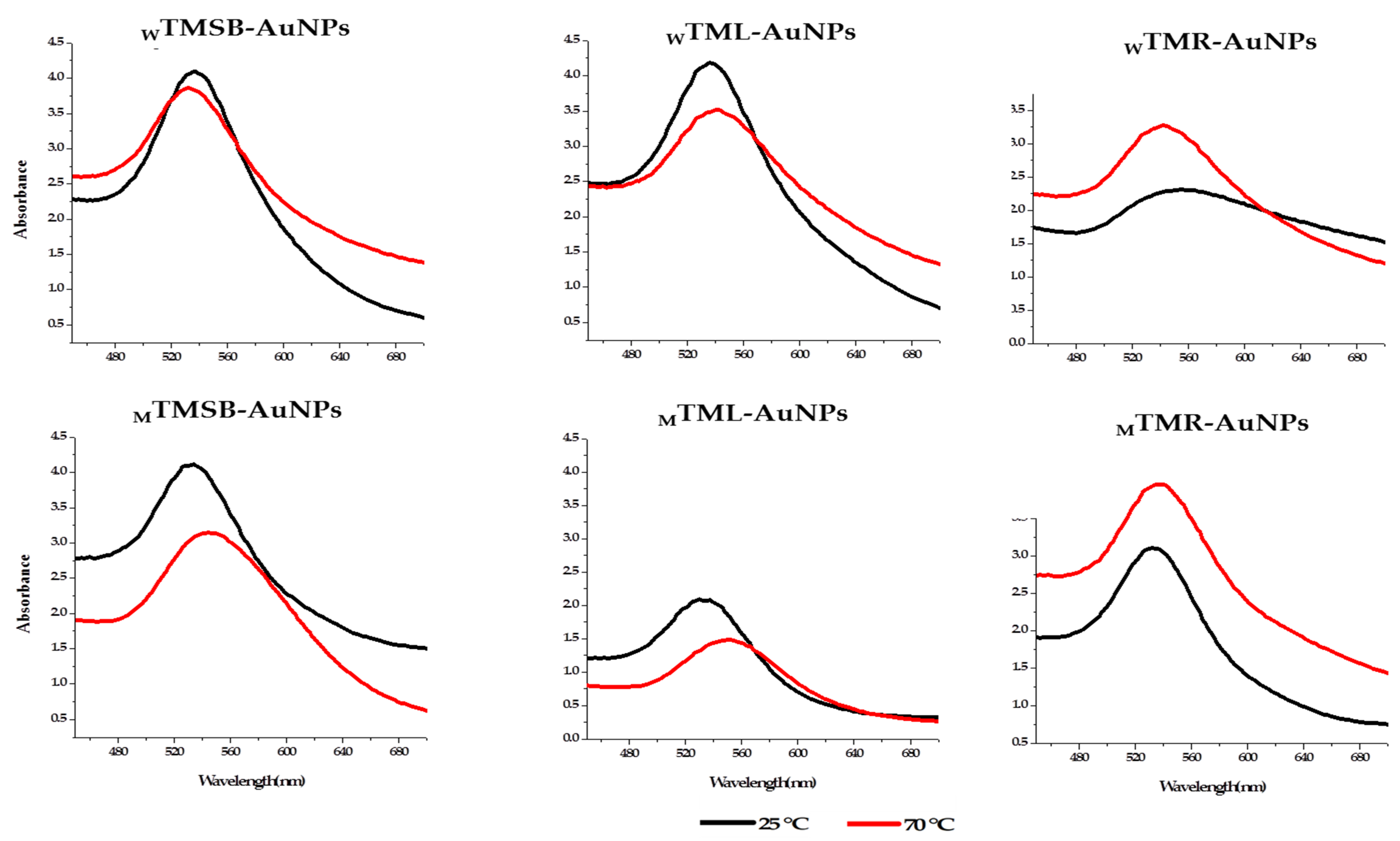
2.2.1. Optimization of TM-AuNP Synthesis and UV–Vis Spectroscopy
2.2.2. DLS Analysis of TM-AuNPs
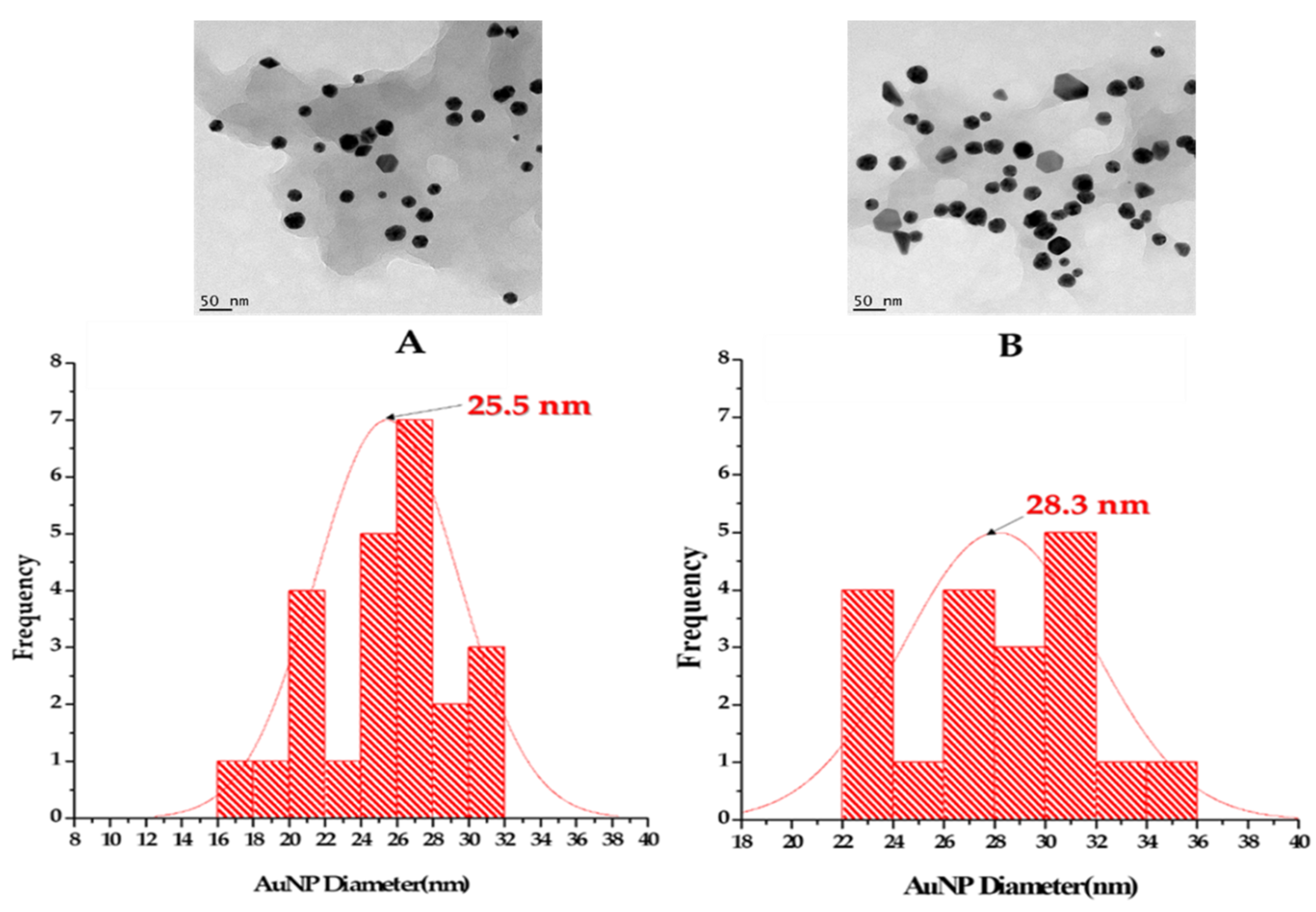
2.2.3. HRTEM, SAED and EDX Analyses
2.2.4. FTIR Analysis of TM-AuNPs
2.3. Effects of TM Extracts and AuNPs on Cancer Cells
3. Materials and Methods
3.1. Collection and Processing of Plant Material
3.2. Plant Extraction and Phytochemical Analysis
3.2.1. Preparation of Crude Extracts
3.2.2. Qualitative Phytochemical Analysis
3.3. Biosynthesis of AuNPs and Characterization
3.3.1. Green Synthesis of AuNPs
3.3.2. Characterization of TM-AuNPs
UV–Visible Spectroscopy
DLS Analysis
TM-AuNPs HRTEM, EDX and SAED Pattern Analysis
FTIR Spectroscopy Measurements
3.4. Cell Viability Using MTT Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shalom, J.; Cock, I.E. Terminalia ferdinandiana Exell. Fruit and leaf extracts inhibit proliferation and induce apoptosis in selected human cancer cell lines. Nutr. Cancer 2018, 70, 579–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngouana, K.T.; Mbouna, J.C.D.; Kuipou, T.R.M.; Tchuenmogne, M.A.T.; Zeuko’o, M.E.; Ngouana, V.; Mallié, M.; Bertout, S.; Boyom, F.F. Potent and synergistic extract combinations from Terminalia catappa, Terminalia mantaly and Monodora tenuifolia against pathogenic yeasts. Medicines 2015, 2, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Majoumouo, M.S.; Dube, A.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from Terminalia mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef] [Green Version]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects of Gold Nanoparticles synthesized from Hypoxis hemerocallidea aqueous extract and hypoxoside on macrophage and natural killer cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankamwar, B. Biosynthesis of gold nanoparticles (Green-gold) using leaf extract of Terminalia Catappa. J. Chem. 2010, 7, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Dudhane, A.A.; Waghmode, S.R.; Dama, L.B.; Mhaindarkar, V.P.; Sonawane, A.; Katariya, S. Synthesis and Characterization of gold nanoparticles using plant extract of Terminalia arjuna with antibacterial activity. J. Nanosci. Nanotechnol. 2019, 15, 75–82. [Google Scholar]
- Mitra, M.; Bandyopadhyay, A.; Datta, G.; Nandi, D.K. Protective Role of Green synthesized gold nanoparticles using Terminalia arjuna against acetaminophen induced hematological alterations in male Wistar rats. J. Nanomed. Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Annavaram, V.; Posa, V.R.; Lakshmi, D.V.; Sumalatha, J.; Somala, A.R. Terminalia bellirica fruit extract mediated synthesis of gold nanoparticles (AuNPs) and studies on antimicrobial and antioxidant activity. Inorg. Nano-Metal Chem. 2016, 47, 681–687. [Google Scholar] [CrossRef]
- Lee, S.Y.; Krishnamurthy, S.; Cho, C.-W.; Yun, Y.-S. Biosynthesis of gold nanoparticles using Ocimum sanctum extracts by solvents with different polarity. ACS Sustain. Chem. Eng. 2016, 4, 2651–2659. [Google Scholar] [CrossRef]
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.; Ruwanthika, R.W.D.; De Silva, R.M.; Udagama, P. honey mediated green synthesis of nanoparticles: New era of safe nanotechnology. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef] [PubMed]
- Jafarizad, A.; Safaee, K.; Gharibian, S.; Omidi, Y.; Ekinci, D. Biosynthesis and in-vitro study of gold nanoparticles using Mentha and Pelargonium extracts. Procedia Mater. Sci. 2015, 11, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Al-Marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Tremel, W.; et al. Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (Miswak) extract. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlin, M.; Vladimir, B. Stability of nanoparticles suspensions in different biologically relevant media. Dig. J. Nanomater. Biostruct. 2012, 7, 1389–1400. [Google Scholar]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of bacteria associated with wound infection by biocompatible green synthesized gold nanoparticles from south african plant extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Simchi, A.; Imani, M. Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J. Phys. Chem. C 2009, 113, 9573–9580. [Google Scholar] [CrossRef]
- De Aberasturi, D.J.; Serrano-Montes, A.B.; Liz-Marzán, L.M. modern applications of plasmonic nanoparticles: From energy to health. Adv. Opt. Mater. 2015, 3, 602–617. [Google Scholar] [CrossRef]
- Suvarna, S.; Das, U.; Kc, S.; Mishra, S.; Sudarshan, M.; Das Saha, K.; Dey, S.; Chakraborty, A.; Yerol, N. Synthesis of a novel glucose capped gold nanoparticle as a better theranostic candidate. PLoS ONE 2017, 12, e0178202. [Google Scholar] [CrossRef]
- Peeyush, K.; Sapna, M.; Malik, A.; Santosh, S. Insecticidal properties of Mentha species: A review. Ind. Crops. Prod. 2011, 34, 802–817. [Google Scholar]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Hiremath, J.; Singh, A.K.; Mathew, J. Optimization and Characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gräfe, C.; Weidner, A.; Lühe, M.V.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle–cell interaction. Int. J. Biochem. Cell Boil. 2016, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Z.; Jia, K.; Zhang, W.; Dang, M. Rabdosia rubescens Linn: Green synthesis of gold nanoparticles and their anticancer effects against human lung cancer cells A549. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, N.; Yasin, H.M.; Younus, M.; Ahmed, W.; Rehman, N.U.; Zakaullah, M.; Iqbal, M.Z. Synthesis and spectroscopic characterization of gold nanoparticles via plasma-liquid interaction technique. AIP Adv. 2018, 8, 015130. [Google Scholar] [CrossRef]
- Naz, F.; Koul, V.; Srivastava, A.; Gupta, Y.K.; Dinda, A.K. Biokinetics of ultrafine gold nanoparticles (AuNPs) relating to redistribution and urinary excretion: A long-term in vivo study. J. Drug Target. 2016, 24, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Kesarla, M.K.; Mandal, B.K.; Bandapalli, P.R. Gold nanoparticles by Terminalia bellirica aqueous extract–a rapid green method. J. Exp. Nanosci. 2014, 9, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Ajdari, Z.; Rahman, H.S.; Shameli, K.; Abdullah, R.; Ghani, M.A.; Yeap, S.K.; Abbasiliasi, S.; Ajdari, D.; Ariff, A.B. Novel Gold Nanoparticles Reduced by Sargassum glaucescens: Preparation, Characterization and Anticancer Activity. Molecules 2016, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Mohammadhosseini, M.; Nejati-Koshki, K.; Abadi, A.J.N.; Moafi, H.F.; Akbarzadeh, A.; Farshbaf, M. Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug Res. 2017, 67, 77–87. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Webster, T.J. Growth process and anticancer properties of gold nanorods. J. Biomed. Mater. Res. Part A 2017, 105, 2616–2621. [Google Scholar] [CrossRef]
- Banu, H.; Renuka, N.; Faheem, S.; Ismail, R.; Singh, V.; Saadatmand, Z.; Khan, S.S.; Narayanan, K.; Raheem, A.; Premkumar, K.; et al. Gold and silver nanoparticles biomimetically synthesized using Date Palm pollen extract-induce apoptosis and regulate p53 and Bcl-2 expression in human breast adenocarcinoma cells. Boil. Trace Element Res. 2018, 186, 122–134. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thipe, V.C.; Panjtan Amiri, K.; Bloebaum, P.; Raphael Karikachery, A.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edison, T.J.I.; Sethuraman, M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process. Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Singh, C. Biocompatible synthesis of silver and gold nanoparticles using leaf extract of Dalbergia sissoo. Adv. Mater. Lett. 2012, 3, 279–285. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Dehghanizade, S.; Arasteh, J.; Mirzaie, A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol. 2017, 46, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiri, Y.; Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Rao, Y.; Inwati, G.K.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Futur. Sci. OA 2017, 3, FSO239. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kim, S.Y.; Kim, J.Y.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.-H. Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-κB and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomed. 2019, 14, 2945. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, L.; Arunachalam, J. Microwave-Assisted green synthesis of small gold nanoparticles using aqueous Garlic (Allium sativum) extract: Their application as antibiotic carriers. Int. J. Green Nanotechnol. 2012, 4, 163–173. [Google Scholar] [CrossRef]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int. J. Nanomed. 2016, 11, 3691. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, P.; Yan, L.; Liu, L. Synthesis of gold nanoparticles with Solanum xanthocarpum extract and their in vitro anticancer potential on nasopharyngeal carcinoma cells. Int. J. Nanomed. 2018, 13, 7047–7059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef] [Green Version]
- Grabinski, C.M. Nanoparticle Deposition and Dosimetry for In Vitro Toxicology. Ph.D. Thesis, Doctor of Philosophy. Case Western Reserve University, Cleveland, OH, USA, May 2015; 134p. [Google Scholar]
- Chanda, N.; Shukla, R.; Zambre, A.; Mekapothula, S.; Kulkarni, R.R.; Katti, K.; Bhattacharyya, K.; Fent, G.M.; Casteel, S.W.; Boote, E.J.; et al. An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom ct imaging and photoacoustic detection of cancerous cells. Pharm. Res. 2010, 28, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Moraes, C.M.; De Paula, E.; Rosa, A.H.; Fraceto, L.F. Physicochemical stability of poly(lactide-co-glycolide) nanocapsules containing the local anesthetic Bupivacaine. J. Braz. Chem. Soc. 2010, 21, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Lyklema, J.; Leeuwen, V.H.P.; Minor, M. DLVO–theory, a dynamic re–interpretation. Adv. Colloid Interface Sci. 1999, 83, 33–69. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Patel, V.R. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.M.; Mandal, B.K.; Sinha, M.; Krishnakumar, V. Terminalia chebula mediated green and rapid synthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Gopinath, K. Green synthesis, characterization of silver, gold and bimetallic nanoparticles using bark extract of Terminalia arjuna and their larvicidal activity against malaria vector, anopheles stephensi. Int. J. Recent Sci. Res. 2013, 4, 904–910. [Google Scholar]
- Singh, M.; McKenzie, K.; Ma, X. Effect of dimethyl sulfoxide on in vitro proliferation of skin fibroblast cells. J. Biotech Res. 2017, 8, 78–82. [Google Scholar]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Kostarelos, K. Cytotoxic Assessment of Carbon Nanotube Interaction with Cell Cultures. Biomed. Nanotechnol. Hum. Press. 2011, 726, 299–312. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Jangholi, E.; Jafari, M.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Kolagar, H.G.; Jalil, S.K.; et al. Comparison of In Vitro Cytotoxicity and Apoptogenic Activity of Magnesium Chloride and Cisplatin as Conventional Chemotherapeutic Agents in the MCF-7 Cell Line. Asian Pac. J. Cancer Prev. 2016, 17, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Nandagopal, S.; Kumar, G.A.; Dhanalakshmi, D.P.; Prakash, P. Bioprospecting the antibacterial and anticancer activities of silver nanoparticles synthesized using Terminalia chebula seed extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 36837. [Google Scholar]
- Kaur, S.; Michael, H.; Arora, S.; Härkönen, P.L.; Kumar, S. The in vitro cytotoxic and apoptotic activity of Triphala—An Indian herbal drug. J. Ethnopharmacol. 2005, 97, 15–20. [Google Scholar] [CrossRef]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlaja, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. Fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Paino, I.M.M.; Marangoni, V.S.; de Oliveira, R.C.S.; Antunes, L.M.G.; Zucolotto, V. Cytotoxicity and genotoxicity of gold nanoparticles in human hepatocellular carcinoma and peripheral blood mononuclear cells. Toxicol. Lett. 2012, 215, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Moses, S.L.; Edwards, V.M.; Brantley, E. Cytotoxicity in MCF-7 and MDA-MB-231 Breast Cancer Cells, without Harming MCF-10A Healthy Cells. J. Nanomed. Nanotechnol. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Abel, E.E.; Poonga, P.R.J.; Panicker, S.G. Characterization and in vitro studies on anticancer, antioxidant activity against colon cancer cell line of gold nanoparticles capped with Cassia tora SM leaf extract. Appl. Nanosci. 2016, 6, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, K.D.; Arun, L.B.; Annamalai, S.K.; Arunachalam, A.M. Biofunctionalized gold nanoparticles synthesis from Gymnema sylvestre and its preliminary anticancer activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 423–430. [Google Scholar]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Electron. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Gautam, R.; Priyadarsini, E.; Rajamani, P.; Pradhan, S.N.; Saravanan, M.; Meena, R. Piper betle: Augmented Synthesis of Gold Nanoparticles and Its In-vitro Cytotoxicity Assessment on HeLa and HEK293 Cells. J. Clust. Sci. 2019, 31, 133–145. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods. A Guide of Modern Techniques of Plants Analysis; Chapman and Hall: London, UK, 1976; p. 150. [Google Scholar]
- Sofowora, A.; Odebeyi. Medicinal Plants and Traditional Medicinal in Africa, 2nd ed.; Spectrum Book Ltd.: Ibadan, Nigeria, 1993; pp. 20–70. [Google Scholar]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [Green Version]
- Mmola, M.; Le Roes-Hill, M.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.; Beukes, D.R.; Antunes, E.; Roes-Hill, M. Enhanced Antimicrobial and Anticancer Activity of Silver and Gold Nanoparticles Synthesised Using Sargassum incisifolium Aqueous Extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the TM-extracts are available on request from the authors. |
| TM Extracts | Alkaloids | Phenolic content | Flavonoids | Tannins | Anthocyanins | Anthraquinones | Steroids | Triterpenes | Glucosides | Saponins |
|---|---|---|---|---|---|---|---|---|---|---|
| WTMSB | + | + | + | + | - | + | + | + | + | + |
| MTMSB | + | + | + | - | - | + | - | - | + | + |
| WTMR | + | + | + | + | - | + | - | - | + | + |
| MTMR | + | + | + | - | - | - | - | - | + | + |
| WTML | + | + | + | + | - | + | + | + | + | + |
| MTML | + | + | + | + | - | - | - | + | + | - |
| AuNPs | AuNPs at 25 °C | AuNPs at 70 °C | ||
|---|---|---|---|---|
| OC | λmax | OC | λmax | |
| (mg/mL) | (nm) | (mg/mL) | (nm) | |
| WTMSB-AuNPs | 1.56 | 545 | 1.56 | 540 |
| MTMSB-AuNPs | 1.56 | 550 | 1.56 | 535 |
| WTMR-AuNPs | 6.25 | 560 | 6.25 | 550 |
| MTMR-AuNPs | 1.56 | 540 | 1.56 | 550 |
| WTML-AuNPs | 1.56 | 540 | 1.56 | 545 |
| MTML-AuNPs | 3.12 | 544 | 1.56 | 544 |
| TM-AuNPs | AuNPs at 25 °C | AuNPs at 70 °C | ||||
|---|---|---|---|---|---|---|
| PD (nm) | Pdi | ZP (mv) | PD (nm) | Pdi | ZP (mv) | |
| WTMSB-AuNPs | 39 | 0.5 | −27 | 57 | 0.3 | −28 |
| MTMSB-AuNPs | 44 | 0.5 | −36 | 52 | 0.5 | −29 |
| WTMR-AuNPs | 66 | 0.7 | −32 | 79 | 0.8 | −29 |
| MTMR-AuNPs | 44 | 0.4 | −30 | 48 | 0.5 | −32 |
| WTML-AuNPs | 43 | 0.6 | −37 | 44 | 0.7 | −35 |
| MTML-AuNPs | 55 | 0.4 | −29 | 51 | 0.5 | −10 |
| AuNPs | AuNP Core Size (nm) | |
|---|---|---|
| 25 °C | 70 °C | |
| WTMSB-AuNPs | 35.5 | 43.0 |
| MTMSB-AuNPs | 25.5 | 28.3 |
| WTML-AuNPs | 26.5 | 21.5 |
| MTML-AuNPs | 23.5 | 25.0 |
| WTMR-AuNPs | 32.0 | 33.5 |
| MTMR-AuNPs | 26.0 | 29.5 |
| Treatments | IC50 Values (µg/mL) | ||
|---|---|---|---|
| HepG2 | Caco-2 | MCF-7 | |
| WTML | 61.19 ± 0.00 | 90.19 ± 0.12 | 66.84 ± 0.01 |
| MTML | 75.07 ± 0.01 | 87.34 ± 0.00 | 72.44 ± 0.00 |
| WTMSB | 93.73 ± 0.00 | 62.66 ± 0.01 | 49.23 ± 0.00 |
| MTMSB | 41.28 ± 0.02 | 76.37 ± 0.01 | 19.73 ± 0.02 |
| WTMR | 90.47 ± 0.01 | 73.03 ± 0.00 | 43.30 ± 0.00 |
| MTMR | 43.24 ± 0.13 | 89.02 ± 0.00 | 2.73 ± 0.01 |
| WTML-AuNPs (25 °C) | 63.09 ± 0.00 | 5.71 ± 0.03 | 6.56 ± 0.01 |
| WTML-AuNPs (70 °C) | 41.74 ± 0.06 | 5.71 ± 0.31 | 32.59 ± 0.10 |
| MTML-AuNPs (25 °C) | 38.75 ± 0.01 | 41.20 ± 0.03 | 15.37 ± 0.06 |
| MTML-AuNPs (70 °C) | 85.07 ± 0.05 | 18.37 ± 0.41 | 54.56 ± 0.02 |
| WTMSB-AuNPs (25 °C) | 66.29 ± 0.01 | 20.34 ± 0.05 | 65.15 ± 0.02 |
| WTMSB-AuNPs (70 °C) | 63.09 ± 0.21 | 7.04 ± 0.01 | 1.77 ± 0.01 |
| MTMSB-AuNPs (25 °C) | 30.56 ± 0.10 | 75.83 ± 0.01 | 54.46 ± 0.01 |
| MTMSB-AuNPs (70 °C) | 36.26 ± 0.07 | 6.56 ± 0.05 | 1.24 ± 0.00 |
| MTMR-AuNPs (25 °C) | 0.18 ± 0.01 | 23.58 ± 0.02 | 3.36 ± 0.20 |
| MTMR-AuNPs (70 °C) | 90.85 ± 0.08 | 31.51 ± 0.03 | 6.23 ± 0.01 |
| WTMR –AuNPs (25 °C) | 88.59 ± 0.11 | 4.74 ± 0.01 | 1.24 ± 0.01 |
| WTMR-AuNPs (70 °C) | 58.03 ± 0.41 | 40.42 ± 0.04 | 6.29 ± 0.01 |
| % Cell Viability | |||
| 10% DMSO | 17.04 ± 0.06 | 34.24 ± 0.22 | 55.23 ± 0.14 |
| 100 µg/mL Cisplatin | 20.6 ± 0.34 | 39.2 ± 1.67 | 1.9 ± 0.04 |
| AuNPs | CC50 (µg/mL) | Selectivity Index (CC50/IC50) | ||
|---|---|---|---|---|
| KMST-6 | MCF-7 | HepG2 | Caco-2 | |
| MTMR-AuNPs (25 °C) | 275.7 ± 0.010 | 82.05 ± 0.010 | 1531.66 ± 0.100 | 11.69 ± 0.500 |
| MTMSB-AuNPs (70 °C) | 334.5 ± 0.020 | 269.75 ± 0.100 | 9.23 ± 0.110 | 50.99 ± 0.100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. https://doi.org/10.3390/molecules25194469
Majoumouo MS, Sharma JR, Sibuyi NRS, Tincho MB, Boyom FF, Meyer M. Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules. 2020; 25(19):4469. https://doi.org/10.3390/molecules25194469
Chicago/Turabian StyleMajoumouo, Michele S., Jyoti R. Sharma, Nicole R. S. Sibuyi, Marius B. Tincho, Fabrice F. Boyom, and Mervin Meyer. 2020. "Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells" Molecules 25, no. 19: 4469. https://doi.org/10.3390/molecules25194469
APA StyleMajoumouo, M. S., Sharma, J. R., Sibuyi, N. R. S., Tincho, M. B., Boyom, F. F., & Meyer, M. (2020). Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules, 25(19), 4469. https://doi.org/10.3390/molecules25194469






