Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials
Abstract
1. Introduction
2. Polysaccharide Nanomaterials
2.1. Cellulose Nanomaterials
2.2. Chitin Nanomaterials
2.3. Starch Nanomaterials
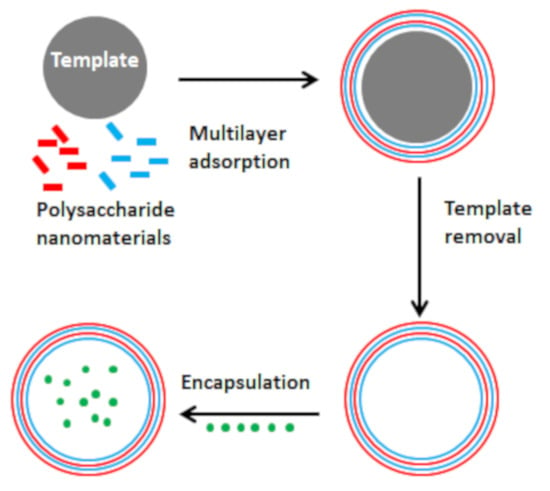
3. Methods of Microcapsule Fabrication
4. Microcapsules Based on Polysaccharide Nanomaterials
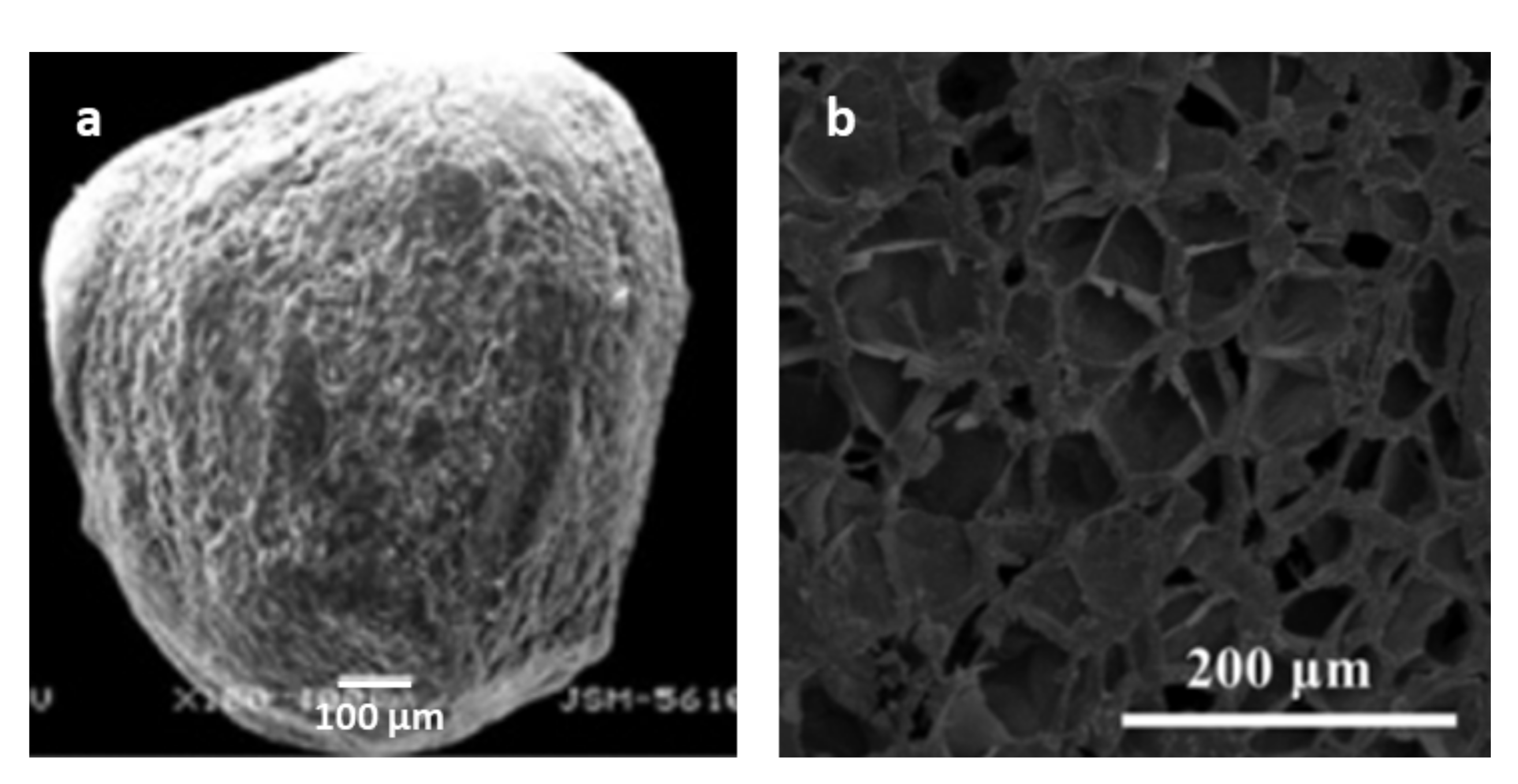
4.1. Ion Cross-Linked Polysaccharide Microcapsules Reinforced with Polysaccharide Nanomaterials
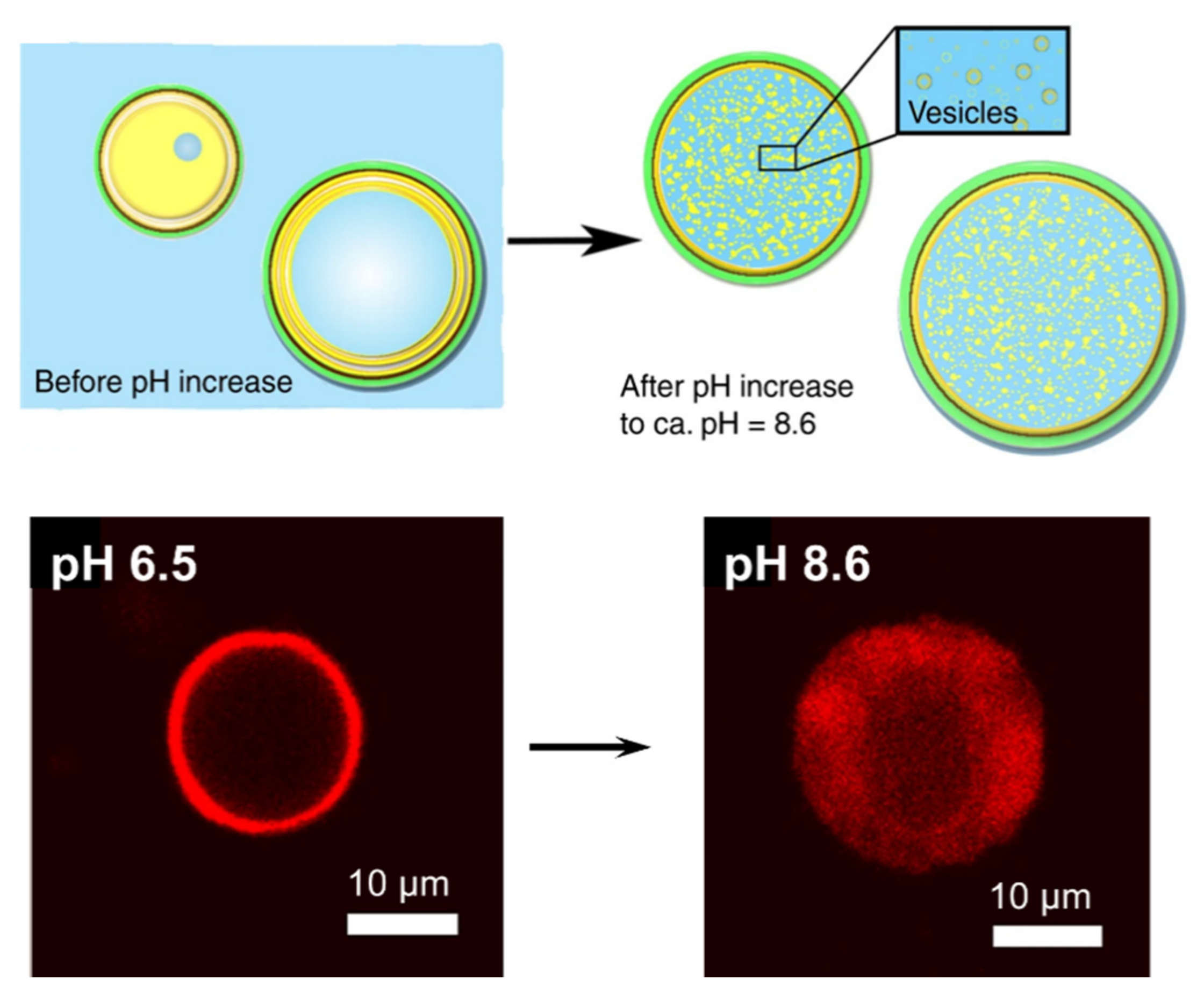
4.2. Biomimetic Polysaccharide Nanomaterials Microcapsules Stabilized by Electrostatic and H-Bond Interactions
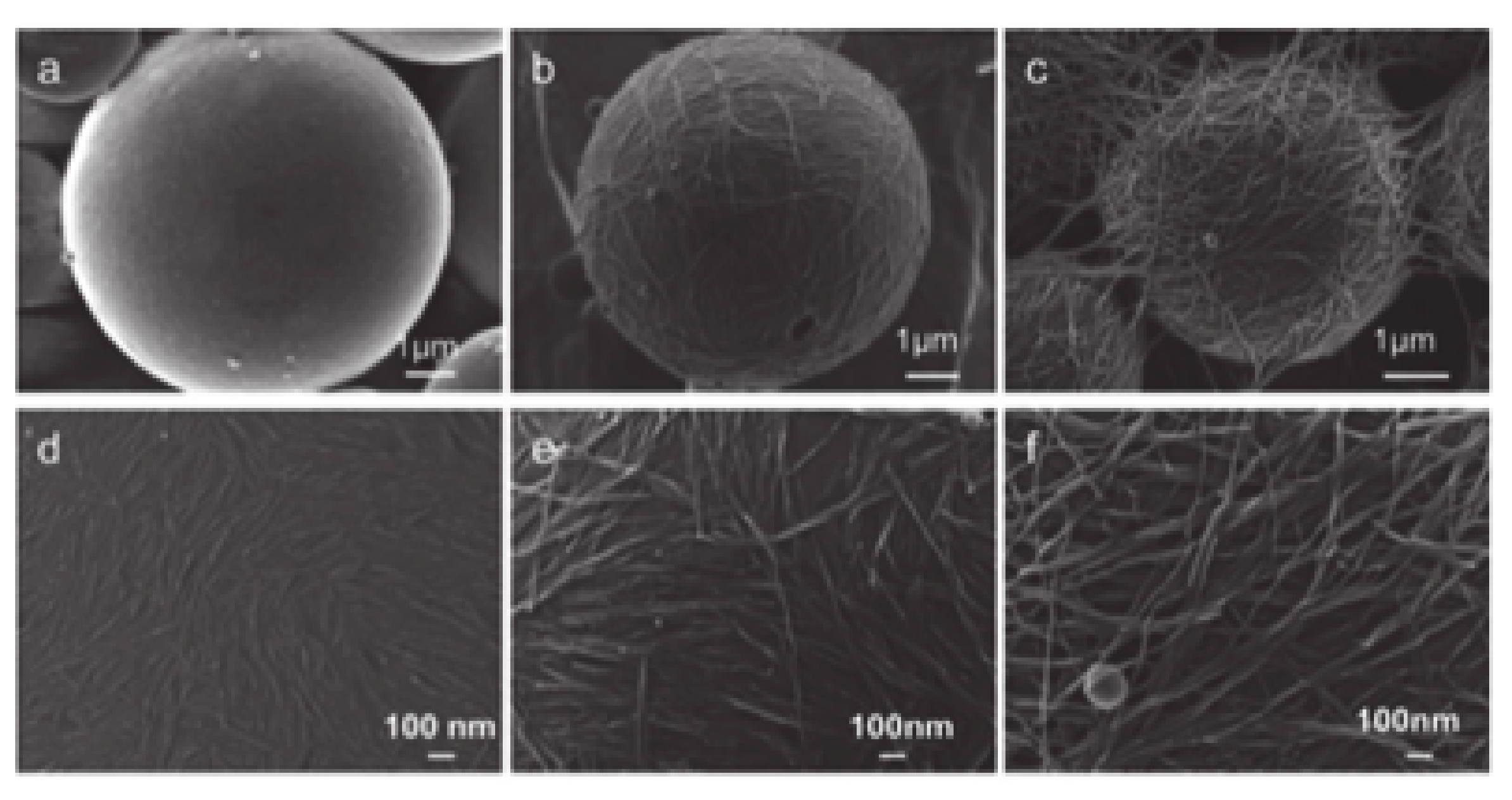
4.3. Microcapsules Prepared by In Situ Core Formation Stabilized by Polysaccharide Nanomaterials
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A Review of Applications in the Food and Pharmaceutical Industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.; Moldão-Martins, M.; Alves, V. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application--a review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef]
- Hu, X.P.; Shasha, B.S.; McGuire, M.R.; Prokopy, R.J. Controlled release of sugar and toxicant from a novel device for controlling pest insects. J. Control. Release 1998, 50, 257–265. [Google Scholar] [CrossRef]
- Han, N.X.; Xing, F. A Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2016, 10. [Google Scholar] [CrossRef]
- Urbas, R.; Milošević, R.; Kašiković, N.; Pavlović, Ž.; Elesini, U.S. Microcapsules application in graphic arts industry: A review on the state-of-the-art. Iran. Polym. J. 2017, 26, 541–561. [Google Scholar] [CrossRef]
- Jia, Y.; Feng, X.; Li, J. Polysaccharides-Based Microcapsules. In Supramolecular Chemistry of Biomimetic Systems; Li, J., Ed.; Springer Singapore: Singapore, 2017; pp. 63–84. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sukhorukov, G.B.; Möhwald, H. The pros and cons of polyelectrolyte capsules in drug delivery. Expert Opin. Drug Deliv. 2009, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, H.; Schroën, K.; Boom, R. Biodegradable polymeric microcapsules: Preparation and properties. Chem. Eng. J. 2011, 169, 1–10. [Google Scholar] [CrossRef]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Feng, X.; Du, C.; Li, J. Molecular Assembly of Polysaccharide-Based Microcapsules and Their Biomedical Applications. Chem. Rec. 2016, 16, 1991–2004. [Google Scholar] [CrossRef]
- Carrick, C.; Ruda, M.; Pettersson, B.; Larsson, P.T.; Wågberg, L. Hollow cellulose capsules from CO2 saturated cellulose solutions—their preparation and characterization. RSC Adv. 2013, 3, 2462. [Google Scholar] [CrossRef]
- Carrick, C.; Larsson, P.A.; Brismar, H.; Aidun, C.; Wågberg, L. Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv. 2014, 4, 19061–19067. [Google Scholar] [CrossRef]
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2006, 14, 49–64. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Jewitt, T.S.; Horie, C.V. The long-term stability of cellulose ester coatings. Polym. Degrad. Stab. 1989, 26, 221–229. [Google Scholar] [CrossRef]
- Hussain, S.A.; Abdelkader, H.; Abdullah, N.; Kmaruddin, S. Review on micro-encapsulation with Chitosan for pharmaceuticals applications. MOJ Curr. Res. Rev. 2018, 1, 77–84. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, J.Z.; Malaki Nik, A. Chapter 17—Starch-Based Microencapsulation. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Swaston, UK, 2018; pp. 661–690. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Yin, Q.; Pan, S.; Yang, R.; Liu, D.; Ouyang, Y. Cellulose nanocrystals obtained from office waste paper and their potential application in PET packing materials. Carbohydr. Polym. 2018, 181, 376–385. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kochhar, T.; Sood, A.; Singh, B. Conversion of Potato Starch and Peel Waste to High Value Nanocrystals. Potato Res. 2018, 61, 341–351. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Wang, J.; Guan, A.; Naguib, H.E. Chitin nano-whiskers (CNWs) as a bio-based bio-degradable reinforcement for epoxy: Evaluation of the impact of CNWs on the morphological, fracture, mechanical, dynamic mechanical, and thermal characteristics of DGEBA epoxy resin. RSC Adv. 2019, 9, 11063–11076. [Google Scholar] [CrossRef]
- Lee, W.J.; Clancy, A.J.; Kontturi, E.; Bismarck, A.; Shaffer, M.S. Strong and Stiff: High-Performance Cellulose Nanocrystal/Poly(vinyl alcohol) Composite Fibers. ACS Appl. Mater. Interfaces 2016, 8, 31500–31504. [Google Scholar] [CrossRef]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wagberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef]
- Carsi, M.; Sanchis, M.J.; Gómez, C.M.; Rodriguez, S.; G Torres, F. Effect of Chitin Whiskers on the Molecular Dynamics of Carrageenan-Based Nanocomposites. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part. B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Kamel, S.; A Khattab, T. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; Al-ghamdi, A.A.; Soufan, W.H.; Abdelsalam, E.M.; Allam, A.A. Novel halochromic cellulose nanowhiskers from rice straw: Visual detection of urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- López-López, E.A.; Hernández-Gallegos, M.A.; Cornejo-Mazón, M.; Hernández-Sánchez, H. Polysaccharide-Based Nanoparticles. In Food Nanoscience and Nanotechnology; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002. [Google Scholar] [CrossRef]
- Gao, K.; Shao, Z.; Li, J.; Wang, X.; Peng, X.; Wang, W.; Wang, F. Cellulose nanofiber–graphene all solid-state flexible supercapacitors. J. Mater. Chem. A 2013, 1, 63–67. [Google Scholar] [CrossRef]
- Goodrich, J.D.; Winter, W.T. α-Chitin Nanocrystals Prepared from Shrimp Shells and Their Specific Surface Area Measurement. Biomacromolecules 2007, 8, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Wang, S.T.; Chang, K.B.; Tsai, M.L. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, H.; Zhang, L. Role of starch nanocrystals and cellulose whiskers in synergistic reinforcement of waterborne polyurethane. Carbohydr. Polym. 2010, 80, 665–671. [Google Scholar] [CrossRef]
- Melida, H.; Sandoval-Sierra, J.V.; Dieguez-Uribeondo, J.; Bulone, V. Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryot. Cell 2013, 12, 194–203. [Google Scholar] [CrossRef]
- Ek, R.; Gustafsson, C.; Nutt, A.; Iversen, T.; Nyström, C. Cellulose powder from Cladophora sp. algae. J. Mol. Recognit. 1998, 11, 263–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Lindström, M.E.; Li, J. Tunicate cellulose nanocrystals: Preparation, neat films and nanocomposite films with glucomannans. Carbohydr. Polym. 2015, 117, 286–296. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid Crystal Systems from fibrillar Polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Stef-Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Li, J.; Revol, J.F.; Marchessault, R.H. Effect of degree of deacetylation of chitin on the properties of chitin crystallites. J. Appl. Polym. Sci. 1997, 65, 373–380. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation of α-Chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Gedanken, A. Eco-Friendly and Facile Preparation of Spherical Chitin Nanoparticles. ChemistrySelect 2018, 3, 10787–10791. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and application of starch nanoparticles for nanocomposites: A review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Dufresne, A. Polymer Nanocomposites from Biological Sources. Encycl. Nanosci. Nanotechnol. 2007, 21, 59–83. [Google Scholar]
- Jackson, D.S. STARCH | Structure, Properties, and Determination. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5561–5567. [Google Scholar] [CrossRef]
- Dai, L.; Li, C.; Zhang, J.; Cheng, F. Preparation and characterization of starch nanocrystals combining ball milling with acid hydrolysis. Carbohydr. Polym. 2018, 180, 122–127. [Google Scholar] [CrossRef]
- Putaux, J.-L.; Molina-Boisseau, S.; Momaur, T.; Dufresne, A. Platelet Nanocrystals Resulting from the Disruption of Waxy Maize Starch Granules by Acid Hydrolysis. Biomacromolecules 2003, 4, 1198–1202. [Google Scholar] [CrossRef]
- Wei, B.; Xu, X.; Jin, Z.; Tian, Y. Surface chemical compositions and dispersity of starch nanocrystals formed by sulfuric and hydrochloric acid hydrolysis. PLoS ONE 2014, 9, e86024. [Google Scholar] [CrossRef]
- Visakh, P.M. Chapter 1 Starch: State-of-the-Art, New Challenges and Opportunities. In Starch-Based Blends, Composites and Nanocomposites; The Royal Society of Chemistry: London, UK, 2016; pp. 1–16. [Google Scholar]
- Xu, Y.; Sismour, E.N.; Grizzard, C.; Thomas, M.; Pestov, D.; Huba, Z.; Wang, T.; Bhardwaj, H.L. Morphological, Structural, and Thermal Properties of Starch Nanocrystals Affected by Different Botanic Origins. Cereal Chem. J. 2014, 91, 383–388. [Google Scholar] [CrossRef]
- Odeniyi, M.A.; Omoteso, O.A.; Adepoju, A.O.; Jaiyeoba, K.T. Starch nanoparticles in drug delivery: A review. Polim. W Med. 2018, 48, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Lee, D.W. Fabrication and characterization of microcapsules with polyamide–polyurea as hybrid shell. J. Mater. Sci. 2012, 47, 2040–2044. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Yang, F.; Ma, S.; Zong, W.; Luo, N.; Lv, M.; Hu, Y.; Zhou, L.; Han, X. Fabrication of pH sensitive microcapsules using soft templates and their application to drug release. RSC Adv. 2015, 5, 51271–51277. [Google Scholar] [CrossRef]
- Li, J.; Stover, H.D. Pickering emulsion templated layer-by-layer assembly for making microcapsules. Langmuir ACS J. Surf. Colloids 2010, 26, 15554–15560. [Google Scholar] [CrossRef]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Oza, K.P.; Frank, S.G. Microcrystalline Cellulose Stabilized emulsions. J. Dispers. Sci. Technol. 1986, 7, 543–561. [Google Scholar] [CrossRef]
- Oza, K.; Frank, S. Multiple Emulsions Stabilized by Colloidal Microcrystalline Cellulose. J. Dispers. Sci. Technol. 1989, 10, 163–185. [Google Scholar] [CrossRef]
- Ougiya, H.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F. Emulsion-stabilizing Effect of Bacterial Cellulose. Biosci. Biotechnol. Biochem. 1997, 61, 1541–1545. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir ACS J. Surf. Colloids 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Andresen, M.; Stenius, P. Water-in-oil Emulsions Stabilized by Hydrophobized Microfibrillated Cellulose. J. Dispers. Sci. Technol. 2007, 28, 837–844. [Google Scholar] [CrossRef]
- Blaker, J.J.; Lee, K.-Y.; Li, X.; Menner, A.; Bismarck, A. Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates. Green Chem. 2009, 11, 1321–1326. [Google Scholar] [CrossRef]
- Sebe, G.; Ham-Pichavant, F.; Pecastaings, G. Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate. Biomacromolecules 2013, 14, 2937–2944. [Google Scholar] [CrossRef]
- Salajková, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir ACS J. Surf. Colloids 2014, 30, 9327–9335. [Google Scholar] [CrossRef]
- Ben Ayed, E.; Cochereau, R.; Dechance, C.; Capron, I.; Nicolai, T.; Benyahia, L. Water-In-Water Emulsion Gels Stabilized by Cellulose Nanocrystals. Langmuir ACS J. Surf. Colloids 2018, 34, 6887–6893. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Bian, W.; Feng, L.; Wu, Z.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsions with regenerated chitin nanofibers. Food Chem. 2015, 183, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch Stärke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Starch nanocrystals as particle stabilisers of oil-in-water emulsions. J. Sci. Food Agric. 2014, 94, 1802–1807. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Liu, C.; Li, Y.; Lu, C.; Wang, P. Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr. Polym. 2012, 88, 1358–1363. [Google Scholar] [CrossRef]
- Song, X.; Pei, Y.; Zhu, W.; Fu, D.; Ren, H. Particle-stabilizers modified from indica rice starches differing in amylose content. Food Chem. 2014, 153, 74–80. [Google Scholar] [CrossRef]
- Marefati, A.; Sjöö, M.; Timgren, A.; Dejmek, P.; Rayner, M. Fabrication of encapsulated oil powders from starch granule stabilized W/O/W Pickering emulsions by freeze-drying. Food Hydrocoll. 2015, 51, 261–271. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.-W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Sontti, S.G.; Atta, A. Numerical Insights on Controlled Droplet Formation in a Microfluidic Flow-Focusing Device. Ind. Eng. Chem. Res. 2019, 59, 3702–3716. [Google Scholar] [CrossRef]
- Tong, W.; Song, X.; Gao, C. Layer-by-layer assembly of microcapsules and their biomedical applications. Chem. Soc. Rev. 2012, 41, 6103–6124. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Eyley, S.; Schütz, C.; van Gorp, H.; Rosenfeldt, S.; Van den Mooter, G.; Thielemans, W. Thermodynamic Study of the Interaction of Bovine Serum Albumin and Amino Acids with Cellulose Nanocrystals. Langmuir ACS J. Surf. Colloids 2017, 33, 5473–5481. [Google Scholar] [CrossRef]
- Lombardo, S.; Chen, P.; Larsson, P.A.; Thielemans, W.; Wohlert, J.; Svagan, A.J. Toward Improved Understanding of the Interactions between Poorly Soluble Drugs and Cellulose Nanofibers. Langmuir ACS J. Surf. Colloids 2018, 34, 5464–5473. [Google Scholar] [CrossRef] [PubMed]
- Cranston, E.D.; Gray, D.G. Polyelectrolyte Multilayer Films Containing Cellulose: A Review. In Model Cellulosic Surfaces; American Chemical Society: Washington, DC, USA, 2009; Volume 1019, pp. 95–114. [Google Scholar]
- Villares, A.; Moreau, C.; Capron, I.; Cathala, B. Chitin nanocrystal-xyloglucan multilayer thin films. Biomacromolecules 2014, 15, 188–194. [Google Scholar] [CrossRef]
- Qi, Z.D.; Saito, T.; Fan, Y.; Isogai, A. Multifunctional coating films by layer-by-layer deposition of cellulose and chitin nanofibrils. Biomacromolecules 2012, 13, 553–558. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable Nanocomposite Films Based on Sodium Alginate and Cellulose Nanofibrils. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Lemahieu, L.; Bras, J.; Tiquet, P.; Augier, S.; Dufresne, A. Extrusion of Nanocellulose-Reinforced Nanocomposites Using the Dispersed Nano-Objects Protective Encapsulation (DOPE) Process. Macromol. Mater. Eng. 2011, 296, 984–991. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Huq, T.; Riedl, B.; Bouchard, J.; Salmieri, S.; Lacroix, M. Microencapsulation of nisin in alginate-cellulose nanocrystal (CNC) microbeads for prolonged efficacy against Listeria monocytogenes. Cellulose 2014, 21, 4309–4321. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Jamshidian, M.; Salmieri, S.; Desjardins, N.; Sahraoui, A.; Lacroix, M. Effect of cellulose nanocrystals on thyme essential oil release from alginate beads: Study of antimicrobial activity against Listeria innocua and ground meat shelf life in combination with gamma irradiation. Cellulose 2019, 26, 5247–5265. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, W.; Wang, Y.; Ke, C.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 112–120. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Hui, L.; Shang, Z.; Yuan, S.; Dai, L.; Liu, P.; Liu, X.; Ni, Y. Lignin-containing cellulose nanocrystals/sodium alginate beads as highly effective adsorbents for cationic organic dyes. Int. J. Biol. Macromol. 2019, 139, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Gencer, A.; Schutz, C.; Van Rie, J.; Eyley, S.; Thielemans, W. Thermodynamic Study of Ion-Driven Aggregation of Cellulose Nanocrystals. Biomacromolecules 2019, 20, 3181–3190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Zwieniecki, M.A. Physical Limits to Leaf Size in Tall Trees. Phys. Rev. Lett. 2013, 110. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Rodriguez, S.A.; Allemann, E.; Fessi, H.; Doelker, E. Polymeric Nanoparticles for Oral Delivery of Drugs and Vaccines: A Critical Evaluation of In Vivo Studies. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 419–464. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Ye, C.; Malak, S.T.; Hu, K.; Wu, W.; Tsukruk, V.V. Cellulose Nanocrystal Microcapsules as Tunable Cages for Nano- and Microparticles. ACS Nano 2015, 9, 10887–10895. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Yao, K.; Andersson, R.L.; Mullertz, A.; Svagan, A.J. Bioinspired Layer-by-Layer Microcapsules Based on Cellulose Nanofibers with Switchable Permeability. Biomacromolecules 2017, 18, 1401–1410. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Svagan, A.J. Bioinspired capsules based on nanocellulose, xyloglucan and pectin—The influence of capsule wall composition on permeability properties. Acta Biomater. 2018, 69, 196–205. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Wieland, D.C.F.; Riazanova, A.V.; Dedinaite, A.; Gunther Pomorski, T.; Cardenas, M.; Svagan, A.J. Primary cell wall inspired micro containers as a step towards a synthetic plant cell. Nat. Commun. 2020, 11, 958. [Google Scholar] [CrossRef]
- Zykwinska, A.; Thibault, J.-F.; Ralet, M.-C. Competitive binding of pectin and xyloglucan with primary cell wall cellulose. Carbohydr. Polym. 2008, 74, 957–961. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Riazanova, A.V.; Wu, Q.; Crespo, G.A.; Svagan, A.J. Porous Cellulose Nanofiber-Based Microcapsules for Biomolecular Sensing. ACS Appl. Mater. Interfaces 2018, 10, 41146–41154. [Google Scholar] [CrossRef] [PubMed]
- Mele, S.; Soderman, O.; Ljusberg-Wahren, H.; Thuresson, K.; Monduzzi, M.; Nylander, T. Phase behavior in the biologically important oleic acid/sodium oleate/water system. Chem. Phys. Lipids 2018, 211, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Mukhopadhyay, S.; Rokhlenko, Y.; Nejati, S.; Boltyanskiy, R.; Choo, Y.; Loewenberga, M.; Osuji, C.O. Highly stiff yet elastic microcapsules incorporating cellulose nanofibrils. Soft Matter 2017, 13, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Babayekhorasani, F.; Spicer, P.T. Soft Bacterial Cellulose Microcapsules with Adaptable Shapes. Biomacromolecules 2019, 20, 4437–4446. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Chen, P.; Rojas, R.; Hajian, A.; Berglund, L. Cellulose nanofibers enable paraffin encapsulation and the formation of stable thermal regulation nanocomposites. Nano Energy 2017, 34, 541–548. [Google Scholar] [CrossRef]
- Levin, D.; Saem, S.; Osorio, D.A.; Cerf, A.; Cranston, E.D.; Moran-Mirabal, J.M. Green Templating of Ultraporous Cross-Linked Cellulose Nanocrystal Microparticles. Chem. Mater. 2018, 30, 8040–8051. [Google Scholar] [CrossRef]
- Jativa, F.; Schütz, C.; Bergström, L.; Zhang, X.; Wicklein, B. Confined self-assembly of cellulose nanocrystals in a shrinking droplet. Soft Matter 2015, 11, 5374–5380. [Google Scholar] [CrossRef]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical Self-Assembly of Cellulose Nanocrystals in a Confined Geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Modulation of starch nanoparticle surface characteristics for the facile construction of recyclable Pickering interfacial enzymatic catalysis. Green Chem. 2019, 21, 2412–2427. [Google Scholar] [CrossRef]
- Jimenez-Saelices, C.; Trongsatitkul, T.; Lourdin, D.; Capron, I. Chitin Pickering Emulsion for Oil Inclusion in Composite Films. Carbohydr. Polym. 2020, 242, 116366. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Jimenez Saelices, C.; Capron, I. Design of Pickering Micro- and Nanoemulsions Based on the Structural Characteristics of Nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Kuang, Y.; Guo, S.; Mo, L.; Ni, Y. Stabilization of Pickering emulsions with cellulose nanofibers derived from oil palm fruit bunch. Cellulose 2019, 27, 839–851. [Google Scholar] [CrossRef]
- Saidane, D.; Perrin, E.; Cherhal, F.; Guellec, F.; Capron, I. Some modification of cellulose nanocrystals for functional Pickering emulsions. Philos. Trans. Ser. AMath. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Nypelo, T.; Rodriguez-Abreu, C.; Kolen’ko, Y.V.; Rivas, J.; Rojas, O.J. Microbeads and hollow microcapsules obtained by self-assembly of pickering magneto-responsive cellulose nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 16851–16858. [Google Scholar] [CrossRef]
- Du, W.; Guo, J.; Li, H.; Gao, Y. Heterogeneously Modified Cellulose Nanocrystals-Stabilized Pickering Emulsion: Preparation and Their Template Application for the Creation of PS Microspheres with Amino-Rich Surfaces. ACS Sustain. Chem. Eng. 2017, 5, 7514–7523. [Google Scholar] [CrossRef]
- Werner, A.; Schmitt, V.; Sèbe, G.; Héroguez, V. Synthesis of surfactant-free micro- and nanolatexes from Pickering emulsions stabilized by acetylated cellulose nanocrystals. Polym. Chem. 2017, 8, 6064–6072. [Google Scholar] [CrossRef]
- Zhang, Y.; Karimkhani, V.; Makowski, B.T.; Samaranayake, G.; Rowan, S.J. Nanoemulsions and Nanolatexes Stabilized by Hydrophobically Functionalized Cellulose Nanocrystals. Macromolecules 2017, 50, 6032–6042. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Dubé, M.A.; Cranston, E.D. Cellulose Nanocrystals and Methyl Cellulose as Costabilizers for Nanocomposite Latexes with Double Morphology. ACS Sustain. Chem. Eng. 2017, 5, 10509–10517. [Google Scholar] [CrossRef]
- Jiménez Saelices, C.; Save, M.; Capron, I. Synthesis of latex stabilized by unmodified cellulose nanocrystals: The effect of monomers on particle size. Polym. Chem. 2019, 10, 727–737. [Google Scholar] [CrossRef]
- Butler, L.N.; Fellows, C.M.; Gilbert, R.G. Effect of surfactants used for binder synthesis on the properties of latex paints. Prog. Org. Coat. 2005, 53, 112–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; Gabriel, M.S.; Teixeira Neto, A.A.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric hollow microcapsules (PHM) via cellulose nanocrystal stabilized Pickering emulsion polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, Y.; Pun, J.; Mohamed Osman, A.S.; Tam, K.C. Polydopamine microcapsules from cellulose nanocrystal stabilized Pickering emulsions for essential oil and pesticide encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 403–413. [Google Scholar] [CrossRef]
- Zhang, Z.; Tam, K.C.; Wang, X.; Sèbe, G. Inverse Pickering Emulsions Stabilized by Cinnamate Modified Cellulose Nanocrystals as Templates to Prepare Silica Colloidosomes. ACS Sustain. Chem. Eng. 2018, 6, 2583–2590. [Google Scholar] [CrossRef]
- Svagan, A.J.; Musyanovych, A.; Kappl, M.; Bernhardt, M.; Glasser, G.; Wohnhaas, C.; Berglund, L.A.; Risbo, J.; Landfester, K. Cellulose nanofiber/nanocrystal reinforced capsules: A fast and facile approach toward assembly of liquid-core capsules with high mechanical stability. Biomacromolecules 2014, 15, 1852–1859. [Google Scholar] [CrossRef]
- Svagan, A.J.; Bender Koch, C.; Hedenqvist, M.S.; Nilsson, F.; Glasser, G.; Baluschev, S.; Andersen, M.L. Liquid-core nanocellulose-shell capsules with tunable oxygen permeability. Carbohydr. Polym. 2016, 136, 292–299. [Google Scholar] [CrossRef]
- Nordenstrom, M.; Riazanova, A.V.; Jarn, M.; Paulraj, T.; Turner, C.; Strom, V.; Olsson, R.T.; Svagan, A.J. Superamphiphobic coatings based on liquid-core microcapsules with engineered capsule walls and functionality. Sci. Rep. 2018, 8, 3647. [Google Scholar] [CrossRef]
- Han, S.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. Fabrication of melamine–urea–formaldehyde/paraffin microcapsules modified with cellulose nanocrystals via in situ polymerization. J. Mater. Sci. 2019, 54, 7383–7396. [Google Scholar] [CrossRef]
- Perrin, E.; Bizot, H.; Cathala, B.; Capron, I. Chitin nanocrystals for Pickering high internal phase emulsions. Biomacromolecules 2014, 15, 3766–3771. [Google Scholar] [CrossRef]
- Haaj, S.B.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystal stabilized Pickering emulsion polymerization for nanocomposites with improved performance. ACS Appl. Mater. Interfaces 2014, 6, 8263–8273. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, C.; Zou, S.; Liu, H.; Tong, Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. [Google Scholar] [CrossRef]


| Nanomaterial | Encapsulated Specie | Properties Studied | Ref |
|---|---|---|---|
| CNC ChiNC SNC | Theophilline | Swelling in water - loading and release | [117] |
| CNC | Probiotics | swelling and dissolution under simulated gastrointestinal conditions | [122] |
| CNC | Nisin | loading and release | [123] |
| CNC | Thyme oil | loading and release | [124] |
| Bacterial CNC | α-Calcidol | loading and release | [125] |
| CNC | Methylene blue | Thermodynamics of interactions | [126] |
| CNC (containing lignin) | Methylene blue | Thermodynamics of interactions | [128] |
| CNC | Pb2+ | Thermodynamics of interactions | [127] |
| Template Used | Coating Components | Encapsulated Species | Properties Studied | Size (µm) | Ref |
|---|---|---|---|---|---|
| Melamine formaldeyde | (chitosan /CNC)n | Doxorubicin hydrochloride | Loading and release | 3.3–3.5 | [133] |
| SiO2 | PEI(CNC)n | Polystyrene beads | Permeability in water | 3.8 ± 0.5 | [134] |
| CaCO3 | CNF/AP/ XyG (AP/CNF)5AP/CNF | Dextran | Permeability in water | 16 ± 4 | [135] |
| CaCO3 | (CNF-XyG)5 (CNF/XyG/CNF/AP)2/CNF/XG | Dextran BSA | Permeability in water/NaCl and cell growth media | 16 ± 4 | [136] |
| CaCO3 | (CNF/XyG/CNF/AP)2CNF | Dextran | Permeability in biological buffer - GOx loading efficiency – enzyme activity | 12 ± 2 | [139] |
| Oil-in-water emulsion | CNF/Pectin | Porosity – pH dependent structure and expansion | 27 | [137] | |
| Water-in-oil emulsion | CNC/cationic polymer | Mechanical properties | 303 ± 3.4 | [141] | |
| Oil-in-water emulsion | Bacterial cellulose | Porosity, mechanical properties | from 100 to few cm | [142] | |
| Oil-in-water emulsion | CNF | Paraffin | Mechanical and thermal properties | 5–10 | [143] |
| Oil-in-water emulsion | ChiNC | Paraffin | 2–5 | [148] | |
| Water-in-oil | CNC(SO4) CNC(aldehyde)-CNC (hydrazone) | Swelling, porosity, self-assembly | > 300 | [144] | |
| Water-in- toluene/ethanol | CNC(COOH) | Self-assembly | 300–800 | [145] | |
| Water-in-hexadecane | CNC(SO4) | Self-assembly | ≈ 20 | [146] | |
| n-Heptane-in-water | SNC | enzyme | Catalytic activity | 5–30 | [147] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.; Villares, A. Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules 2020, 25, 4420. https://doi.org/10.3390/molecules25194420
Lombardo S, Villares A. Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules. 2020; 25(19):4420. https://doi.org/10.3390/molecules25194420
Chicago/Turabian StyleLombardo, Salvatore, and Ana Villares. 2020. "Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials" Molecules 25, no. 19: 4420. https://doi.org/10.3390/molecules25194420
APA StyleLombardo, S., & Villares, A. (2020). Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules, 25(19), 4420. https://doi.org/10.3390/molecules25194420