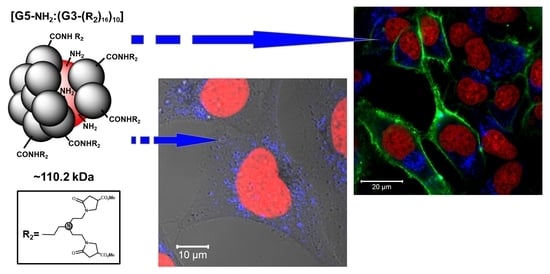
Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells †
Abstract
1. Introduction
1.1. Historical Overview
1.2. Dendrimer Properties of Value in Drug Delivery and Nanomedicine
1.3. Core-Shell Tecto(dendrimers); Synthesis and Property Evaluation for Life Science Applications
2. Results
2.1. Synthesis
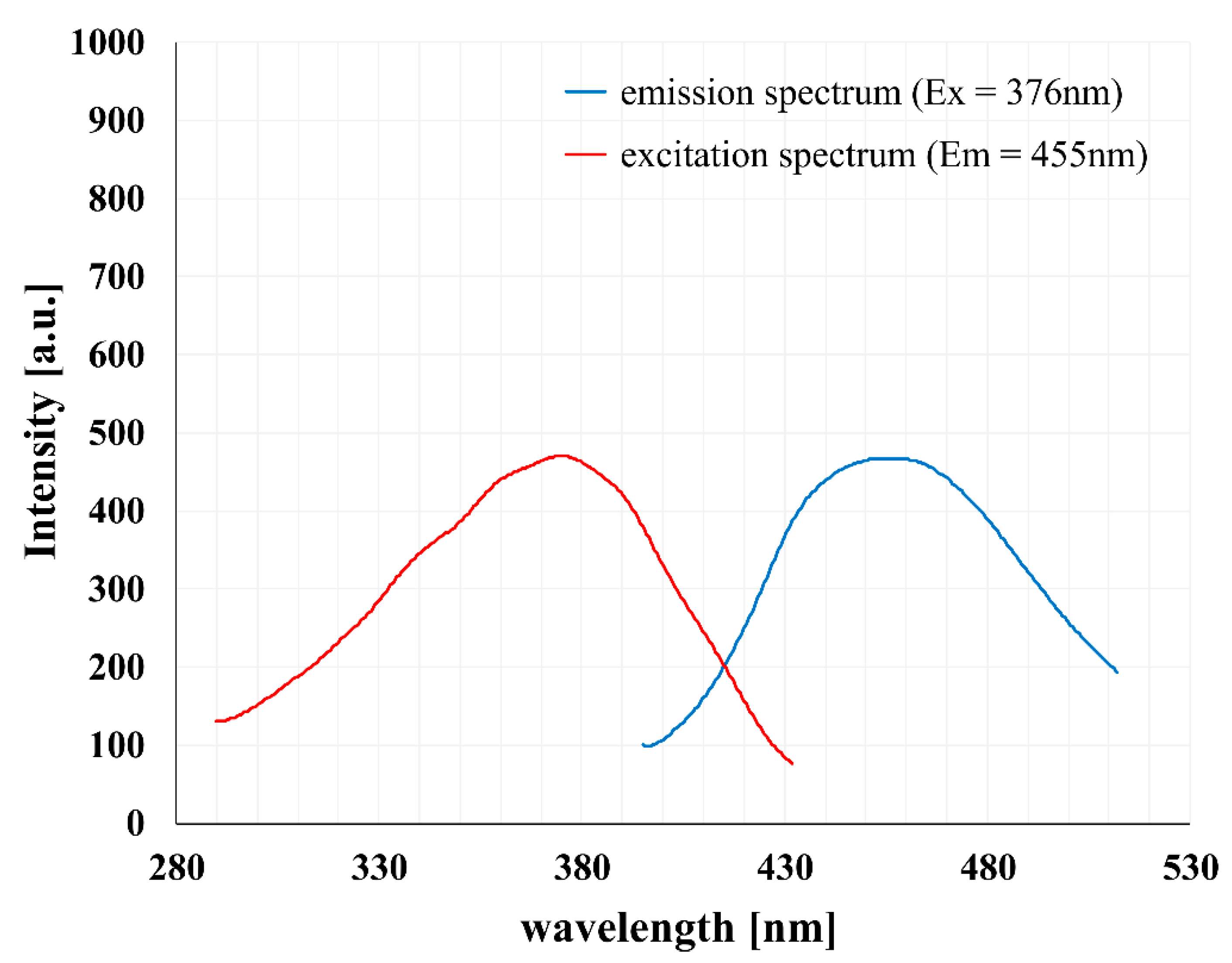
2.2. Selected Physicochemical Properties of PAMAM [G5:G3-(TREN)-N-(4-carbomethoxy) pyrrolidone]10 Terminated Tecto(dendrimer)
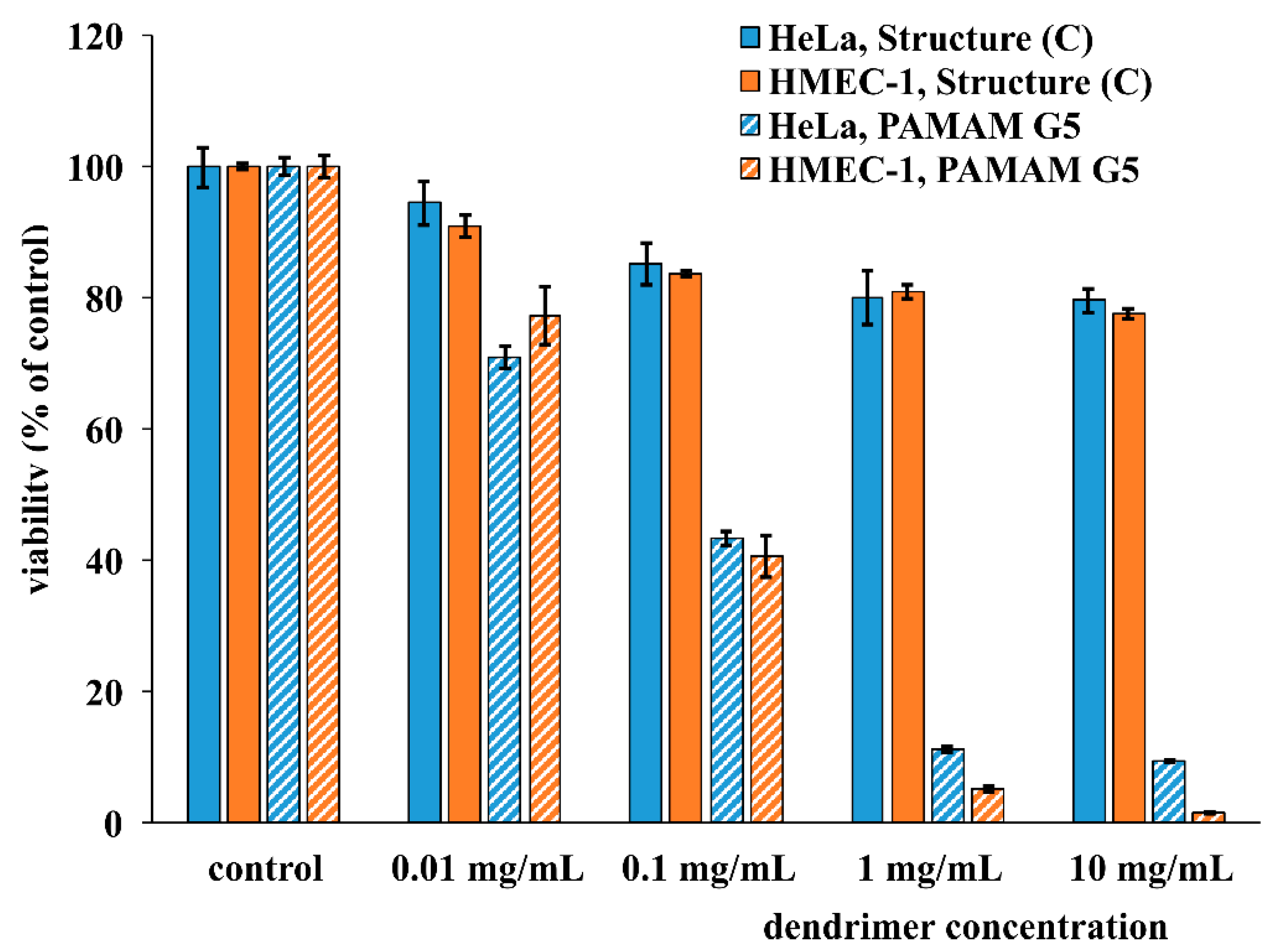
2.3. Cytotoxicity of PAMAM [G5:G3-(TREN)]-N-(4-carbomethoxy) Pyrrolidone Terminated Tecto(dendrimer) (C)
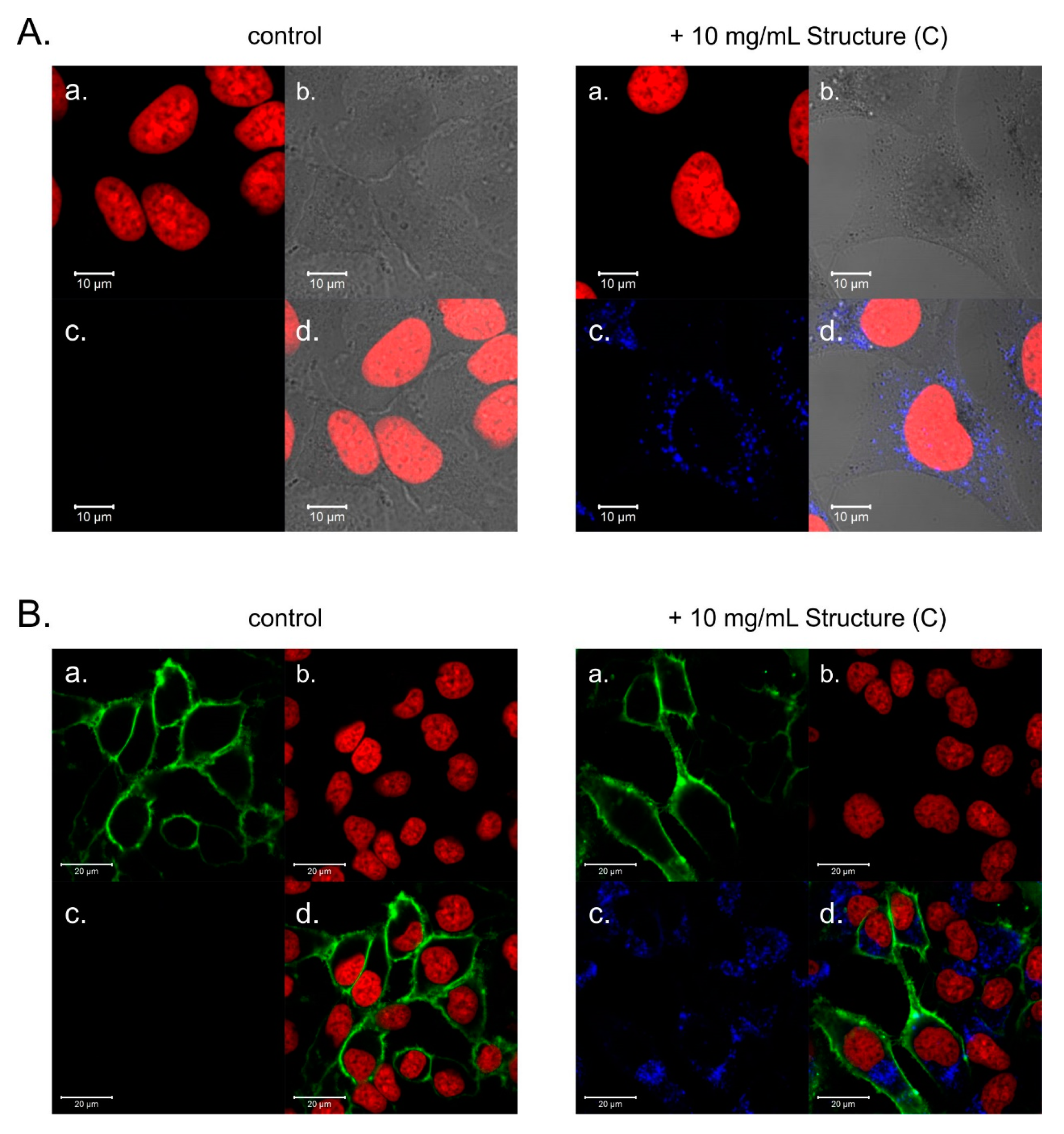
2.4. Internalization of PAMAM [G5:G3-(TREN)]-N-(4-carbomethoxy) Pyrrolidone Terminated Tecto(dendrimer) (C) into HeLa Cells
2.5. Potential of PAMAM [G5:G3-(TREN)]-N-(4-carbomethoxy) Pyrrolidone Terminated Tecto(dendrimer) (C) for DNA Transfection into HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Synthesis of Modified PAMAM G5:G2.5 Tecto (Dendrimer)
4.1.1. Synthesis of PAMAM, [G5:G2.5-(CO2Me)]10, Structure (A)
4.1.2. Synthesis of PAMAM, [G5:G3-(TREN)]10, Structure (B)
4.1.3. Synthesis of PAMAM, [G5:G3-(TREN)-N-(4-Carbomethoxy) Pyrrolidone]10, Structure (C)
4.2. Cell Culture
4.3. Ninhydrin Assay
4.4. Physical Characterization of PAMAM [G5:G3-(TREN)]-N-(4-carbomethoxy) pyrrolidone]10 Terminated Tecto(dendrimer) Structure (C)
4.5. Cytotoxicity Assay
4.6. Transfection with (G5) Amine Terminated PAMAM Dendrimers
4.7. Confocal Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part. A: Polym. Chem. 2020, 40, 2719–2728. [Google Scholar] [CrossRef]
- Kallos, G.J.; Tomalia, D.A.; Hedstrand, D.M.; Lewis, S.; Zhou, J. Molecular weight determination of a polyamidoamine Starburst polymer by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1991, 5, 383–386. [Google Scholar] [CrossRef]
- Müller, R.; Laschober, C.; Szymanski, W.W.; Allmaier, G. Determination of molecular weight, particle size, and density of high number generation PAMAM dendrimers using MALDI−TOF−MS and nES−GEMMA. Macromolecules 2007, 40, 5599–5605. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-0-521-51580-1. [Google Scholar]
- Launay, N.; Caminade, A.-M.; Lahana, R.; Majoral, J.-P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Ed. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A systematic framework and nanoperiodic concept for unifying nanoscience: Hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive mendeleev-like nanoperiodic tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Ficker, M.; Petersen, J.F.; Hansen, J.S.; Christensen, J.B. Guest-host chemistry with dendrimers—binding of carboxylates in aqueous solution. PloS ONE 2015, 10, e0138706. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The role of branch cell symmetry and other critical nanoscale design parameters in the determination of dendrimer encapsulation properties. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.C.; Lawless, L.J. Supramolecular chemistry of dendrimers. In Dendrimers IV: Metal Coordination, Self Assembly, Catalysis; Vögtle, F., Schalley, C.A., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2001; pp. 95–120. ISBN 978-3-540-45003-0. [Google Scholar]
- Lazniewska, J.; Milowska, K.; Gabryelak, T. Dendrimers—revolutionary drugs for infectious diseases. Wires Nanomed. Nanobiotechnology 2012, 4, 469–491. [Google Scholar] [CrossRef]
- Matthews, B.R.; Holan, G. Anionic or Cationic Dendrimer Antimicrobial or Autiprotozoan Compositions. U.S. Patent No. 6,464,971, 15 October 2002. [Google Scholar]
- Janaszewska, A.; Gorzkiewicz, M.; Ficker, M.; Petersen, J.F.; Paolucci, V.; Christensen, J.B.; Klajnert-Maculewicz, B. Pyrrolidone modification prevents PAMAM dendrimers from activation of pro-inflammatory signaling pathways in human monocytes. Mol. Pharm. 2018, 15, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Laskar, P.; Altwaijry, N.; Kewcharoenvong, P.; Irving, C.; Robb, G.; Pickard, B.S.; Dufès, C. PEGylation of polypropylenimine dendrimers: Effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Sci. Rep. 2018, 8, 9410. [Google Scholar] [CrossRef] [PubMed]
- Padié, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.-M.; Majoral, J.-P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7. [Google Scholar] [CrossRef]
- Jatczak-Pawlik, I.; Gorzkiewicz, M.; Studzian, M.; Appelhans, D.; Voit, B.; Pulaski, L.; Klajnert-Maculewicz, B. Sugar-modified poly(propylene imine) dendrimers stimulate the NF-κB pathway in a myeloid cell line. Pharm. Res. 2017, 34, 136–147. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Hedstrand, D.M.; Nixon, L.S. Pyrrolidone Derivatives, Oligomers and Polymers. Patent WO 2016/040962 A1, 17 March 2016. [Google Scholar]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomedicine 2013, 9, 461–464. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Studzian, M.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Klajnert-Maculewicz, B. PAMAM dendrimer with 4-carbomethoxypyrrolidone—In vitro assessment of neurotoxicity. Nanomedicine 2015, 11, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Konopka, M.; Janaszewska, A.; Johnson, K.A.M.; Hedstrand, D.; Tomalia, D.A.; Klajnert-Maculewicz, B. Determination of non-traditional intrinsic fluorescence (NTIF) emission sites in 1-(4-carbomethoxypyrrolidone)-PAMAM dendrimers using CNDP-based quenching studies. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Studzian, M.; Pułaski, Ł.; Tomalia, D.A.; Klajnert-Maculewicz, B. Non-traditional intrinsic luminescence (NTIL): Dynamic quenching demonstrates the presence of two distinct fluorophore types associated with NTIL behavior in pyrrolidone-terminated PAMAM dendrimers. J. Phys. Chem. C 2019, 123, 18007–18016. [Google Scholar] [CrossRef]
- Janaszewska, A.; Studzian, M.; Petersen, J.F.; Ficker, M.; Paolucci, V.; Christensen, J.B.; Tomalia, D.A.; Klajnert-Maculewicz, B. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups-its uptake, efflux, and location in a cell. Colloids Surf. B Biointerfaces 2017, 159, 211–216. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.-M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-traditional intrinsic luminescence: Inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Larson, C.L.; Tucker, S.A. Intrinsic fluorescence of carboxylate-terminated polyamido amine dendrimers. Appl. Spectrosc. 2001, 55, 679–683. [Google Scholar] [CrossRef]
- Varnavski, O.; Ispasoiu, R.G.; Balogh, L.; Tomalia, D.; Goodson, T. Ultrafast time-resolved photoluminescence from novel metal–dendrimer nanocomposites. J. Chem. Phys. 2001, 114, 1962–1965. [Google Scholar] [CrossRef]
- Khopade, A.J.; Möhwald, H. Statistical megamer morphologies and materials from PAMAM dendrimers. Macromol. Rapid Commun. 2005, 26, 445–449. [Google Scholar] [CrossRef]
- Magalhães, T.M.; Guerra, R.C.; San Gil, R.A.d.S.; Valente, A.P.; Simão, R.A.; Soares, B.G.; Mendes, T.d.C.; Pyrrho, A.d.S.; Sousa, V.P.d.; Rodrigues-Furtado, V.L. PAMAM dendrimer hydrogel film—Biocompatible material to an efficient dermal delivery of drugs. J. Nanopart. Res. 2017, 19, 277. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Uppuluri, S.; Swanson, D.R.; Li, J. Dendrimers as reactive modules for the synthesis of new structure-controlled, higher-complexity megamers. Pure Appl. Chem. 2000, 72, 2343–2358. [Google Scholar] [CrossRef]
- Uppuluri, S.; Swanson, D.R.; Piehler, L.T.; Li, J.; Hagnauer, G.L.; Tomalia, D.A. Core-shell tecto(dendrimers): I. Synthesis and characterization of saturated shell models. Adv. Mater. 2000, 12, 796–800. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Brothers, H.M.; Piehler, L.T.; Durst, H.D.; Swanson, D.R. Partial shell-filled core-shell tecto(dendrimers): A strategy to surface differentiated nano-clefts and cusps. Proc. Natl. Acad. Sci. USA 2002, 99, 5081–5087. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.L.; Rakesh, L.; Tomalia, D.A. The random parking of spheres on spheres. J. Chem. Phys. 1996, 105, 3245–3249. [Google Scholar] [CrossRef]
- Song, C.; Shen, M.; Rodrigues, J.; Mignani, S.; Majoral, J.-P.; Shi, X. Superstructured poly(amidoamine) dendrimer-based nanoconstructs as platforms for cancer nanomedicine: A concise review. Coord. Chem. Rev. 2020, 421, 213463. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as Encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo de Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Myers, V.S.; Weir, M.G.; Carino, E.V.; Yancey, D.F.; Pande, S.; Crooks, M.R. Dendrimer-encapsulated nanoparticles: New synthetic and characterization methods and catalytic applications. Chem. Sci. 2011, 2, 1632–1646. [Google Scholar] [CrossRef]
- Hernández Ramírez, R.E.; Lijanova, I.V.; Likhanova, N.V.; Olivares Xometl, O.; Hernández Herrera, A.; Federico Chávez Alcalá, J.; Trejo Chavero, O. Synthesis of PAMAM dendrimers with porphyrin core and functionalized periphery as templates of metal composite materials and their toxicity evaluation. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Leriche, E.-D.; Afonso, C.; Lange, C.M.; Grossel, M.C.; Truong, L.; Coadou, G.; Oulyadi, H.; Loutelier-Bourhis, C. Glycine-modified polyamidoamine dendrimers: Synthesis and structural characterization using nuclear magnetic resonance, ion-mobility mass spectrometry and capillary electrophoresis. Rsc Adv. 2013, 4, 1744–1753. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, W.; Song, Y.; Duan, Y.; Wang, L.; Steinhoff, G.; Kong, D.; Yu, Y. Polyamidoamine dendrimers with a modified Pentaerythritol core having high efficiency and low cytotoxicity as gene carriers. Biomacromolecules 2009, 10, 617–622. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, M.A.; Vaidyanathan, S.; Banaszak Holl, M.M. PAMAM dendrimers as quantized building blocks for novel nanostructures. Soft Matter 2013, 9. [Google Scholar] [CrossRef]
- Raviña, M.; de la Fuente, M.; Correa, J.; Sousa-Herves, A.; Pinto, J.; Fernandez-Megia, E.; Riguera, R.; Sanchez, A.; Alonso, M.J. Core-shell dendriplexes with sterically induced stoichiometry for gene delivery. Macromolecules 2010, 43, 6953–6961. [Google Scholar] [CrossRef]
- Welch, P.M.; Welch, C.F. Tecto-dendrimers: A study of covalently bound nanospheres. Macromolecules 2009, 42, 7571–7578. [Google Scholar] [CrossRef]
- Lukowiak, M.C.; Thota, B.N.S.; Haag, R. Dendritic core-shell systems as soft drug delivery nanocarriers. Biotechnol. Adv. 2015, 33, 1327–1341. [Google Scholar] [CrossRef]
- Schilrreff, P.; Cervini, G.; Romero, E.L.; Morilla, M.J. Enhanced antimelanoma activity of methotrexate and zoledronic acid within polymeric sandwiches. Colloids Surf. B Biointerfaces 2014, 122, 19–29. [Google Scholar] [CrossRef]
- Schilrreff, P.; Mundiña-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Selective cytotoxicity of PAMAM G5 core—PAMAM G2.5 shell tecto-dendrimers on melanoma cells. Int J. Nanomed. 2012, 7, 4121–4133. [Google Scholar] [CrossRef]
- Chu, C.-C.; Imae, T. Fluorescence investigations of oxygen-doped simple amine compared with fluorescent PAMAM dendrimer. Macromol. Rapid Commun. 2009, 30, 89–93. [Google Scholar] [CrossRef]
- Huang, J.-F.; Luo, H.; Liang, C.; Sun, I.; Baker, G.A.; Dai, S. Hydrophobic brønsted acid-base ionic liquids based on PAMAM dendrimers with high proton conductivity and blue photoluminescence. J. Am. Chem. Soc. 2005, 127, 12784–12785. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qian, Y. A study using quantum chemical theory methods on the intrinsic fluorescence emission and the possible emission mechanisms of PAMAM. RSC Adv. 2014, 4, 58788–58794. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. Poly(amidoamine) dendrimers as a pharmaceutical excipient. Are we there yet? J. Pharm. Sci. 2018, 107, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. -Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Saovapakhiran, A.; D’Emanuele, A.; Attwood, D.; Penny, J. Surface modification of PAMAM dendrimers modulates the mechanism of cellular internalization. Bioconjug. Chem. 2009, 20, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, H.; Li, L.; Cheng, Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat. Commun. 2014, 5, 3053. [Google Scholar] [CrossRef]
- Kretzmann, J.A.; Ho, D.; Evans, C.W.; Plani-Lam, J.H.C.; Garcia-Bloj, B.; Mohamed, A.E.; O’Mara, M.L.; Ford, E.; Tan, D.E.K.; Lister, R.; et al. Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem. Sci. 2017, 8, 2923–2930. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| PAMAM [G5G3-(TREN)]-N-(4-carbomethoxy) pyrrolidone terminated; tecto(dendrimer)(C) | 0.43 ± 0.01 | 5.86 ± 0.22 | 2.35 ± 0.03 | 44.22 ± 0.77 | 7.11 ± 0.06 | 49.93 ± 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studzian, M.; Działak, P.; Pułaski, Ł.; Hedstrand, D.M.; Tomalia, D.A.; Klajnert-Maculewicz, B. Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells . Molecules 2020, 25, 4406. https://doi.org/10.3390/molecules25194406
Studzian M, Działak P, Pułaski Ł, Hedstrand DM, Tomalia DA, Klajnert-Maculewicz B. Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells . Molecules. 2020; 25(19):4406. https://doi.org/10.3390/molecules25194406
Chicago/Turabian StyleStudzian, Maciej, Paula Działak, Łukasz Pułaski, David M. Hedstrand, Donald A. Tomalia, and Barbara Klajnert-Maculewicz. 2020. "Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells " Molecules 25, no. 19: 4406. https://doi.org/10.3390/molecules25194406
APA StyleStudzian, M., Działak, P., Pułaski, Ł., Hedstrand, D. M., Tomalia, D. A., & Klajnert-Maculewicz, B. (2020). Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells . Molecules, 25(19), 4406. https://doi.org/10.3390/molecules25194406