Synthesis of Hydrotalcites from Waste Steel Slag with [Bmim]OH Intercalated for the Transesterification of Glycerol Carbonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ionic Liquid
2.1.1. 13C-NMR analysis of Ionic Liquid
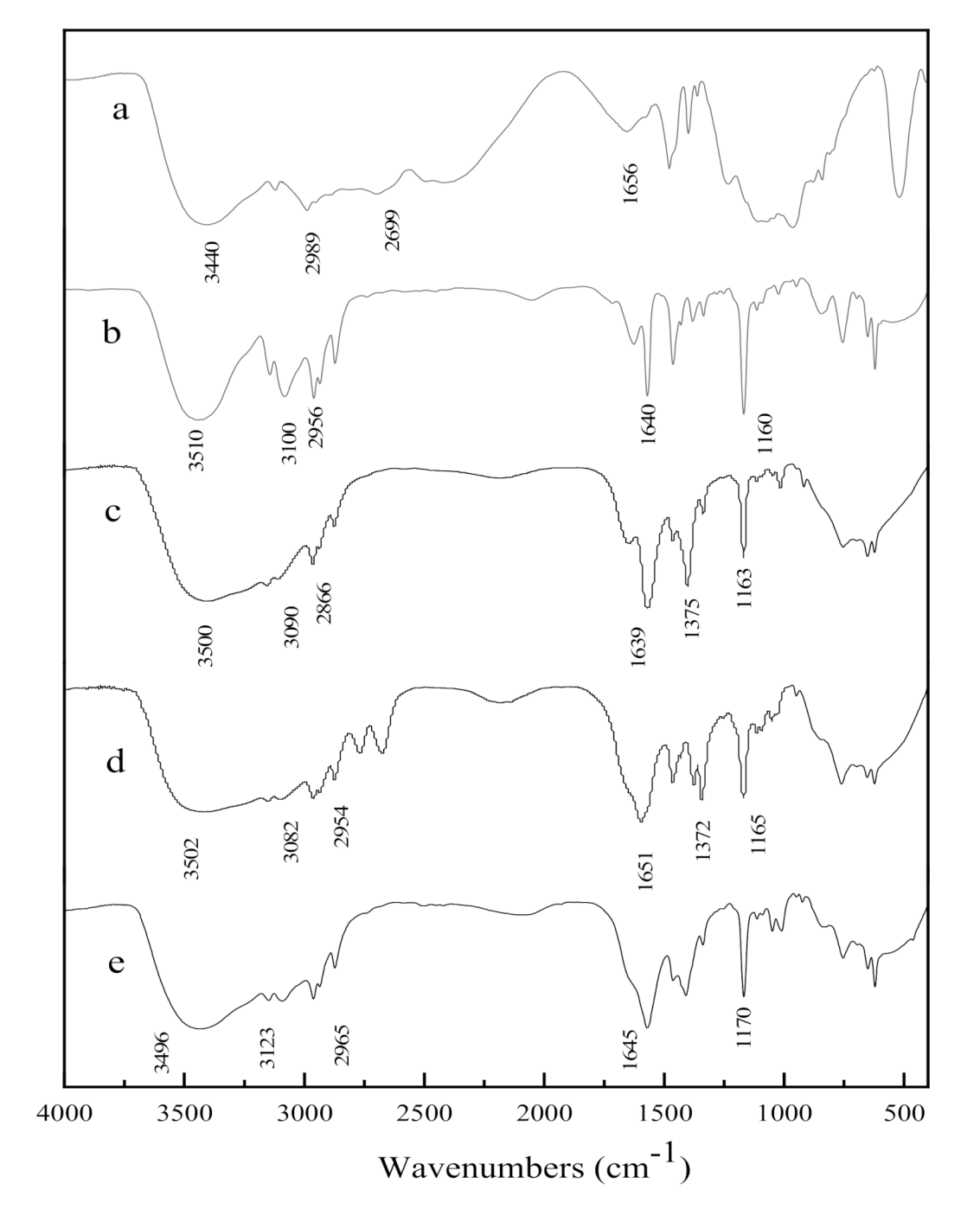
2.1.2. FT-IR Analysis of Ionic Liquid
2.1.3. Results of Basicity of Ionic Liquids
2.2. Characterization of IL-CaMgAl
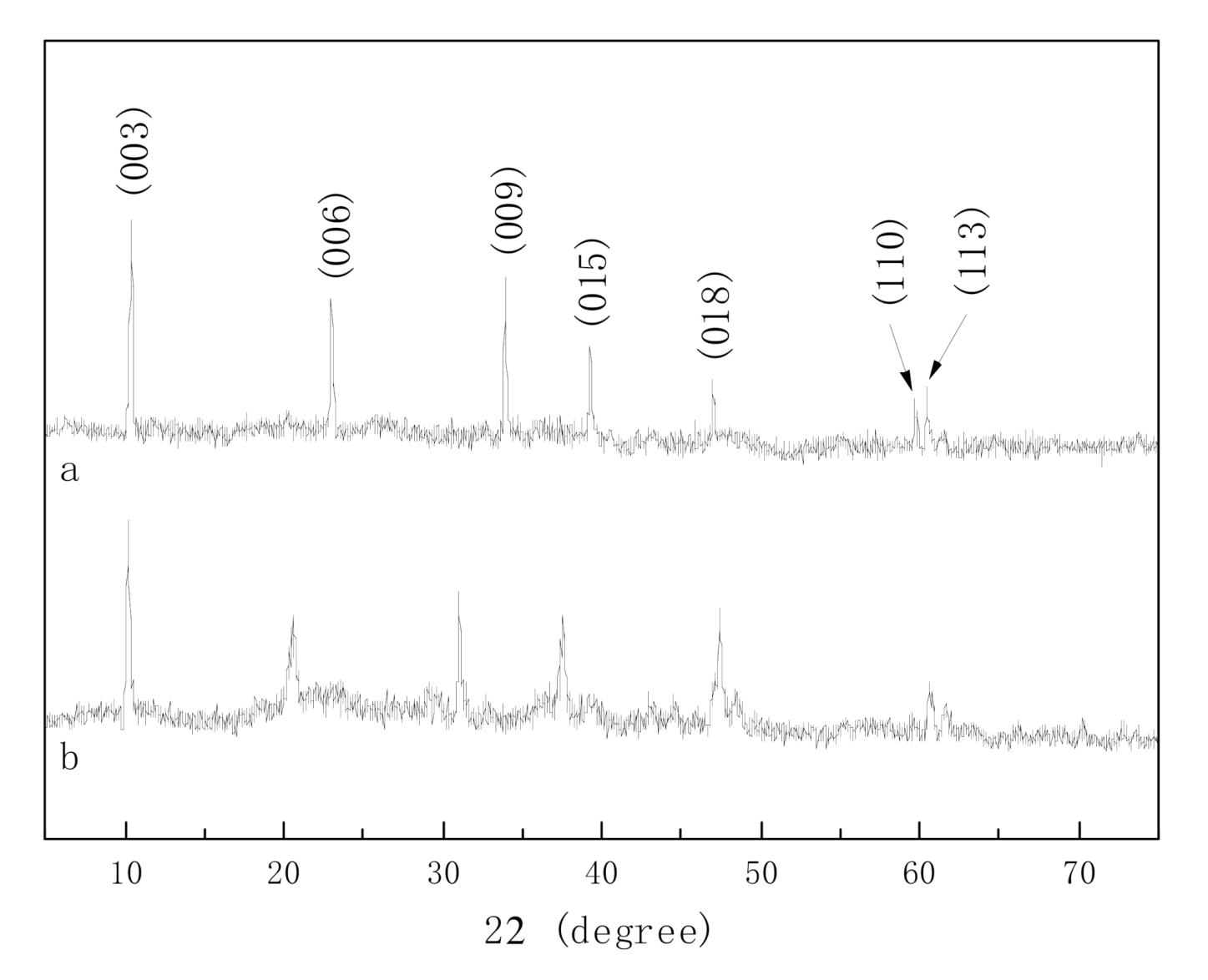
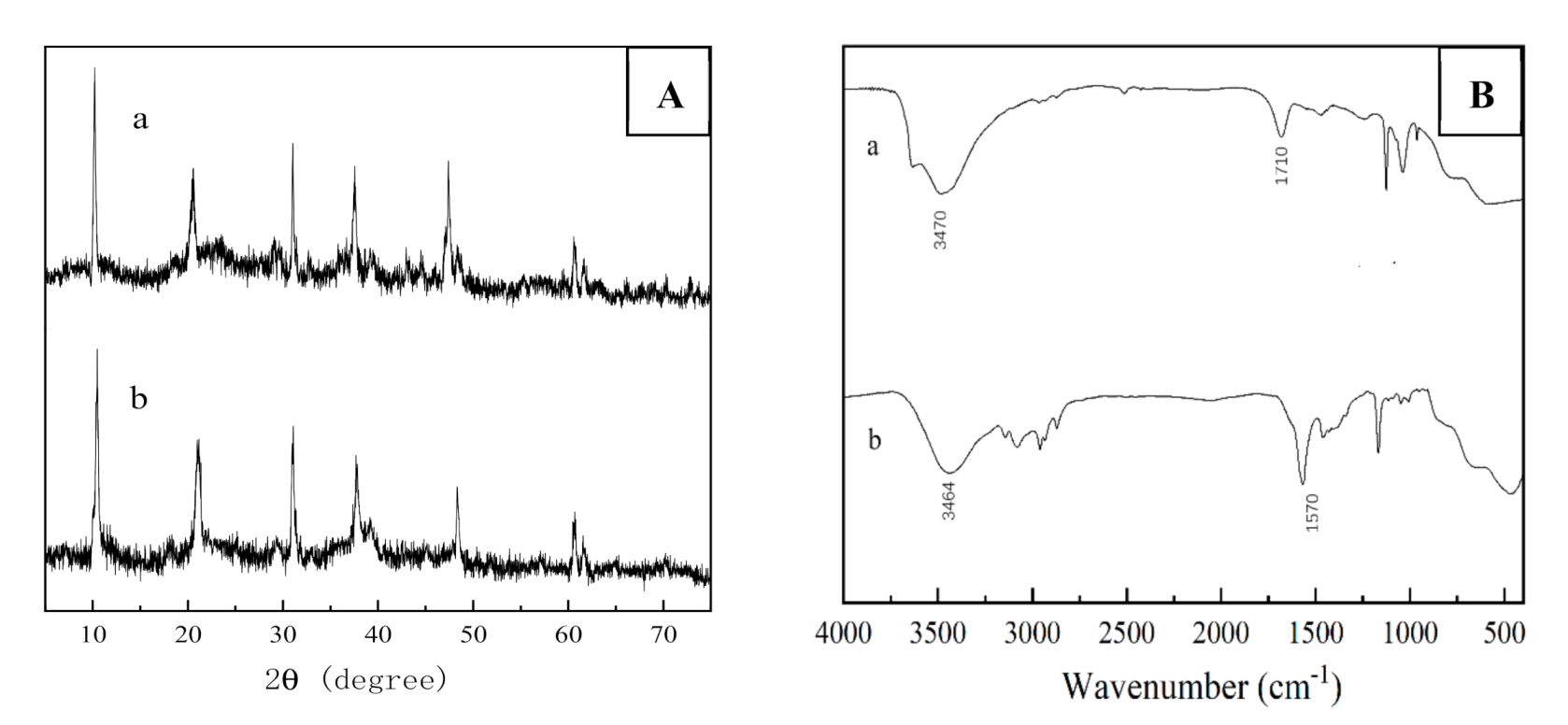
2.2.1. XRD Analysis
2.2.2. FT-IR Analysis
2.2.3. Element Analysis
2.2.4. SEM Analysis
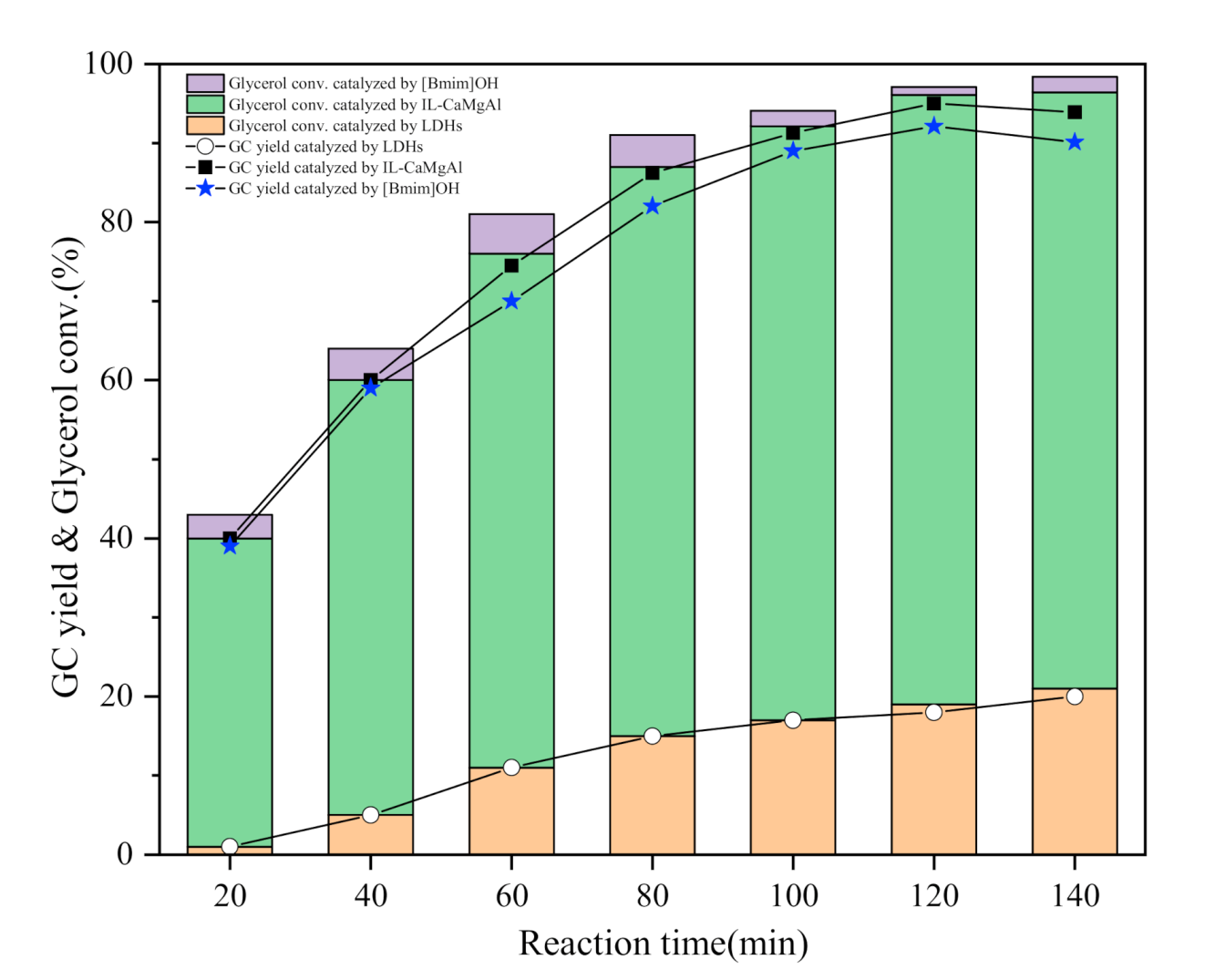
2.3. Transesterification of Glycerol and Dimethyl Carbonate
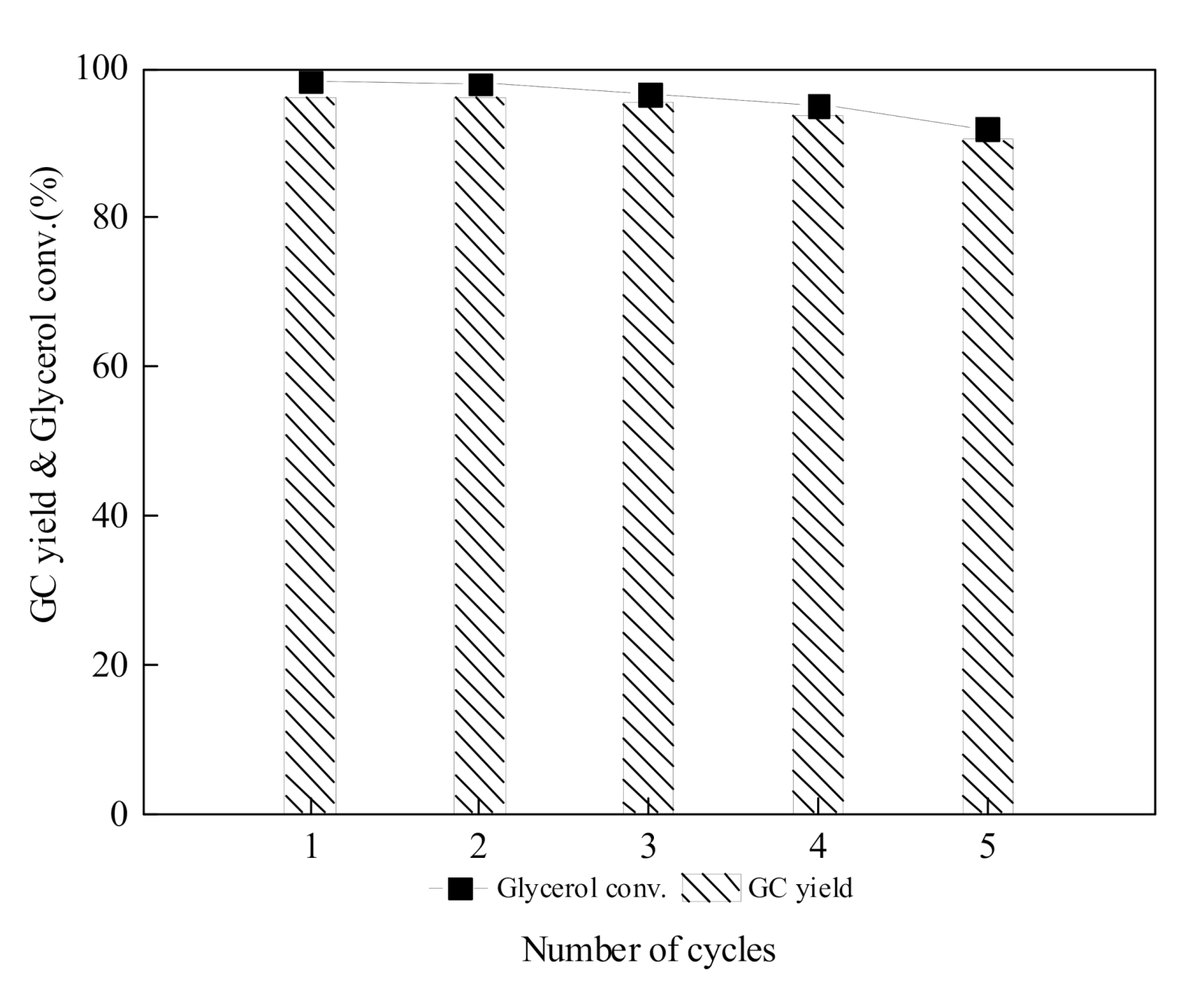
2.4. Reusability Test of IL-CaMgAl
3. Experimental
3.1. Preparation of Ionic Liquid
3.2. Preparation of Ca-Mg-Al Hydrotalcites Intercalated by [Bmim]OH
3.3. Characterization Methods
3.4. Transesterification Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tae, S.J.; Morita, K. Immobilization of Cr (VI) in stainless steel slag and Cd, As, and Pb in wastewater using blast furnace slag via a hydrothermal treatment. Met. Mater. Int. 2017, 23, 576–581. [Google Scholar] [CrossRef]
- Yüksel, İ. A review of steel slag usage in construction industry for sustainable development. Environ. Dev. Sustain. 2017, 19, 369–384. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Hu, J. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3. Chin. J. Chem. Eng. 2017, 26, 583–592. [Google Scholar] [CrossRef]
- Purohit, A.; Satapathy, A. Dry sliding wear characteristics of epoxy composites filled with steel industry slag and sludge particles: A comparative study. Mater. Today Proc. 2018, 5, 11906–11913. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Li, H. Sorption of Cr(VI) on Mg-Al-Fe layered double hydroxides synthesized by a mechanochemical method. RSC Adv. 2014, 4, 46823–46830. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Jia, Y. Three-dimensional hierarchical flower-like Mg-Al-layered double hydroxides: Highly efficient adsorbents for As(V) and Cr(VI) removal. Nanoscale 2012, 4, 3466–3474. [Google Scholar] [CrossRef]
- Hussein, M.Z.B.; Zainal, Z.; Yahaya, A.H. Controlled release of a plant growth regulator, α-naphthaleneacetate from the lamella of Zn-Al-layered double hydroxide nanocomposite. J. Control. Release 2012, 82, 417–427. [Google Scholar] [CrossRef]
- Nowicki, J.; Lach, J.; Organek, M.; Sabura, E. Transesterification of rapeseed oil to biodiesel over Zr-doped MgAl hydrotalcites. Appl. Catal. A 2016, 524, 17–24. [Google Scholar] [CrossRef]
- Wan, Y.; Lei, Y.; Lan, G.; Liu, D.; Li, G.; Bai, R. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over DABCO embedded porous organic polymer as a bifunctional and robust Catalyst. Appl. Catal. A 2018, 562, 267–275. [Google Scholar] [CrossRef]
- Wang, S.; Wan, J.; Sun, P.; Xu, L.; Okoye, P.U.; Li, S. Disposable baby diapers waste derived catalyst for synthesizing glycerol carbonate by the transesterification of glycerol with dimethyl carbonate. J. Cleaner Prod. 2019, 211, 330–341. [Google Scholar] [CrossRef]
- Liu, J.; He, D. Transformation of CO2 with glycerol to glycerol carbonate by a novel ZnWO4-ZnO catalyst. J. CO2 Util. 2018, 26, 370–379. [Google Scholar] [CrossRef]
- Chaves, D.M.; Márcio, J.; Da, S. A selective synthesis of glycerol carbonate from glycerol and urea over Sn(OH)2: A solid and recyclable in situ generated catalyst. New J. Chem. 2019, 43, 3698–3706. [Google Scholar] [CrossRef]
- Olkiewica, M.; Plechkova, N.V.; Earle, M.J.; Fabregat, A.; Stüber, F. Biodiesel production from sewage sludge lipids catalysed by Brønsted acidic ionic liquids. Appl. Catal. B 2016, 181, 738–746. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Fan, M.; Jiang, P. Catalytic performance of a novel amphiphilic alkaline ionic liquid for biodiesel production: Influence of basicity and conductivity. Renew. Energ. 2016, 86, 99–105. [Google Scholar] [CrossRef]
- Shirani, M.; Semnani, A.; Habibollahi, S. Synthesis and application of magnetic NaY zeolite composite immobilized with ionic liquid for adsorption desulfurization of fuel using response surface methodology. J. Porous Mat. 2016, 23, 701–712. [Google Scholar] [CrossRef]
- Yao, H.; Wang, G.; Zuo, C. Deep hydrodenitrification of pyridine by solid catalyst coupling with ionic liquids under mild conditions. Green Chem. 2017, 19, 1692–1700. [Google Scholar] [CrossRef]
- Duan, J.; Sun, Y.; Shi, L. Three different types of heterocycle of nitrogen-containing alkaline ionic liquids treatment of acid oil to remove naphthenic acids. Catal Today. 2013, 212, 180–185. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Kim, T.K.; Lee, S.D.; Ahn, B.S. CaO-Catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate: Isolation and characterization of an active Ca species. Appl. Catal. A 2011, 401, 220–225. [Google Scholar] [CrossRef]
- Wang, S.; Hao, P.; Li, S.; Zhang, A.; Guan, Y.; Zhang, L. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by calcined silicates. Appl. Catal. A 2017, 542, 174–181. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Kabir, G.; Hameed, B.H. Synthesis of glycerol carbonate from biodiesel by-product glycerol over calcined Dolomite. J. Taiwan Inst. Chem. Eng. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Pan, S.; Zheng, L.; Nie, R.; Xia, S.; Chen, P.; Hou, Z. Transesterification of glycerol with dimethyl carbonate to glycerol carbonate over Na–Based Zeolites. Chin. J. Catal. 2012, 33, 1772–1777. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Ca-Mg-Al hydrotalcites intercalated by [Bmim]OH and pure hydrotalcites are available from the authors. |



| Catalyst | Glycerol Conv. (%) | GC Yield (%) | Basic Amount (mmol/g) | Basic Strength (H_) |
|---|---|---|---|---|
| [Bmim]HCOO | 81.6 | 81.1 | 0.87 | H_ < 9.8 |
| [Bmim][CH3COO] | 90.5 | 90.3 | 0.92 | 9.8 < H_ < 15.0 |
| [Bmim]OH | 97.5 | 92.1 | 1.17 | 18.4 < H_ < 26.5 |
| [Bmim]Br | 75.2 | 75.0 | 0.69 | H_ < 9.8 |
| ChOH | 96.5 | 91.4 | 1.09 | 18.4 < H_ < 26.5 |
| Element (wt.%) | C | N | O | Mg | Al | Ca | Others |
|---|---|---|---|---|---|---|---|
| CaMgAl | 6.11 | NA * | 45.49 | 4.04 | 9.99 | 28.74 | 5.63 |
| IL-CaMgAl | 12.30 | 5.25 | 41.93 | 3.66 | 7.98 | 27.53 | 1.35 |
| Catalyst | Catalyst Dosage (wt.%) | Reaction Temperature (℃) | Molar Ratio of DMC to Glycerol | Reaction Time (min) | GC Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| CaO | 3 | 75 | 2:1 | 30 | 90.2 | [18] |
| Na2SiO3-200 | 5 | 75 | 4:1 | 150 | 95.5 | [19] |
| Calcined Dolomite | 6 | 75 | 3:1 | 90 | 94.0 | [20] |
| NaY zeolite | 10 | 70 | 3:1 | 240 | 80.0 | [21] |
| IL-CaMgAl | 3 | 75 | 3:1 | 90 | 96.2 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Yang, J.; Xu, X. Synthesis of Hydrotalcites from Waste Steel Slag with [Bmim]OH Intercalated for the Transesterification of Glycerol Carbonate. Molecules 2020, 25, 4355. https://doi.org/10.3390/molecules25194355
Liu G, Yang J, Xu X. Synthesis of Hydrotalcites from Waste Steel Slag with [Bmim]OH Intercalated for the Transesterification of Glycerol Carbonate. Molecules. 2020; 25(19):4355. https://doi.org/10.3390/molecules25194355
Chicago/Turabian StyleLiu, Guanhao, Jingyi Yang, and Xinru Xu. 2020. "Synthesis of Hydrotalcites from Waste Steel Slag with [Bmim]OH Intercalated for the Transesterification of Glycerol Carbonate" Molecules 25, no. 19: 4355. https://doi.org/10.3390/molecules25194355
APA StyleLiu, G., Yang, J., & Xu, X. (2020). Synthesis of Hydrotalcites from Waste Steel Slag with [Bmim]OH Intercalated for the Transesterification of Glycerol Carbonate. Molecules, 25(19), 4355. https://doi.org/10.3390/molecules25194355





