Abstract
The digestion of flaxseed polysaccharides (FSP) in simulated saliva, gastric and small intestine conditions was assessed, as well as in vitro fermentation of FSP by human gut microbiota. FSP was not degraded in the simulated digestive systems (there was no change in molecular weight or content of reducing sugars), indicating that ingested FSP would reach the large intestine intact. Changes in carbohydrate content, reducing sugars and culture pH suggested that FSP could be broken down and used by gut microbiota. FSP modulated the composition and structure of the gut microbiota by altering the Firmicutes/Bacteroidetes ratio and increasing the relative abundances of Prevotella, Phascolarctobacterium, Clostridium and Megamonas, which can degrade polysaccharides. Meanwhile, FSP fermentation increased the concentration of short-chain fatty acids, especially propionic and butyric acids. Our results indicate that FSP might be developed as a functional food that benefits gut health.
1. Introduction
The gut microbiota, consisting of numerous microbial species, is considered a vital organ that plays a pivotal role in host health [1]. Numerous studies have indicated that human gut microbiota can exert numerous benefits to the host health including nutrient absorption, glucose tolerance, and pathogen defense [2,3]. Growing evidence has demonstrated that plant polysaccharides cannot be digested by upper digestive tract, but can reach the distal intestine and be degraded by gut microbiota [4]. The fermentation of polysaccharides and their interaction with gut microbiota are closely related to human health. For example, polysaccharides extracted from soybean was found to promote the production of acetic acid and the relative richness of Bifidobacterium. In addition, polysaccharide fermentation can further improve the intestinal micro-ecosystem by inhibiting certain harmful bacteria in the host [5]. Recently, some scientists have proposed that using polysaccharides as a new therapeutic strategy to regulate gut microbiota for improving human health and preventing diseases [6,7].
Bioactive polysaccharide intake regulates the composition and metabolites of the gut microbiota [8]. Most polysaccharides modulate the diversity of gut microbiota by decreasing the ratio of Firmicutes to Bacteroidetes, which might be beneficial for regulating host lipid metabolism [9,10]. Polysaccharides could also protect against dysbiosis of the gut microbiota and inflammatory bowel disease by modulating the multiformity of the intestinal flora [11]. Moreover, the major metabolites arising from the microbial fermentation of dietary fiber, short-chain fatty acids (SCFAs), play a vital role in providing energy for epithelial cells [12] and maintaining the epithelial barrier. SCFAs can not only modulate immune response to prevent colorectal cancer, but also regulate the characteristics of metabolic syndrome [13,14].
Flaxseed (Linum usitatissimum L.) contains various of biological compounds such as n-3 fatty acids, good quality dietary fiber and bioactive lignans, which was considered as a good functional food ingredients [15,16]. The main soluble dietary fiber extracted from flaxseed is a heterogeneous polysaccharide, which consist of neutral arabinoxylan and acidic rhamnose [17]. Flaxseed polysaccharide (FSP) exhibited good antioxidant activities when scavenging radicals in vitro [18]. In addition, FSP intake can reduce the elevated blood glucose and bodyweight gain induced by high-fat diet in rats [19].
Our previous work demonstrated that FSP alleviated metabolic syndrome by modulating the gut microbiota and increasing the content of SCFAs in high-fat-diet mice [20]. However, there has been little study regarding the digestion and fermentation characteristics of FSP.
Therefore, in this study, in vitro digestion and fermentation models were conducted to investigate the digestion and fermentation traits of FSP. The interaction with gut microbiota and production of SCFAs owing to FSP fermentation were evaluated. This study provides a scientific basis for the bioactivity of FSP and its effects on promoting gut health.
2. Results and Discussion
2.1. Change of FSP on Digestion in Simulated Saliva
In the first stage of digestion, the primary function of the saliva is to convert food into small particles with the aid of alpha-amylase. Salivary α-amylase hydrolyses a-(1→4)-glycosidic bonds [21], facilitating the digestion of starch and other carbohydrates. Digestion of FSP by human saliva was studied in this work. As shown in Figure 1a,b, the molecular peak of FSP showed no significant change after saliva digestion, indicated by the retention time and response value. Furthermore, the CR values of FSP solutions had no obvious difference before and after digestion (Table 1), showing that FSP was not hydrolyzed in saliva; this might be attributed to the structure of FSP. This may be due to the fact that the glycoside bonds in carbohydrates cannot be degraded by alpha-amylase [22]. It has been reported that FSP composed of at least 7 kinds of monosaccharides and can be divided into neutral polysaccharides and acidic polysaccharides parts. It has complex helical conformation and both α and β glycoside configurations [23], and thus was hard to be digested by α-amylase in saliva.
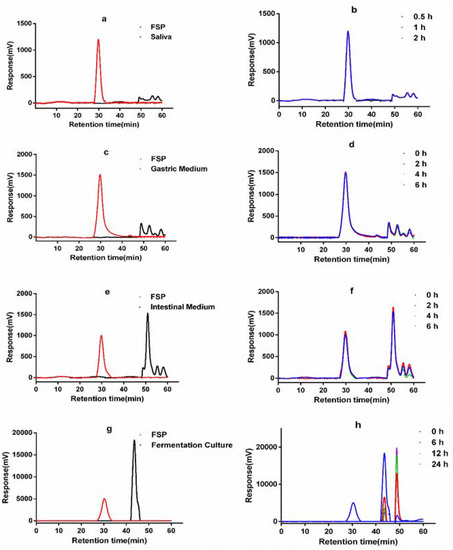
Figure 1.
HPGPC chromatograms of FSP before and after saliva (a,b), simulated gastric (c,d), small intestinal (e,f) digestion and fermentation by human gut microbiota (g,h).

Table 1.
Reducing sugar of FSP during digestion of saliva, gastric and small intestine.
2.2. Change of FSP on Simulated Gastric Digestion
On treatment by simulated gastric juice, the retention time and response values (Figure 1c,d) of FSP were almost constant. There was no obvious change of FSP molecular weight during gastric digestion. The CR content of FSP showed no change (Table 1). These data suggested that the gastric juice failed to degrade the FSP. The results of the current study were consistent with previously reported studies of polysaccharides from Fuzhuan brick tea [24]. However, it has been reported that gastric juice could lead to the degradation of polysaccharides from seeds of Plantago asiatica L. [25]. The digestibility and absorption characteristics of polysaccharides are influenced by their molecular weight, monosaccharide composition, glucoside bonding mode, and conformation. Our data indicated that the huge molecular weight and glucoside bonds of FSP hindered its degradation in saliva and gastric juice.
2.3. Change of FSP in Simulated Intestinal Digestion
The retention time indicating the molecular weight of FSP in the simulated small intestine juice was 30.2 min (Figure 1e). As shown in Figure 1f, both the molecular peaks of FSP and intestine medium did not change during 6 h incubation. The content of reducing sugars also remained unchanged (Table 1) during the simulated intestinal digestion. Thus, these results showed that the upper digestive tract had no effect on FSP. Here, the interaction between FSP and small intestinal microbiota was not considered. However, it is well known that the small intestine microbiota can also degrade and ferment diet-derived simple carbohydrates into organic acids, aldehydes, alcohols, and gases [26]. In vitro digestion is used to study the changes of FSP in the gastrointestinal juice, but it is difficult to study the effect of small intestinal microbiota on FSP. Thus, we focus on the degradation process of FSP in the fecal microbiota.
2.4. Change of FSP in In Vitro Fermentation by Human Gut Microbiota
Figure 1g showed the molecular peaks of FSP and fermentation medium. As displayed in Figure 1h, the peak of FSP disappeared after 6 h of fermentation, while peaks representing the FSP fragments appeared at 44.92 and 49.56 min, demonstrating that FSP was totally degraded into smaller fragments. As shown in Figure 2b, the initial concentrations of reducing sugar and total carbohydrate in FSP fermentation culture were 0.523 and 6.819 mg/mL, respectively. Thereafter, the concentration of reducing sugar increased to 0.625 mg/mL at 12 h and then began to decline, while the concentration of total carbohydrate decreased significantly from 0 to 12 h of fermentation
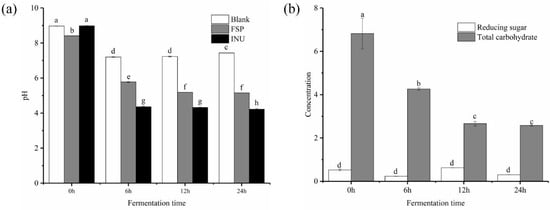
Figure 2.
Shift of pH during in vitro fermentation (a) and change of contents of total carbohydrate and reducing sugar during fermentation (b). a–h means significantly different (p < 0.05) by a Tukey test in the same time between each two group.
The degradation and use of polysaccharides by human fecal microbiota can lead to changes of monosaccharide composition [27]. We detected the residual monosaccharide composition of FSP at different fermentation time points. As shown in Table 2, after 24 h of fermentation, the percentages of xylose and arabinose were significantly increased. The amount of xylose increased rapidly, with a maximum content of 71.8%. The arabinose content reached 22.7%. The consumption rate of rhamnose was higher than that of other monosaccharides (decreased from 32.1% to 0% within 24 h). All the results demonstrated that FSP was degraded and used by gut microbiota.

Table 2.
Compositions and contents of residual monosaccharides at different time points of fermentation.
The pH change of each group during anaerobic incubation reflects fermentation processes. As shown in Figure 2a, the pH value of all tested groups significantly (p < 0.05) declined from during fermentation process. In the FSP group, the pH decreased from 8.92 ± 0.01 to 5.78 ± 0.02 after 24 h fermentation and then remained stable. The pH of the INU group dropped sharply from 9.83 ± 0.01 to 4.36 ± 0.02 after 24 h fermentation, significantly lower than that of the FSP group. This may be due to the unequal amount of SCFAs produced by FSP and INU during the fermentation. Previous studies have shown that lowering the pH value in the gut lumen can inhibit the propagation of certain harmful microorganisms and improve the propagation of certain beneficial colonic microbiota [28,29].
The FTIR spectrums of FSP fermentation fractions were illustrated in Figure 3. The broad absorption peak around 3400–3200 cm−1 was attributed to the stretching of O-H. This characteristic peak firstly moved toward the longer wavelength (3288 cm−1) after fermentation for 12 h and 24 h, compared with 6 h (3281 cm−1). the absorption peak at 1634 cm−1 might be stretching vibration of the deprotonated carboxylic group from amide I [30], indicating all fermentation fractions have glucosamine. After fermentation for 24 h, there existed some differences in absorption wavelength and intensity among different digestion phases. The absorption peaks centered at 1413 cm−1 was due to the presence of -CH2, which existed in the fermentation productions of 6–24 h, and not in fermentation productions of 0 h. The four absorption bands around 1100–1010 cm−1 were assigned to pyranose rings, representing characteristic peaks for carbohydrate [31], and the absorption peak disappears after 6 h of fermentation. Unique primary protein features found in peptide bonds include amide I and amide II (~60% N-H bending vibration, ~40% C-N stretching vibration; ca. 1550 cm−1). After fermentation for 12 h and 24 h, a new band appearing at 1559 cm−1 could be attributed to protein [32,33]. There is no such peak in the infrared results of pure FSP (data not shown); thus, this functional group for protein might indicated the production of protein metabolite by proliferated bacterium. Differences from 1413 cm−1 to 1039 cm−1 were attributed to the diversity of monosaccharide composition of fermentation fractions [34]. This result suggested the FSP was directly degraded by human fecal microbiota accompanied with the changes of polysaccharides structure and metabolite production of fecal microbiota.
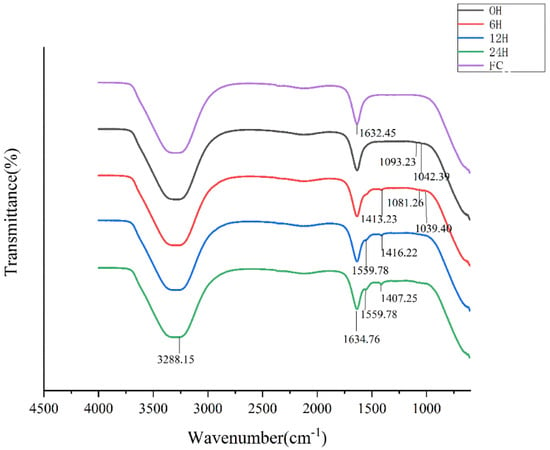
Figure 3.
FT-IR spectra of FSP and its fermentation fractions (FC: Fermentation culture).
2.5. Effects of FSP on SCFA Production
The effect of FSP on the intestinal environment was assessed by measuring the changes in SCFA concentrations during the fermentation process. Previous studies have demonstrated that SCFAs are considered as signaling molecules in miscellaneous cellular pathways. For instance, propionic acid can improve tissue insulin sensitivity and reduce fatty acid content in the liver [35]. Butyric acid is an energy source of intestinal epithelial cells, and exerts beneficial anti-inflammatory and anticarcinogenic effects [36].
The contents of individual and total SCFAs during in vitro fermentation of FSP are summarized in Table 3. Compared with the BLK group, the final concentration of total SCFAs increased 5.2- and 4.6-fold in the INU and FSP groups, respectively, illustrating that both FSP and inulin had a profound influence on production of SCFAs. Butyric, propionic, and acetic acids were the predominant products of FSP fermentation; the levels produced were close to those in the INU group and higher than those in the BLK group. Compared with the BLK group, propionic, butyric, isovaleric, and valeric acids were significantly higher in the FSP group at any fermentation time point (p < 0.05). In the INU group, the concentrations of propionic and n-butyric acid were a little higher than those in the FSP group, while the concentrations of isovaleric and valeric acid were much higher in the FSP group than those in the INU group. These results suggested that FSP and inulin probably promoted growth of different microorganisms producing different kinds of SCFA. The total SCFAs in the FSP group notably increased from 6 to 24 h, suggesting that FSP could slowly modulate the gut microenvironment.

Table 3.
The concentrations of SCFAs in fermentation solutions at different time points of fermentation.
2.6. Effects of FSP on Gut Microbiota
Figure 4a shows the alpha diversity of the gut microbiota community in each group. In this work, the Simpson, Shannon and Pielou indexes were significantly increased in the FSP group compared with the BLK group, indicating that degradation of FSP increased the microbial evenness. However, both FSP and INU treatment failed to elevate the Chao1 index, which might be because some bacterial species that could use the polysaccharide was enriched in the fecal culture, reduces the uniformity of microbial community. Hierarchical clustering showed that the FSP group formed a distinct cluster from the BLK and INU groups (Figure 4b). Principal coordinate analysis (PCoA) was performed to reveal the beta diversity of tested samples. As shown in Figure 4c, there was distinct separation among the four test groups. The first two axes interpreted 43.1% of the total variance (PC1: 24.9% and PC2: 18.2%), suggesting that the microbial community structure was different among the four groups.
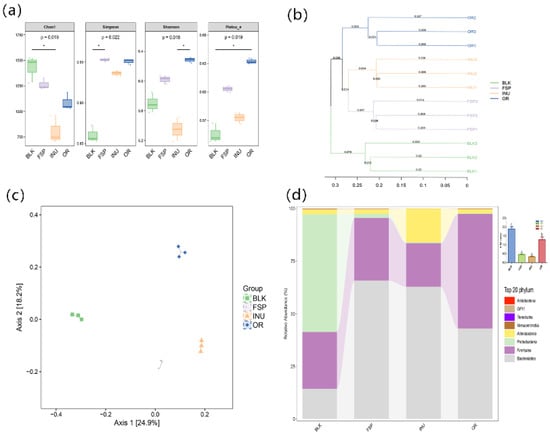
Figure 4.
Compositions of gut microbiota. (a) Alpha diversity analysis of Simpson index and Shannon index, (b) Bray–Curtis clustering analysis, (c) principal component analysis of gut microbiota at the OTU level, (d) taxonomic composition distribution at the phylum level.
FSP modulated the gut microbiota at the phylum level (Figure 4d). Bacteroidetes and Firmicutes were predominant bacterial phyla in the FSP and INU groups. The BLK group contained an average 52% abundance of the phylum Proteobacteria, which was found to be significantly decreased in both the FSP and INU samples. The portion of Bacteroidetes significantly increased in the FSP group compared with the BLK group while Firmicutes displayed the opposite change. The ratio of Firmicutes/Bacteroidetes (F/B) was significantly decreased by FSP fermentation. The F/B ratio is widely considered to be associated with energy harvest, and can characterize the risk of obesity [37]. The decreased F/B ratio caused by FSP fermentation suggested that FSP might have a beneficial effect on host weight loss. It has been demonstrated that FSP decreased the F/B ratio and inhibited obesity in high-fat-diet mice [20]. Additionally, at genus level, fermentation with FSP exhibited a higher Phascolarctobacterium but lower Bifidobacterium and Faecalibacterium in comparison with INU groups (Table 4). A significant growth in the genera Megamonas was observed in the FSP and INU groups.

Table 4.
Composition of the top 20 taxons identified in the tested groups at genus level.
Comparison of variance in relative abundance of gut microbiota was carried out by LEfSe analysis (used to analyze OTUs with relative abundance >0.1%). This demonstrated that a total of 42 OTUs were significantly different in the three groups (Figure 5a). Based on LDA values, 26 OTUs were abundant in the BLK group, 4 OTUs were abundant in the INU group, while 12 OTUs were abundant in the FSP group.
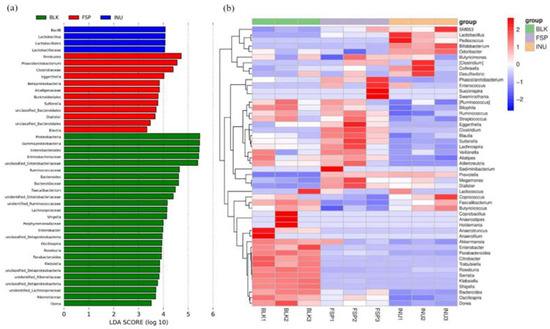
Figure 5.
Comparisons of intra-group variance at OTUs level using LefSe. (a) LDA scores computed for features at OTUs level; (b) heatmap of the relative abundance of OTU (log 10 transformed).
Key genera were notably influenced by FSP and INU treatments compared with the BLK group (Figure 5b). Parabacteroides, Shigella, Bacteroides, Dorea, Citrobacter, and Roseburia were enriched in the BLK group, but this enrichment was not observed in the INU and FSP groups. Notably, Phascolarctobacterium, Clostridium, Sutterella, Ruminococcus and Blautia were enriched in the FSP group. Among them, Ruminococcus and Blautia are SCFA-producing bacteria; they can degrade and use dietary fiber such as cellulose [38].
Composition of the top 20 taxon identified in the tested groups at genus level is shown in Table 4. The relative abundance of Prevotella increased from 3.12% to 60.39% (p < 0.001) and 61.38% (p < 0.001) in the FSP and INU samples, respectively, suggesting that Prevotella might be the principal gut microbiota that degrade and use polysaccharides. Increasing evidence has suggested that Prevotella are beneficial gut microbiota that can improve glucose metabolism [38]. The bloom of Prevotella by FSP suggested it could be used in maintain blood glucose stability in body as reported in mice [20] and rats [39]. Dialister was also stimulated by FSP and inulin both, it has been reported to use pectin as a substrate [40] and produce SCFAs [41].
Recently, Sutterella spp. was demonstrated to be involved in the adhesion of gut microbes to intestinal epithelial cells, and have the potential to regulate immune [42]. Moreover, Phascolarctobacterium was proved to be associated with propionate production through the succinate cycle [43]. This might explain the higher concentration of propionate observed in the FSP group. Clostridium was also detected at comparatively high relative abundance in the FSP group compared to BLK group. Based on previous reports, Clostridium can use various polysaccharides in versatile ways [44]. In addition, after fermentation, Bifidobacterium and Lactobacillus were significantly (p < 0.05) increased in the INU group compared with the BLK group. This result is consistent with previous reports that inulin could promote the proliferation of Bifidobacterium and Lactobacillus [28]. However, these two genera were not increased by FSP fermentation, indicating that FSP displayed a different pattern of regulation of the gut microbiota compared with inulin. Altogether, the fermentation of FSP modulated the microbial community structure and increased the microbial diversity, suggesting that FSP has potential prebiotic potency.
3. Materials and Methods
3.1. Materials
Golden flaxseed was purchased from Zhangye, Ganshu Province, China. Pancreatin, vitamin K and monosaccharide standards (including ribose (Rib), xylose (Xyl), mannose (Man), galacturonic acid (GalA), glucose (Glc) and galactose (Gal)) were purchased from Sigma Chemical Co. (St. Louis, MO, USA.) Bile salt was obtained from Solarbio Science & Technology Co.Ltd. (Beijing, China). SCFAs standards were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals were analytical grade.
3.2. Preparation of Polysaccharide
FSP was extracted from raw flaxseed using the method of Romain et al. [45] with modification. Firstly, the golden flaxseed was cleaned with distilled water. Then, flaxseeds were dissolved with distilled water (1:10, w/w) and stirred at 600 rpm at 60 °C for 2 h. The extracts were centrifuged at 4000 rpm for 10 min at 25 °C. The supernatants were concentrated with three volumes of 95% ethanol at 4 °C overnight to precipitate the polysaccharide. The polysaccharide was then collected by centrifuging at 5000 rpm for 15 min and finally dried in the freeze-dryer.
3.3. Simulated Saliva Digestion
The simulated saliva digestion of FSP was prepared following the reported method [25] with slight modification. First, FSP was dissolved in distilled water at 5 mg/mL concentration. Then, 4.0 mL of FSP solution (FSP group) was mixed with 4.0 mL of saliva and incubated in water bath oscillator (160 rpm, 37 °C). Equal volumes of distilled water (BLK group) and inulin (INU group) were used as blank control and positive control, respectively. During the digestion (at 0.5, 1, 1.5 and 2 h), 1.0 mL mixture was withdrawn, boiled in water bath for 5 min to deactivate salivary amylase. Each sample was replicated three times.
3.4. Simulated Gastric Digestion
The simulated gastric digestion was conducted as described the study by Fu et al. [46]. The gastric electrolyte solution (GES) was composed of 1.55 g NaCl, 0.55 g KCl, 0.075 g CaCl2·2H2O and 0.3 g NaHCO3 in 500 mL distilled water, and the solution was adjusted the pH to 2.0 by 0.1 M HCl solution. Then, gastric lipase (25.0 mg), gastric pepsin (23.6 mg) and 1 M CH3COONa (2 mL, pH 5.0) were added to 1 L of GES, affording the simulated gastric juice. After that, 50.0 mL of FSP solution (5.0 mg/mL) was prepared with equal volume gastric juice and incubated in water bath (37 °C, 160 rpm), while the control group was treated in a similar manner. After 0, 2, 4, and 6 h, samples (5 mL) were separately taken out and then deactivated enzymes for 5 min. Each experiment was replicated independently three times.
3.5. Simulated Small Intestinal Digestion
Simulated intestinal digestion of FSP was conducted as previous reports by Tedeschi et al. [47]. Briefly, the simulated small intestinal electrolyte solution (IES, 300 mL) involving 1.62 g NaCl, 0.195 g KCl and 0.099 g CaCl2•2H2O was prepared. Then, 50 g pancreatin solution (7%, w/w), 100.0 g bile salt (4%, w/w) and 6.5 mg trypsin were added into IES, and the pH was adjusted to 7.0. Subsequently, the digested gastric digestion (after 6 h) was mixed with the small intestinal juice at a ratio of 10:3. The resulting mixture was incubated in water bath oscillator (160 rpm) at 37 °C for 6 h. During the digestion of 0, 1, 2, 4 and 6 h, samples (5 mL) were separately taken out and then deactivated enzymes for 5 min.
3.6. In Vitro Fermentation of FSP
3.6.1. Preparation of Fermentation Medium
The basal medium was conducted in accordance with the report by Ding et al. [48]. Briefly, 500 mL of basal medium consisted of 1.0 g peptone, 1.0 g yeast extract, 0.025 g hemin, 0.25 g L-cysteine, 0.25 g bile salts, 0.05 g NaCl, 0.02 g K2HPO4, 0.02 g KH2PO4, 0.005 g MgSO4, 0.005 g CaCl2, 1 g NaHCO3, 0.5 mg resazurin, 1.0 mL Tween-80, and 5 μL vitamin K. After pH was adjusted to 7.0, the fermentation medium was autoclaved at 12 °C for 20 min.
3.6.2. Preparation of Fecal Slurry and Fermentation
Fecal samples were pooled from three healthy volunteers (one male and two females, age 20–30) who remained on regular diet and had not used antibiotics in at least 3 months. Males should weigh no less than 55 kg and women should weigh no less than 45 kg. Volunteers should be in good health and have no history of heart, liver, kidney, digestive tract, nervous system, mental disorders, and metabolic disorders. Firstly, fresh fecal samples were collected in a triangle beaker, mixed and dispersed with 10% sterilized PBS to obtain a 20% (w/v) feces suspension. Feces suspension was filed with three layers of gauze to obtain fecal slurry. Then, 7.0 mL of the fecal slurry was added into 28.0 mL of basal medium containing 350.0 mg FSP or inulin (INU), or not (BLK), respectively. The entire transport and mix process are completed in a clean bench to ensure no contamination. The fermentation process was carried out in an anaerobic tank (Mitsubishi Chemical, Japan) to ensure an aerobic environment. All groups were incubated at 37 °C. Samples were taken out after fermentation at 6, 12 and 24 h for future analysis. All experimental protocols were approved by the Ethical Committee of the faculty of Hubei Biosafety (China, 2019.08.10). All enrolled subjects were able to comply with the study procedures and gave their written informed consent prior to initiation of the trial. Throughout the study, the principles of Declaration of Helsinki and further amendments were fully respected.
3.7. Determinations of pH, Carbohydrate Content and Reducing Sugar
The pH value was measured by a pH meter (Mettler-Toledo Instruments Co., Ltd., Shanghai, China). The total carbohydrate content was measured by the phenol–sulfuric acid method [49] using glucose as standard. The contents of reducing sugar (CR) were evaluated by dinitro salicylic acid (DNS) method [50].
3.8. Determination of SCFA Content
The content of SCFAs was analyzed according to the reported method [51]. In brief, the fermentation products were centrifuged at 4500 rpm for 10 min and filtered through a 0.22 μm membrane filter to obtain supernatant before analysis. Then, the supernatant of 200 ul was mixed with 0.5 uL 2-ethylbutyric acid as internal standard to determine the SCFAS. SCFAs determination was implemented on GC (7890N, Agilent, Santa Clara, CA, USA),) equipped with a DB-FFAP column (30 m × 0.53 mm× 0.50 μm, Agilent) and flame ionization detector. The carrier gas was N2 with split ratio of 1:10. The initial temperature of column was 80 °C, maintained for 0.5 min and increased to 180 °C at a rate of 8 °C/min. The detector temperature was finally kept at 200 °C for 5 min. The SCFAs concentration was analyzed based on standard calibration curves.
3.9. DNA Extraction and Analysis
After fermentation for 24 h, total genomic DNA from fresh slurry (OR) group, BLK group, FSP group and INU group were immediately extracted by using the QIAamp DNA Stool Mini Kit [52]. The V4 region of 16S rRNA of each sample was chosen for amplification and sequenced by Personalbio Bio. Technology (Shanghai, China) Co., Ltd. Bioinformatics analysis mainly included principal component analysis (PCA), cluster analysis and linear discriminant analysis effect size (LEfSe).
3.10. Molecular Weight Determination
The molecular weight of FSP was determined by HPGPC according to the reported method [53]. The chromatographic separation was carried out by a BRT105-104-102 column and Gel column (8 × 300 mm, borui Saccharide, Biotech, Yangzhou, China. Co. Ltd.). The mobile phase was 0.05 M NaCl solution (0.6 mL/min). The column temperature was maintained at 35 ± 0.2 °C. The sample were centrifuged at 12,000 rpm for 10 min, and fermentation products filtered through a 0.22 μm membrane filter before injection. The injection volume was 20 μL. The standard curve was drawn with Dextran standards (1152 Da, 11,600 Da, 23,800 Da, 48,600 Da, 80,900 Da, 148,000 Da, 273,000 Da, 409,800 Da).
3.11. Determination of Free Monosaccharide and Fourier Transform Infrared (FT-IR) Spectra
FSP was added into 3 mol/L trifluoroacetate and hydrolyzed at 110 °C for 4 h. The hydrolysate was then derived with 200 uL sodium carbonate, anhydrous methanol (3–5 mL) according to a previously described method [54]. Analysis was performed on shimadzu GC-MS-QP 2010 (Shimadzu Technologies, Kyoto, Japan). Samples were isolated with RXI-5 SIL MS column (30 m × 0.25 mm × 0.25 μm, Shimadzu). The initial column temperature was 250 °C, held for 15 min. The pressure was 73.0 kPa, the detector temperature was 290 °C. The carrier gas was helium and the flow rate was 1 mL/min. Standard sugars were determined in the same way. The monosaccharide composition in the sample was obtained by comparing it to the HPLC result of standard sugar.
The fermentation time of 0, 6, 12, and 24 h, 1.0 mL of fermentation supernatant was taken out and placed in a diamond ATR module for infrared (FT-IR) spectrometry. The FT-IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in the frequency range of 600–4000 cm−1 at 4 cm−1 resolution using 32 scans for three or more times.
3.12. Statistical Analysis
All the results were expressed as mean ± standard deviations and all estimations were run in triplicate. The results were analyzed using SPSS 25 software by a Tukey′s test. Values were considered to be statistically significant at p < 0.05.
4. Conclusions
FSP cannot be degraded by simulated saliva or gastric or small intestine conditions, so it reaches the large intestine intact. FSP was gradually hydrolyzed and used by human fecal microbiota during fermentation. FSP fermentation exhibited a modulatory effect on the gut microbial community structure by reducing the ratio of Firmicutes to Bacteroidetes. FSP stimulated the growth of beneficial and SCFA-producing bacteria such as Prevotella and Phascolarctobacterium. Moreover, the content of SCFAs, especially acetic and propionic acids, was significantly increased during FSP fermentation. Therefore, all the results suggest that FSP might contribute to gut health and could be a candidate for use as a prebiotic in functional foods.
Author Contributions
Conceptualization, C.Y. and X.Z.; Methodology, X.Z.; Software, Z.Z.; Validation, C.Y., F.H. and Q.H.; Formal Analysis, X.Z.; Investigation, C.Y.; Resources, F.H. and Q.H.; Data Curation, C.Y.; Writing—Original Draft Preparation, X.Z.; Writing—Review & Editing, C.Y.; Visualization, C.Y. and X.Z.; Supervision, C.Y. and Q.H.; Project Administration, C.Y. and Q.H.; Funding Acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Agricultural Sciences (1610172019009), the Natural Science Foundation of Hubei Province (2019CFB342), the Earmarked Fund for China Agriculture Research System (CARS-14), the Agricultural Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-OCRI).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Marialetizia, R.; Cani, P.D.; Claude, K. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2018, 40, 1271–1284. [Google Scholar]
- Rao, T.P.; Quartarone, G. Role of guar fiber in improving digestive health and function. Nutrition 2019, 59, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, A.; Liu, P.; Li, Z. Effects of Fuzhuan Brick-Tea Water Extract on Mice Infected with E. coli O157:H7. Nutrients 2015, 7, 5309–5326. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700485. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Bewick, G.A. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansiontoincrease satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Dhar, A. Flaxseed and Diabetes. Curr. Pharm. Des. 2016, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Biao, Y.; Nan, H.J.; Yao, L.C.; Jie, S.C.; Chun, D.H.; Mcclements, J.D.; Jiang, C.C. Identification and characterization of antioxidant and immune-stimulatory polysaccharides in flaxseed hull. Food Chem. 2020, 315, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Safdar, B.; Pang, Z.; Liu, X.; Ahmad Jatoi, M.; Mehmood, A.; Tayyab Rashid, M.; Ali, N.; Naveed, M. Flaxseed gum: Extraction, bioactive composition, structural characterization, and its potential antioxidant activity. J. Food Biochem. 2020, 44, e13134. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Vieira, J.M.; Andrade, C.C.P.; Santos, T.P.; Okuro, P.K.; Garcia, S.T.; Rodrigues, M.I.; Vicente, A.A.; Cunha, R.L. Flaxseed gum-biopolymers interactions driving rheological behaviour of oropharyngeal dysphagia-oriented products. Food Hydrocoll. 2020, 111, 132–144. [Google Scholar]
- Yang, C.; Xu, Z.; Deng, Q.; Huang, Q.; Wang, X.; Huang, F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res. Int. 2020, 131, 108994. [Google Scholar] [CrossRef]
- Pedersen, A.; Sorensen, C.E.; Proctor, G.B.; Carpenter, G.H. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Davies, G.A. Viscoelasticity of human whole saliva collected after acid and mechanical stimulation. Biorheology 2007, 44, 141–160. [Google Scholar] [PubMed]
- Liang, S.; Li, X.; Ma, X.; Li, A.; Wang, Y.; Reaney, M.J.T.; Shim, Y.Y. A flaxseed heteropolysaccharide stimulates immune responses and inhibits hepatitis B virus. Int. J. Biol. Macromol. 2019, 136, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Artificial simulated saliva, gastric and intestinal digestion of polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2013, 92, 1143–1150. [Google Scholar] [CrossRef]
- Kastl, A.J., Jr.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Liu, H.; Gong, F.; Wei, F.; Wu, H. Artificial simulation of salivary and gastrointestinal digestion, and fermentation by human fecal microbiota, of polysaccharides from Dendrobium aphyllum. RSC Adv. 2018, 8, 13954–13963. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Pintado, M.; Gomes, A.M.; Alonso, J.L.; Parajó, J.C. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J. Funct. Foods 2014, 6, 438–449. [Google Scholar] [CrossRef]
- Ma, G.; Kimatu, B.M.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, J.; Xia, X.; Kan, J. Purification and Structural Identification of Polysaccharides from Bamboo Shoots (Dendrocalamus latiflorus). Int. J. Mol. Sci. 2015, 16, 15560–15577. [Google Scholar] [CrossRef]
- Correa, V.G.; Goncalves, G.A.; de Sa-Nakanishi, A.B.; Ferreira, I.; Barros, L.; Dias, M.I.; Koehnlein, E.A.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Effects of in vitro digestion and in vitro colonic fermentation on stability and functional properties of yerba mate (Ilex paraguariensis A. St. Hil.) beverages. Food Chem. 2017, 237, 453–460. [Google Scholar] [CrossRef]
- Theodoridou, K.; Vail, S.; Yu, P. Explore protein molecular structure in endosperm tissues in newly developed black and yellow type canola seeds by using synchrotron-based Fourier transform infrared microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. Evaluation of the information content in infrared spectra for protein secondary structure determination. Biophys. J. 2006, 90, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Fan, W.; Huo, G.; Li, X.; Yang, L.; Duan, C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the six months of life. J. Microbiol. Biotechnol. 2014, 24, 133–143. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Mai, Y.; Gao, L.; Ou, S.; Wang, Y.; Liu, L.; Peng, X. Flaxseed gum reduces body weight by regulating gut microbiota. J. Funct. Foods 2018, 47, 136–142. [Google Scholar] [CrossRef]
- Wojciechowicz, M.; Heinrichova, K.; Ziolecki, A. A polygalacturonate lyase produced by Lachnospira multiparus isolated from the bovine rumen. J. Gen. Microbiol. 1980, 117, 193–199. [Google Scholar] [CrossRef]
- Jones, M.L.; Ganopolsky, J.G.; Martoni, C.J.; Labbe, A.; Prakash, S. Emerging science of the human microbiome. Gut Microbes 2014, 5, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Kalliomaki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef]
- Li, T.; Long, M.; Gatesoupe, F.J.; Zhang, Q.; Li, A.; Gong, X. Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microb. Ecol. 2015, 69, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Roulard, R.; Petit, E.; Mesnard, F.; Rhazi, L. Molecular investigations of flaxseed mucilage polysaccharides. Int. J. Biol. Macromol. 2016, 86, 840–847. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Tedeschi, C.; Clement, V.; Rouvet, M.; Valles-Pamies, B. Dissolution tests as a tool for predicting bioaccessibility of nutrients during digestion. Food Hydrocoll. 2009, 23, 1228–1235. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol Macromol 2019, 125, 751–760. [Google Scholar] [CrossRef]
- Li, X.; Klaus, B. A novel carboxylesterase from Aspergillus niger and its hydrolysis of succinimide esters. Carlsberg. Res. Commun. 1989, 54, 241–249. [Google Scholar] [CrossRef]
- Toma, R.B.; Leung, H.K. Determination of Reducing Sugars in French Fried Potatoes by 3,5-Dinitrosalicylic Acid. Food Chem. 1987, 23, 29–33. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, alpha-amylase and alpha-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Liu, Y.; Xiao, J.; Su, D.; Yi, Y.; Wang, G.; Wei, Z.; Zhang, M. Characterization and mesenteric lymph node cells-mediated immunomodulatory activity of litchi pulp polysaccharide fractions. Carbohydr. Polym. 2016, 152, 496–503. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).