Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family
Abstract
1. Introduction
2. Results
2.1. Chemical Characteristic of Vegetable Preparations
2.2. Effects of Vegetable Preparations on Hemostatic Parameters of Human Plasma
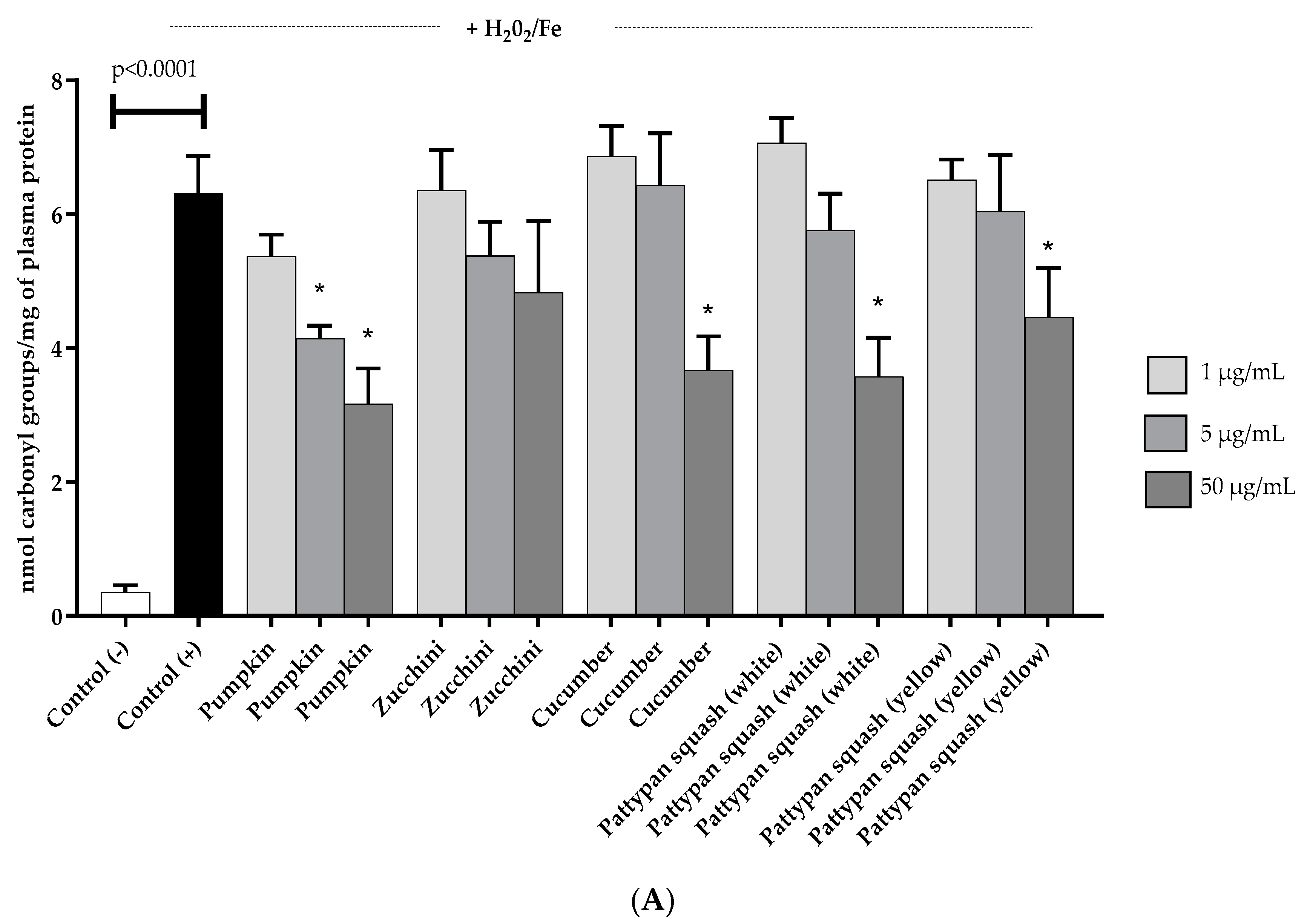
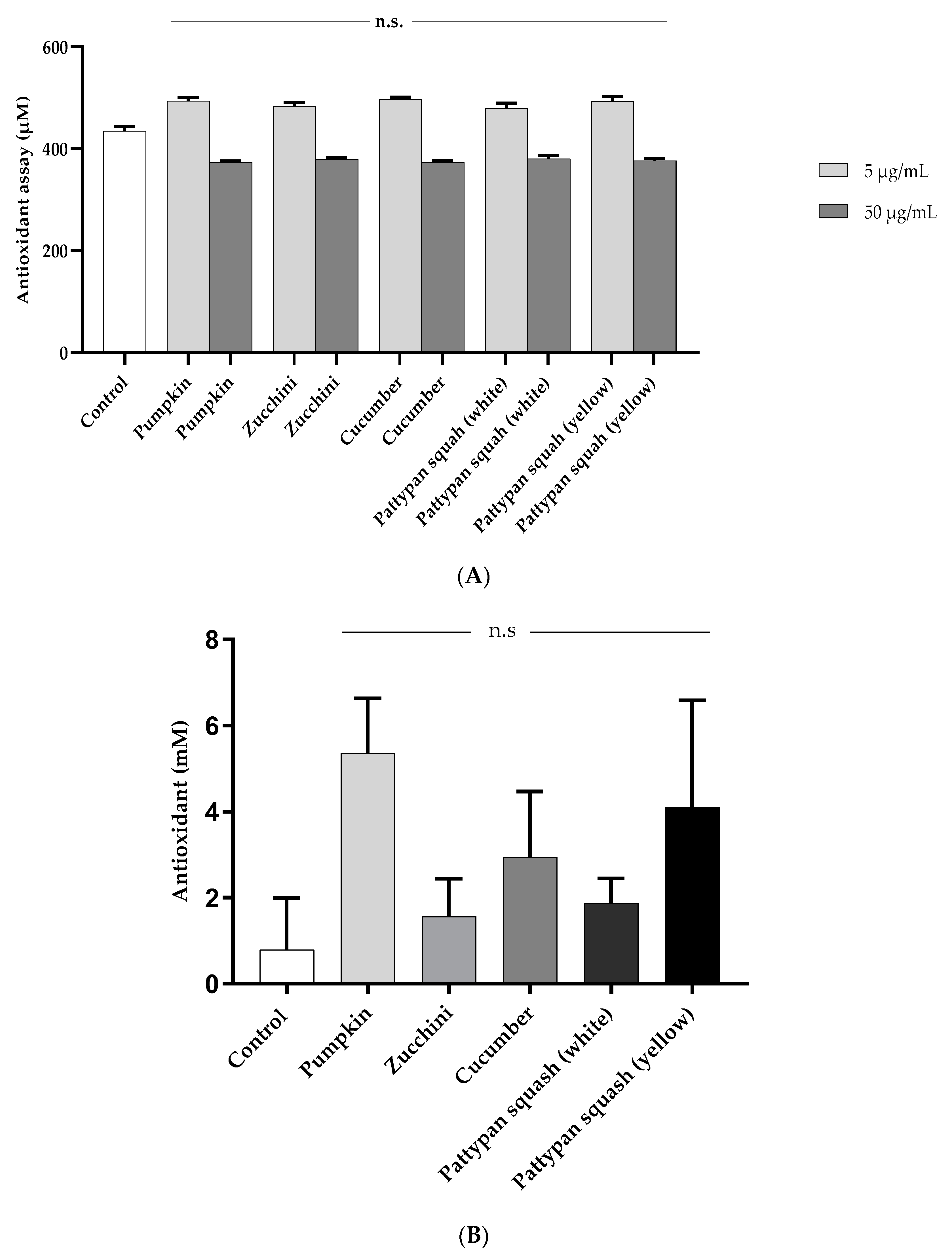
2.3. Effects of Vegetable Preparations on Oxidative Stress Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extraction and Preparation of Vegetable Preparations
4.4. Phytochemical Profiling
4.5. Stock Solutions of Vegetable Preparation
4.6. Human Plasma Isolation
4.7. Markers of Oxidative Stress
4.7.1. Lipid Peroxidation Measurement
4.7.2. Carbonyl Group Measurement
4.7.3. Thiol Group Measurement
4.7.4. TLC-DPPH• Test
4.7.5. Image Processing Procedure
4.7.6. ORAC Assay
4.7.7. Total Antioxidant Capacity of Plasma
4.8. Parameters of Coagulation
4.8.1. Measurement of Prothrombin Time (PT)
4.8.2. Measurement of Thrombin Time (TT)
4.8.3. Measurement of Activated Partial Thromboplastin Time (APTT)
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olas, B. Anti-aggregatory potential of selected vegetables-promising dietary components for the prevention and treatment of cardiovascular disease. Adv. Nutr. 2019, 2, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Koryachkina, S.Y.; Ladnova, O.L.; Godunov, O.A.; Kholodova, E.N.; Lazareva, T.N. The study of physiological effect of fruit and vegetable powders in animal experiments. Vopr. Pitan. 2016, 85, 48–56. [Google Scholar] [PubMed]
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. Vegetables from Cucurbitaceae family and their products; positive effect on human health. Nutrition 2020, 78, 11078. [Google Scholar] [CrossRef]
- Saboo, S.S.; Thorat, P.K.; Tapadiya, G.G.; Khadabadi, S.S. Ancient and recent medicinal use of Cucurbitaceae family. Intern. J. Ther. Applic. 2013, 9, 11–19. [Google Scholar]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef]
- Pastel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Montesano, D.; Roccheti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2010, 22, 81–87. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants-implications for human health? Front. Pharm. 2018, 9, 78. [Google Scholar] [CrossRef]
- Levandi, T.; Püssa, T.; Vaher, M.; Toomik, P.; Kaljurand, M. Oxidation products of free polyunsaturated fatty acids in wheat varieties. Eur. J. Lipid Sci. Technol. 2009, 111, 715–722. [Google Scholar] [CrossRef]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.S.; Young, M.C.M.; Furlan, M.; Eberlin, M.N.; Castro-Gamboa, I.; Cavalheiro, A.J.; da Silva Bolzani, V. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Vicente, M.J.; Cifuentes, A.; Ibanez, E. Characterization by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry of the lipid fraction of Spirulina platensis pressurized ethanol extract. Rapid Com. Mass Spectrometr. 2007, 21, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arráez-Román, D.; Quirantes-Piné, R.; Fernández-Arroyo, S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012, 46, 108–117. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Intern 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Hamdy, S.A.; El Hefnawy, H.M.; Azzam, S.M.; Aboutabl, E.A. Chemical profiling, volatile oil analysis and anticholinesterase activity of Hydrocotyle umbellata L. aerial parts cultivated in Egypt. S. Afr. J. Bot. 2018, 115, 108–112. [Google Scholar] [CrossRef]
- Nowak, P.; Olas, B.; Wachowicz, B. Stres oksydacyjny w przebiegu hemostazy. Post. Biochem. 2010, 3, 239–247. [Google Scholar]
- Kim, D.O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Critic. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Rad. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Thuan, N.D.; Ha, D.T.; Thuong, P.T.; Na, M.K.; Bae, K.; Lee, J.P.; Lee, J.H.; Seo, H.W.; Min, B.S.; Kim, J.C. A phenylpropanoid glycoside with antioxidant activity from picria tel-ferae. Arch. Pharm. Res. 2007, 30, 1062–1066. [Google Scholar] [CrossRef]
- López-Munguía, A.; Hernández-Romero, Y.; Pedraza-Chaverri, J.; Miranda-Molina, A.; Regla, I.; Martínez, A.; Castillo, E. Phenylpropanoid glycoside analogues: Enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS ONE 2011, 6, e20115. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S. Antioxidant and lipoxygenase 554 inhibitory activities of pumpkin seed extracts. Food Res. Int. 2009, 42, 641–646. [Google Scholar] [CrossRef]
- Khennouf, S.; Amira, S.; Arrar, L.; Baghiani, A. effect of some phenolic compounds and quercus tannins on lipid peroxidation. World Appl. Sci. J. 2009, 8, 1144–1149. [Google Scholar]
- Torkova, A.A.; Lisitskaya, K.V.; Filimonov, I.S.; Glazunova, O.A.; Kachalova, G.S.; Golubev, V.N.; Fedorova, T.V. Physicochemical and functional properties of Cucurbita maxima pumpkin pectin and commercial citrus and apple pectins: A comparative evaluation. PLoS ONE 2018, 13, e0204261. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, R.; Kamalinejad, M.; Adiban, H.; Kardan, A.; Keyhanfar, F.; Eskandari, M.R. Cytoprotective effects of pumpkin (Cucurbita moschata) fruit extract against oxidative stress and carbonyl stress. Drug Res. 2017, 67, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of phytocehmicals, antioxidant and burn wound healing activities of Cucurbita muschata Duchesne fruit peel. Iran. J. Basic Med. Sci. 2017, 20, 798–805. [Google Scholar]
- Abarikwu, S.O.; Mgbudom-Okah, C.J.; Onuah, C.L.; Ogunlaja, A. Fluted pumpkin seeds protect against busulfan-induced oxidative stress and testicular injuries in adult mice. Drug Chem. Toxicol. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Hajipour, R.; Hemmati, M.; Moossavi, M.; Mohagiq, Z. The beneficial effects of pumpkin extract on atherogenic lipid, insulin resistance and oxidative stress status in high-fat diet-induced obese rats. J. Complement. Integr. Med. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Stochmal, A.; Olas, B. Assessment of effects of phenolic fractions from leaves and petals of dandelion in selected components of hemostasis. Food Res. Int. 2018, 107, 605–612. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Granum, P.E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal. Biochem. 1980, 109, 156–159. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wachowicz, B. Adenine nucleotides in thrombocytes of birds. Cell Biochem. Funct. 1984, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Druga Twarz Tlenu: Wolne Rodniki w Przyrodzie; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Ando, Y.; Steiner, M. Sulfhydryl and disulfide groups of platelet membranes. I. Determination of sulfhydryl groups. Biochim. Biophys. Acta Biomembr. 1973, 311, 26–37. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulfhydryl and disulfide groups of platelet membranes. II. Determination of disulfide groups. Biochim. Biophys. Acta Biomembr. 1973, 311, 38–44. [Google Scholar] [CrossRef]
- Cieśla, Ł.; Kowalska, I.; Oleszek, W.; Stochmal, A. Free radical scavenging activities of polyphenolic compounds isolated from Medicago sativa and Medicago truncatula assessed by means of thin-layer chromatography DPPH• rapid test. Phytochem. Anal. 2013, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I.; Jędrejek, D.; Cieśla, Ł.; Pecio, Ł.; Masullo, M.; Piacente, S.; Oleszek, W.; Stochmal, A. Isolation, chemical and free radical scavenging characterization of phenolics from Trifolium scabrum L. aerial parts. J. Agric. Food Chem. 2013, 61, 4417–4423. [Google Scholar] [CrossRef]
- Kowalska, I.; Cieśla, Ł.; Oniszczuk, T.; Waksmundzka-Hajnos, M.; Oleszek, W.; Stochmal, A. Comparison of two TLCDPPH-image processing procedures for studying free radical scavenging activity of compounds from selected varieties of Medicago sativa. J. Liquid Chromatogr. Relat. Technol. 2013, 36, 2387–2394. [Google Scholar] [CrossRef]
- Olech, M.; Komsta, Ł.; Nowak, R.; Cieśla, Ł.; Waksmundzka-Hajnos, M. Investigation of antiradical activity of plant material by thin-layer chromatography with image processing. Food Chem. 2012, 132, 549–553. [Google Scholar] [CrossRef]
- Hu, R.; Dunmire, K.M.; Truelock, C.N.; Paulk, C.B.; Aldrich, G.; Li, Y. Antioxidant performances of corn gluten meal and DDGS protein hydrolysates in food, pet food, and feed systems. J. Agric. Food Res. 2020, 2, 100030. [Google Scholar] [CrossRef]
- Mesa, M.D.; Olza, J.; Gonzalez-Anton, C.; Aguilera, C.M.; Moreno-Torres, R.; Jimenez, A.; Perez de la Cruz, A.; Ruperez, A.I.; Gil, A. Changes in Oxidative Stress and Inflammatory Biomarkers in Fragile Adults over Fifty Years of Age and in Elderly People Exclusively Fed Enteral Nutrition. Oxidative Med. Cell. Longev. 2016, 5709312. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, J.; Kołodziejczyk-Czepas, J.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A.; Olas, B. Phenolic fractions from Trifolium pallidum and Trifolium scabrum aerial parts in human plasma protect against changes induced by hyperhomocysteinemia in vitro. Food Chem. Toxicol. 2012, 50, 4023–4027. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of pumpkin (Cucurbita pepo; fruit), zucchini (Cucurbita pepo convar. giromontina), cucumber (Cucumis sativus), white pattypan squash (Cucurbita pepo var. patisoniana) and yellow pattypan squash (Cucurbita pepo var. patisoniana) are available from the authors. |
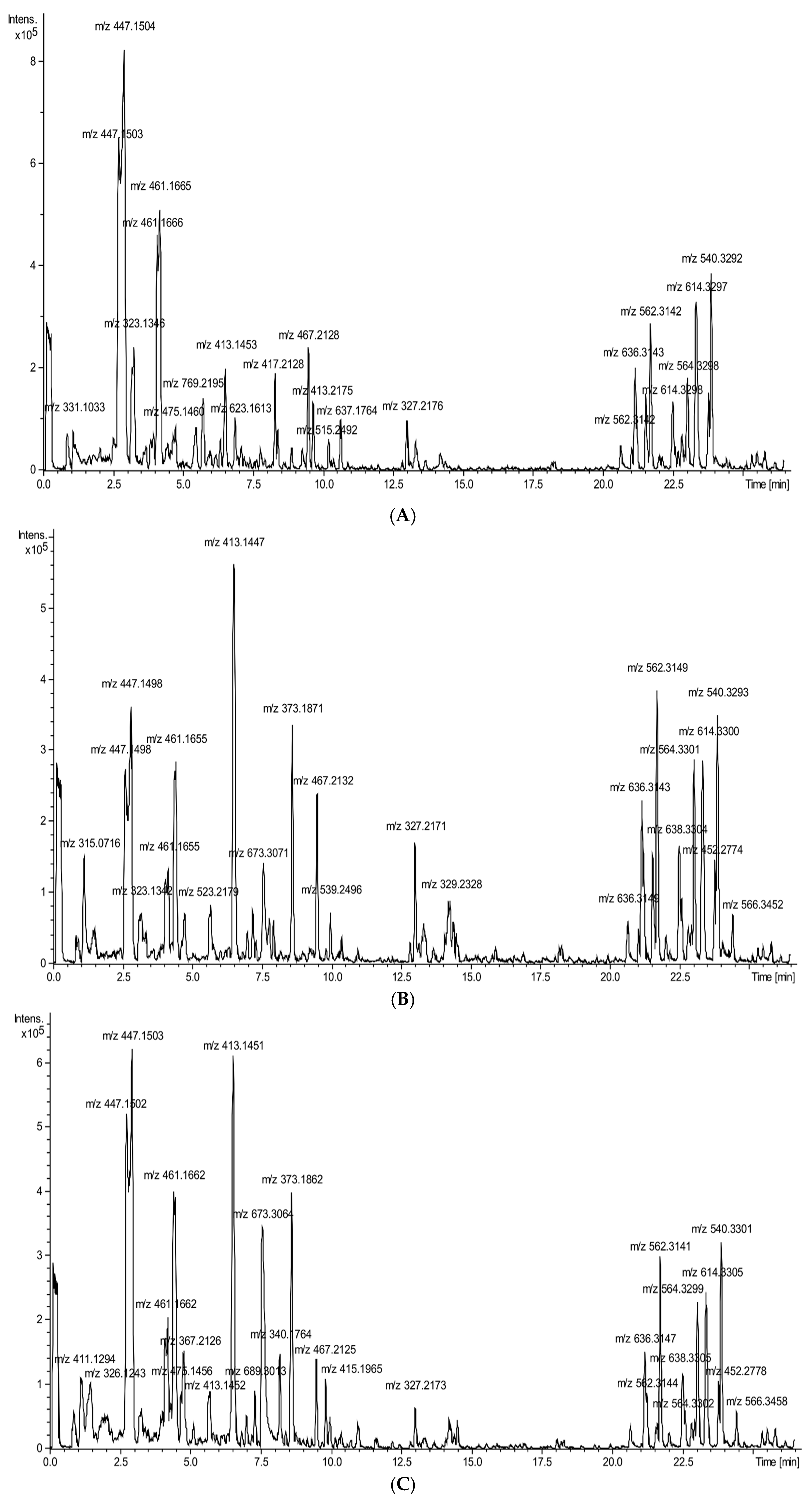
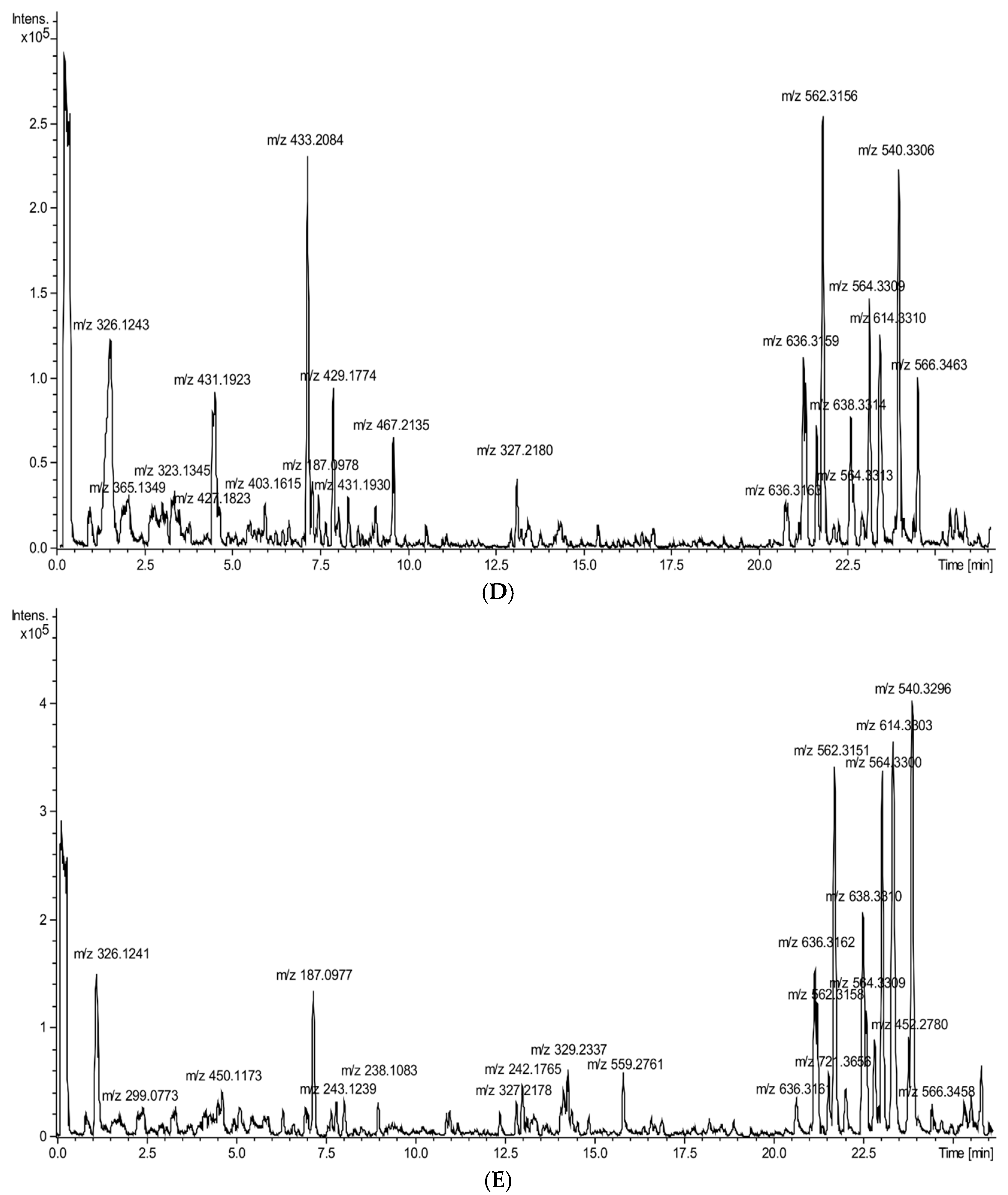


| Nr | Rt (min) | Identified Compound | Compound Class | [M-H], m/z, ESI Neg. | Major MS-MS Fragments | Pumpkin | Cucumber | Zucchini | White Pattypan Squash | Yellow Pattypan Squash |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.14 | 3-(β-D-glucopyranosyloxy)-2-hydroxybenzoic acid | benzoic acid derivative | 315.071649 | 152, 315 | - | - | - | - | + |
| 2 | 1.47 | fructosyl l-phenylalanine | amino acid | 326.124313 | 164, 236, 326 | + | + | - | + | + |
| 3 | 1.87 | l-tryptophan glycoside | amino acid | 365.134743 | 116, 203, 275, 365 | + | - | - | - | - |
| 4 | 2.45 | salicylic acid O-glycoside | phenolic acid | 299.077241 | 137, 299 | - | + | - | - | - |
| 5 | 3.87 | zizybeoside I | phenylethanoid glycoside | 431.155861 | 147, 431 | - | - | + | - | - |
| 6 | 4.23 | forsythoside E (isomer I) | phenylethanoid glycoside | 461.166185 | 147, 309, 461 | - | - | + | + | + |
| 7 | 4.35 | cinncassiol A | diterpenoid | 381.191905 | 289, 381 | - | - | - | + | - |
| 8 | 4.39 | sinapic acid hexoside | phenolic acid | 431.192317 | 223, 385, 431 | + | - | - | - | - |
| 9 | 4.43 | hydrangeifolin I | phenylpropanoid glycoside | 415.160971 | 269, 415, 461 | - | - | - | + | + |
| 10 | 4.55 | shimaurinoside B | megastigmane glycosides | 381.176621 | 249, 381, 427 | + | - | - | - | - |
| 11 | 4.65 | kaempferol derivative | flavonoid | 450.117527 | 145, 285, 450 | - | + | - | - | - |
| 12 | 4.68 | primulaverin derivative | flavonoid | 475.146049 | 133, 295, 323, 475 | - | - | + | - | - |
| 13 | 4.75 | adenostemmoic acid C | diterpenoid | 367.212612 | 287, 303, 367 | - | - | - | - | + |
| 14 | 5.50 | rutin | flavonoid | 609.146766 | 301, 609 | - | - | + | - | - |
| 15 | 5.69 | secoisolariciresinol monoglucoside | lignan | 523.217892 | 165, 361, 523 | - | - | - | + | + |
| 16 | 5.75 | xanthorhamnin | flavonoid | 769.219503 | 299, 314, 769 | - | - | + | - | - |
| 17 | 6.54 | unidentified | iridoid glycoside | 413.145347 | 269, 311, 351, 413 | - | - | + | + | + |
| 18 | 6.88 | isorhamnetin 3-O-rutinoside | flavonoid | 623.161334 | 299, 315, 623 | - | - | + | - | - |
| 19 | 7.21 | azelaic acid | dicarboxylic acid | 187.097798 | 125, 187 | + | + | - | - | + |
| 20 | 8.41 | hesperetin 7-O-(2′′,6′′-di-O-α-rhamnopyranosyl)-β-glucopyranoside | flavonoid | 739.244676 | 295, 471, 559, 739 | - | - | + | - | - |
| 21 | 9.68 | octadecadienoic acid derivative | fatty acid | 413.217479 | 209, 371, 413 | - | - | + | - | - |
| 22 | 10.66 | quercetin 3,3′-dimethyl ether 7-rutinoside | flavonoid | 637.176449 | 299, 313, 329, 637 | - | - | + | - | - |
| 23 | 13.04 | octadecadienoic acid derivative | fatty acid | 327.218013 | 211, 327 | + | - | + | + | + |
| 24 | 14.17 | octadecadienoic acid derivative | fatty acid | 329.233674 | 211, 329 | - | + | + | + | + |
| 25 | 20.68 | glycerophospholipid | lipid | 636.316333 | 277, 474, 636 | - | + | + | + | + |
| 26 | 21.19 | glycerophospholipid | lipid | 636.315949 | 277, 474, 636 | + | + | + | + | + |
| 27 | 21.26 | glycerophospholipid | lipid | 562.315676 | 277, 505, 562 | + | + | + | + | + |
| 28 | 21.57 | γ-linolenic acid derivative | fatty acid | 721.364358 | 277, 397, 721 | - | - | + | - | + |
| 29 | 22.54 | glycerophospholipid | lipid | 638.331427 | 152, 279, 476, 638 | + | + | + | + | + |
| 30 | 22.63 | glycerophospholipid | lipid | 564.331348 | 279, 504, 564 | + | + | + | + | + |
| 31 | 23.03 | γ-linolenic acid derivative | fatty acid | 559.311446 | 277, 559 | - | - | + | - | - |
| 32 | 23.06 | linoleic acid derivative | fatty acid | 564.329757 | 279, 504, 564 | + | + | + | + | + |
| 33 | 23.27 | glycerophospholipid | lipid | 614.330267 | 255, 452, 614 | + | + | + | + | + |
| 34 | 23.82 | glycerophospholipid | lipid | 452.277974 | 255, 452 | - | - | + | + | + |
| 35 | 23.89 | glycerophospholipid | lipid | 540.330582 | 255, 480, 540 | + | + | + | + | + |
| 36 | 24.46 | glycerophospholipid | lipid | 566.346313 | 281, 506, 566 | + | + | - | + | + |
| Preparation | Protein Carbonylation | Thiol Oxidation | Lipid Peroxidation |
|---|---|---|---|
| from fruit with seeds | |||
| Zucchini | No effect | No effect | Positive action-inhibition of this process (anti-oxidative potential) |
| Cucumber | Positive action-inhibition of this process (anti-oxidative potential) | No effect | Positive action-inhibition of this process (anti-oxidative potential) |
| from fruit without seeds | |||
| Pumpkin | Positive action-inhibition of this process (anti-oxidative potential) | No effect | Positive action-inhibition of this process (anti-oxidative potential) |
| White pattypan squash | Positive action-inhibition of this process (anti-oxidative potential) | No effect | Positive action-inhibition of this process (anti-oxidative potential) |
| Yellow pattypan squash | Positive action-inhibition of this process (anti-oxidative potential) | Positive action-inhibition of this process (anti-oxidative potential) | Positive action-inhibition of this process (anti-oxidative potential) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolnik, A.; Kowalska, I.; Soluch, A.; Stochmal, A.; Olas, B. Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family. Molecules 2020, 25, 4326. https://doi.org/10.3390/molecules25184326
Rolnik A, Kowalska I, Soluch A, Stochmal A, Olas B. Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family. Molecules. 2020; 25(18):4326. https://doi.org/10.3390/molecules25184326
Chicago/Turabian StyleRolnik, Agata, Iwona Kowalska, Agata Soluch, Anna Stochmal, and Beata Olas. 2020. "Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family" Molecules 25, no. 18: 4326. https://doi.org/10.3390/molecules25184326
APA StyleRolnik, A., Kowalska, I., Soluch, A., Stochmal, A., & Olas, B. (2020). Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family. Molecules, 25(18), 4326. https://doi.org/10.3390/molecules25184326







