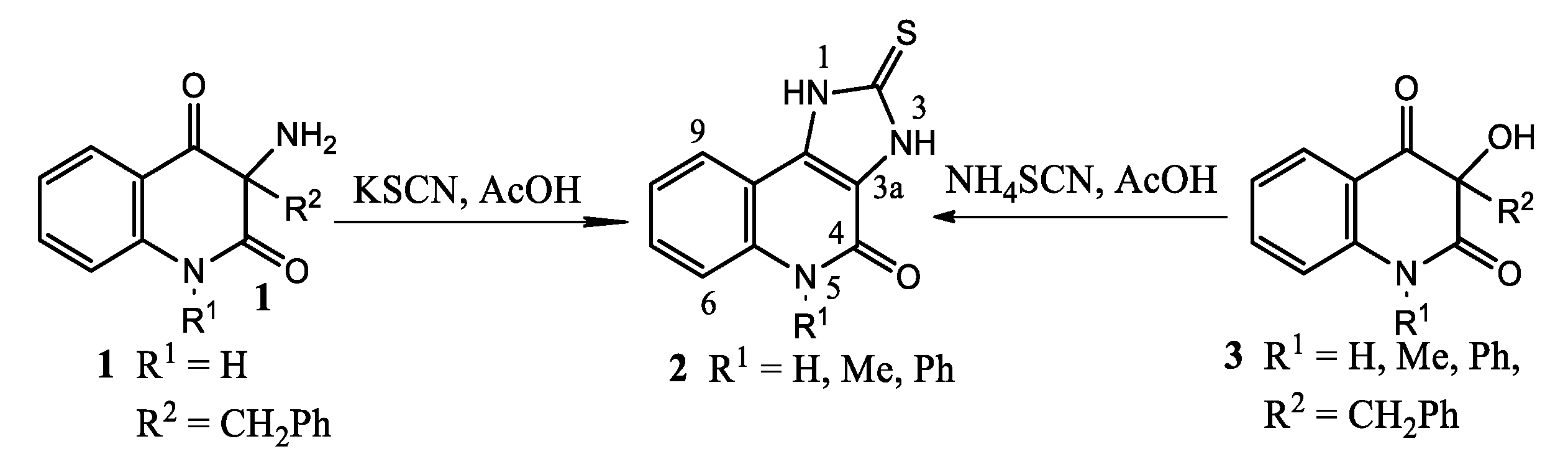
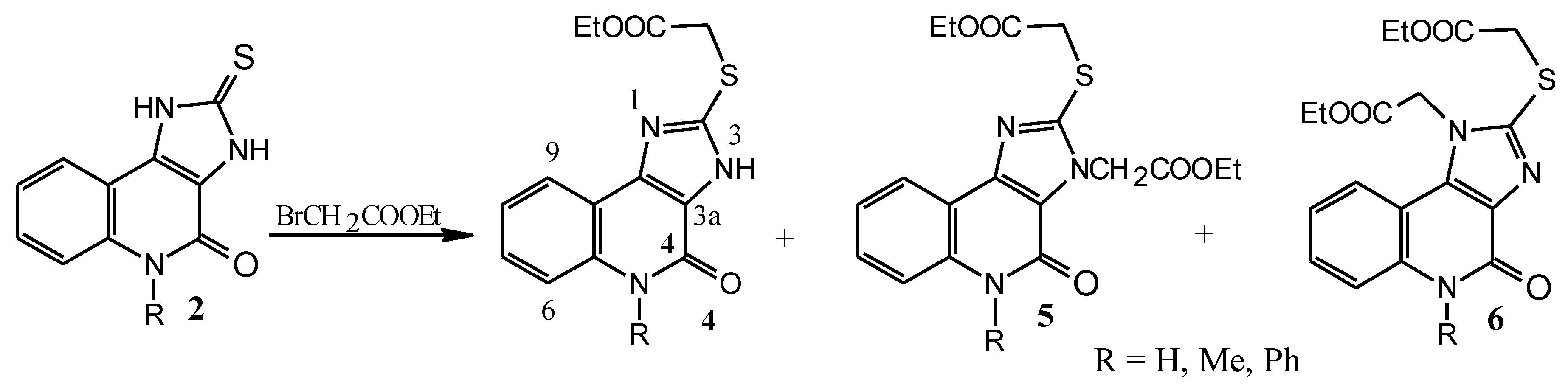
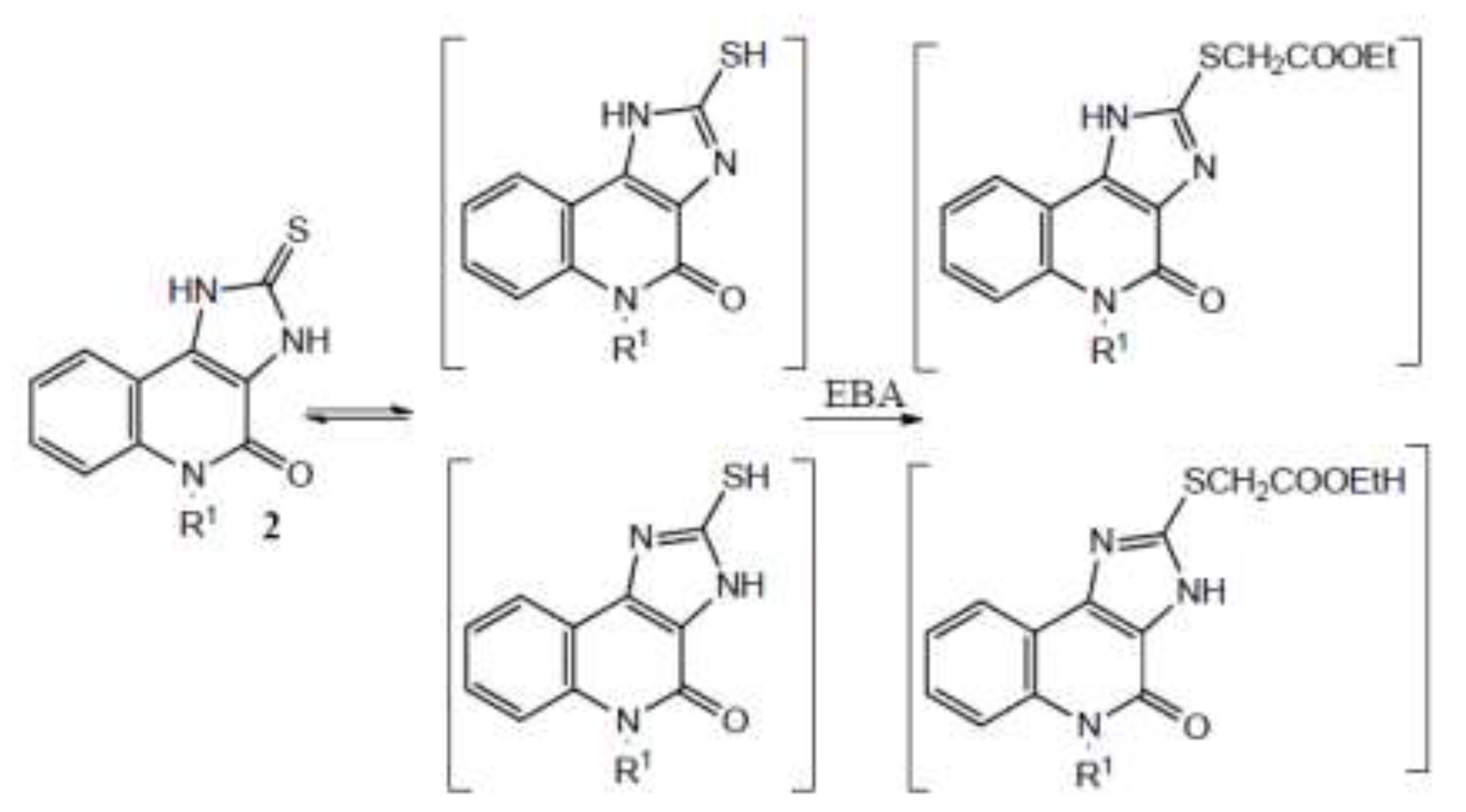
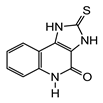
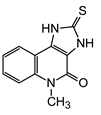
3.2. Syntheses
General Procedure for the Reaction of Compounds 2 with Ethyl Bromoacetate
To the solution of compound 2 (10 mmol) in DMF (40 mL) was added powdered potassium carbonate (40 mmol) and ethyl bromoacetate in quantities given in
Table 1. The mixture was stirred at 40 °C for 4 h and extracted with chloroform (4 × 50 mL). Collected extracts were washed with water (3 × 40 mL), dried with anhydrous sodium sulfate and evaporated to dryness. The residue was column chromatographed. Yields of pure product are given in
Table 1.
Ethyl 2-((4-oxo-4,5-dihydro-3H-imidazo[4,5-c]quinolin-2-yl)thio)acetate (4a). Product characterization: Colorless solid, melting point 218–222 °C (from ethyl acetate). EA: % calcd (found) for C
14H
13N
3O
3S (303.34): C 55.43 (55.35), H 4.32 (4.10), N 13.85 (13.68), S 10.57 (10.27). IR (KBr): 3338, 3085, 3032, 2979, 2918, 1730, 1649, 1549, 1479, 1462, 1421, 1353, 1334, 1307, 1252, 1201, 1172, 1161, 1110, 1026, 969, 932, 895, 868, 851, 817, 776, 750, 720, 685, 660, 640, 600, 562, 503, 468 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, %) EI-MS: 304.04 (32%), 276.04 (7%), 258.03 (100%), 230.04 (95%), 216.02 (18%), 186.07 (16%). ESI-MS (
m/
z): 303 [M + H]
+, 326 [M + Na]
+.
Ethyl 2-((4-oxo-5-methyl-4,5-dihydro-3H-imidazo[4,5-c]-quinolin-2-yl)thio)acetate (4b). Product characterization: Colorless solid, melting point 231–237 °C (from benzene).EA: % calcd (found) for C
15H
15N
3O
3S (317.33): C 56.77 (56.71), H 4.76 (4.68), N 13.24 (13.05), S 10.10 (10.03).IR (KBr): 3441, 3083, 2982, 2926, 1730, 1649, 1573, 1481, 1454, 1427, 1404, 1356, 1301, 1256, 1222, 1176, 1164, 1117, 1028, 975, 949, 901, 868, 818, 756, 736, 711, 697, 679, 654, 571 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, %): 318.09 (9%), 290.06 (7%), 272.05 (100%), 244.05 (61%), 230.04 (18%). HR-MS:[M
+] 318.0905; [M
+ + 1] 319.0939; [M
+ + 3] 320.0861.
Ethyl 2-((4-oxo-5-phenyl-4,5-dihydro-3H-imidazo[4,5-c]-quinolin-2-yl)thio)acetate (4c). Product characterization: Colorless solid, melting point 207–210 °C (from benzene/hexane). EA: % calcd(found) for C
20H
17N
3O
3S (379.39): C 63.31 (63.51), H 4.52 (4.47), N 11.08 (10.96), S 8.45 (8.41). IR (KBr): 3090, 3036, 2975, 2927, 2753, 1722, 1641, 1571, 1491, 1474, 1448, 1413, 1387, 1353, 1307, 1271, 1225, 1183, 1145, 1095, 1073, 1051, 1024, 975, 924, 900, 861, 842, 814, 760, 710, 678, 659, 575, 555, 520, 448 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, %): 380.11 (7%), 352.07 (6%), 290.06 (7%), 334.06 (82%), 306.07 (100%), 292.05 (57%), 265.04 (37%), 259.07 (10%), 237.05 (11%), 205.08 (8%), 186.07 (9%). HR-MS: [M
+] 380.1060; [M
++1] 381.1094; [M
++3] 382.1015
Ethyl 2-((3-(2-ethoxy-2-oxoethyl)-4-oxo-4,5-dihydro-3H-imidazo[4,5-c]quinolin-2-yl)thio)acetate (5a). Product characterization: Colorless solid, melting point 200–204 °C (from ethyl acetate). %). EA: % calcd (found) for C
18H
19N
3O
5S (389.37): C 55.52 (55.68), H 4.92 (4.89), N 10.79 (10.75), S 8.24 (8.17). IR (KBr): 3453, 3165, 3126, 3052, 2986, 2846, 1738, 1662, 1556, 1466, 1436, 1376, 1304, 1224, 1174, 1098, 1025, 965, 898, 875, 762, 724, 701, 681, 669, 597, 512, 456 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, %): 390.11 (10%), 362.08 (8%), 344.07 (25%), 316.4 (15%), 286.06 (37%), 258.03 (100%), 242.04 (40%), 230.04 (98%), 216.02 (12%), 198.07 (17%), 186.07 (40%). HR-MS: [M
+] 390.1114; [M
+ + 1] 391.1148; [M
+ + 3] 392.1069.
Ethyl 2-((3-(2-ethoxy-2-oxoethyl)-4-oxo-5-methyl-4,5-di-hydro-3H-imidazo[4,5-c]quinolin-2-yl)thio)- acetate (5b). Product characterization: Colorless solid, melting point 129–132 °C (from benzene/hexane). EA: % calcd (found) for C
19H
21N
3O
5S (403.45): calcd C 56.57 (56.75), H 5.25 (5.33), N 10.42 (10.46), S 7.95 (7.86). IR (KBr): 3452, 3067, 2984, 2936, 2904, 1734, 1653, 1577, 1503, 1466, 1443, 1416, 1378, 1707, 1264, 1224, 1159, 1117, 1095, 1029, 980, 946, 898, 874, 859, 780, 755, 714, 677, 657, 573, 551, 480 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, %): 404.13 (24%), 376.10 (15%), 358.08 (37%), 330.05 (17%), 300.08 (65%), 272.05 (100%), 256.05 (18%), 242.04 (61%), 230.04 (18%) 200.08 (16%). HR-MS: [M
+] 404.1270; [M
+ + 1] 405.1306; [M
+ + 3] 406.1226.
Ethyl 2-((3-(2-ethoxy-2-oxoethyl)-4-oxo-5-phenyl-4,5-di-hydro-3H-imidazo[4,5-c]quinolin-2-yl)thio)- acetate (5c). Product characterization: Colorless solid, melting point 129–131 °C (from benzene/hexane). EA: % calcd (found) for C
24H
23N
3O
5S (465.46): C 61.93 (61.75), H 4.98 (5.17), N 9.03 (8.96), S 6.89 (6.79). IR (KBr): 3034, 2984, 2934, 1755, 1660, 1576, 1510, 1491m, 1462, 1440, 1417, 1376, 1310, 1261, 1219, 1200, 1175, 1148, 1127, 1096, 1024, 967, 896, 863, 847, 804, 767, 700, 679, 583, 531 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. EI-MS (
m/
z, % 466.14 (22%), 438.11 (10%), 420.10 (24%), 393.07 (10%), 362.10 (48%), 334.06 (100%), 318.08 (10%), 306.07 (32%), 278.06 (14%), 262.10 (14%), 247.08 (8%). HR-MS: [M
+] 466.1429; [M
+ + 1] 467.1464; [M
+ + 3] 468.1385.
Ethyl 2-((1-(2-ethoxy-2-oxoethyl)-4-oxo-4,5-dihydro-1H-imidazo[4,5-c]chinolin-2-yl)thio)acetate (6a). Product characterization: Colorless solid, melting point 235–237 °C (from ethanol). EA: % calcd (found) for C
18H
19N
3O
5S (389.43): C 55.52 (55.43), H 4.92 (4.84), N 10.79 (10.65), S 8.23 (8.07). IR (KBr): 3457, 3174, 3016, 2973, 2876, 1743, 1680, 1549, 1514, 1456, 1428, 1403, 1370, 1350, 1309, 1225, 1174, 1117, 1023, 977, 941, 903, 873, 802, 755, 725, 687, 670, 565, 514 cm
−1.
1H,
13 C{
1H}, and
15N NMR data are referred in
Table 2. ESI-MS: 389 ([M+H]
+, 412 [M+Na]
+, 428 [M+K]
+.
3.3. Analytical Methods
Melting points were determined on a Kofler block (Vienna, Austria).
IR (KBr) spectra were recorded on a Smart OMNI-Transmission Nιcolet iS10 spectrophotometer (Waltham, MA, USA).
The 1H, 13C{1H}, and 15N NMR spectra were recorded on a Bruker Avance III HD 500 spectrometer (500.13 MHz for 1H, 125.76 MHz for 13C{1H}, and 50.68 MHz for 15N) in DMSO-d6. 1H and 13C chemical shifts are given on the δ scale (ppm) and are referenced to internal TMS (δ = 0.0). 15N chemical shifts were referred to external neat CH3NO2 in a co-axial capillary (δ = 0.0). All 2D experiments (gradient-selected (gs)-COSY, gs-TOCSY, gs-NOESY, gs-HMQC, gs-HMQC-TOCSY, gs-HMQC-RELAY, and gs-HMBC) were performed using manufacturer’s software (TOPSPIN 3.5, Rheinstetten, Germany).
The electrospray mass spectra (ESI-MS) were recorded using an amaZon X ion-trap mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with an electrospray ion source. All experiments were conducted in both positive and negative polarity mode. Individual samples (with a concentration of 500 ng·mL−1) were infused into the ESI source as methanol/water (1:1, v/v) solutions via a syringe pump with a constant flow rate of 3 μL·min−1. The other instrumental conditions were as follows: Electrospray voltage of ±4.2 kV, capillary exit voltage of ±140 V, drying gas temperature The GC-MS analyses were measured with Shimadzu GC-MS QP-2010 with quadrupole mass detector and column EQUITY 1 (30 m, 0.32 mm, 1 μm). Temperature program: 100 °C/7 min, 30 °C/min, temperature of spray 250 °C. All experiments were carried out at constant linear rate of 52 cm/s. HR-MS spectra were recorded by micrOTOF-QII (Agilent Technologies, Santa Clara, CA, USA), and calibration was performed with TuneMIX (Agilent Technologies, Santa Clara, CA, USA). Full-mass range 50–750 m/z. Full mass resolution 140,000. MS/MS parameters: Type of HCD fragmentation (high energy collision dissociation), CE (collision energy) 35. Spray voltage positive ion mode 4100 V. Sheath gas 10 arbitrary units. Auxiliary gas 0 arbitrary units. Ion transfer temperature 320 °C. Vaporisation temperature 250 °C.
Column chromatography was carried out on silica gel (Merck, grade 60, 70–230 mesh) using successive mixtures of chloroform/ethanol (in ratios from 99:1 to 8:2) (
Figure S1) or benzene/ethyl acetate (in ratios from 99:1 to 8:2) (
Figure S2).
Reactions as well as the course of separation and also the purity of all substances were monitored by TLC (elution systems benzene/ethyl acetate (4:1) (
Figure S3), chloroform/ethanol (9:1 and 1:1) (
Figures S4 and S5), and chloroform/ethyl acetate (7:3) (
Figure S6) on Alugram
® SIL G/UV
254 foils (Macherey-Nagel, (Dueren, Germany).
Elemental analyses (C, H, N, S) of the obtained compounds were performed with an analyser Vario EL III C, H, N from Elementar Company (Langenselbold, Germany).
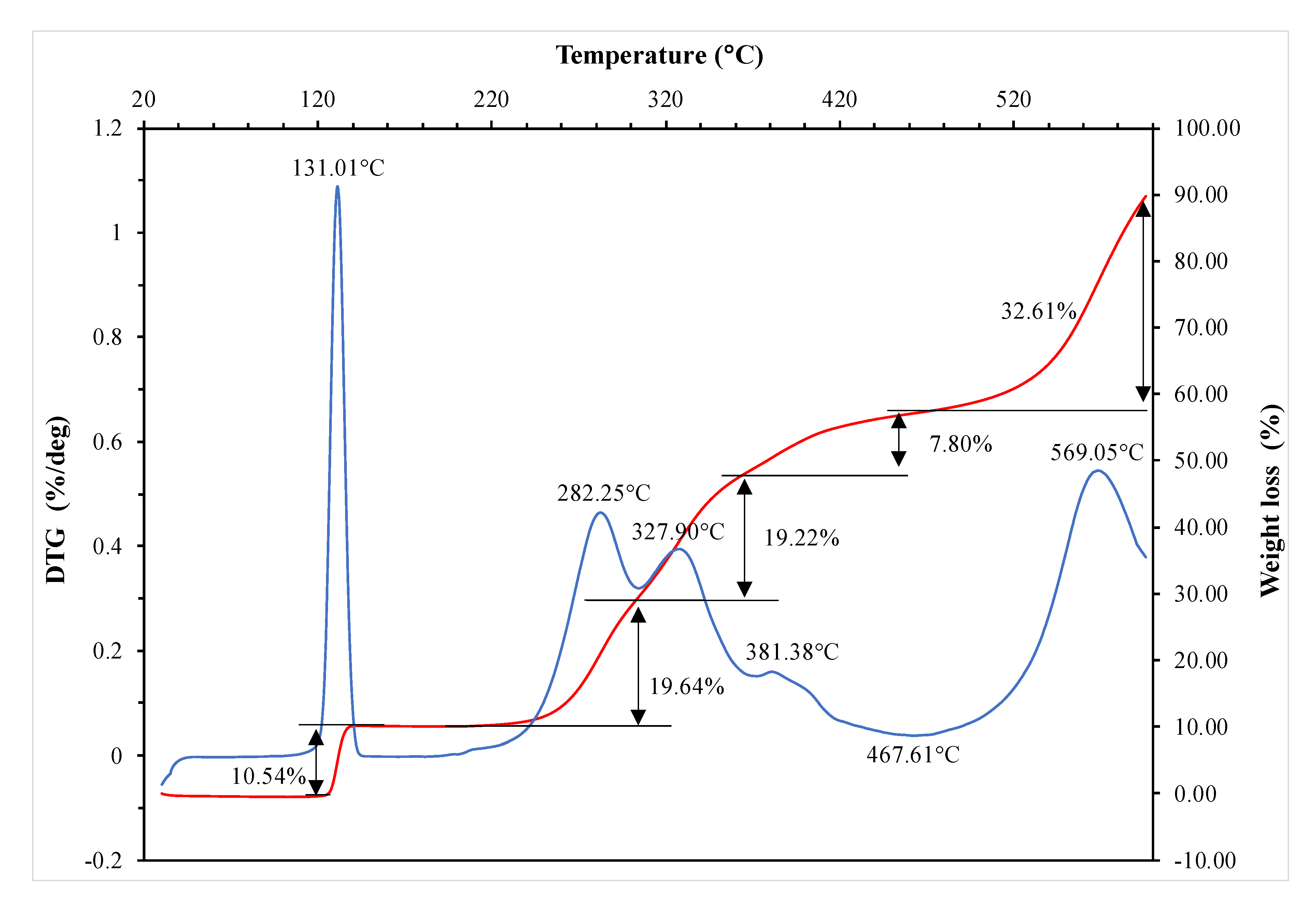
The thermogravimetric analysis of mono- and diesters was performed in the ceramic crucible using microthermogravimeter Mettler Toledo 851e Prague, Czech republic). The measurement conditions were as follows: Sample weight: 7–8 mg, temperature range: 20–600 °C, atmosphere: Argon, heating rate 10 °C/min.
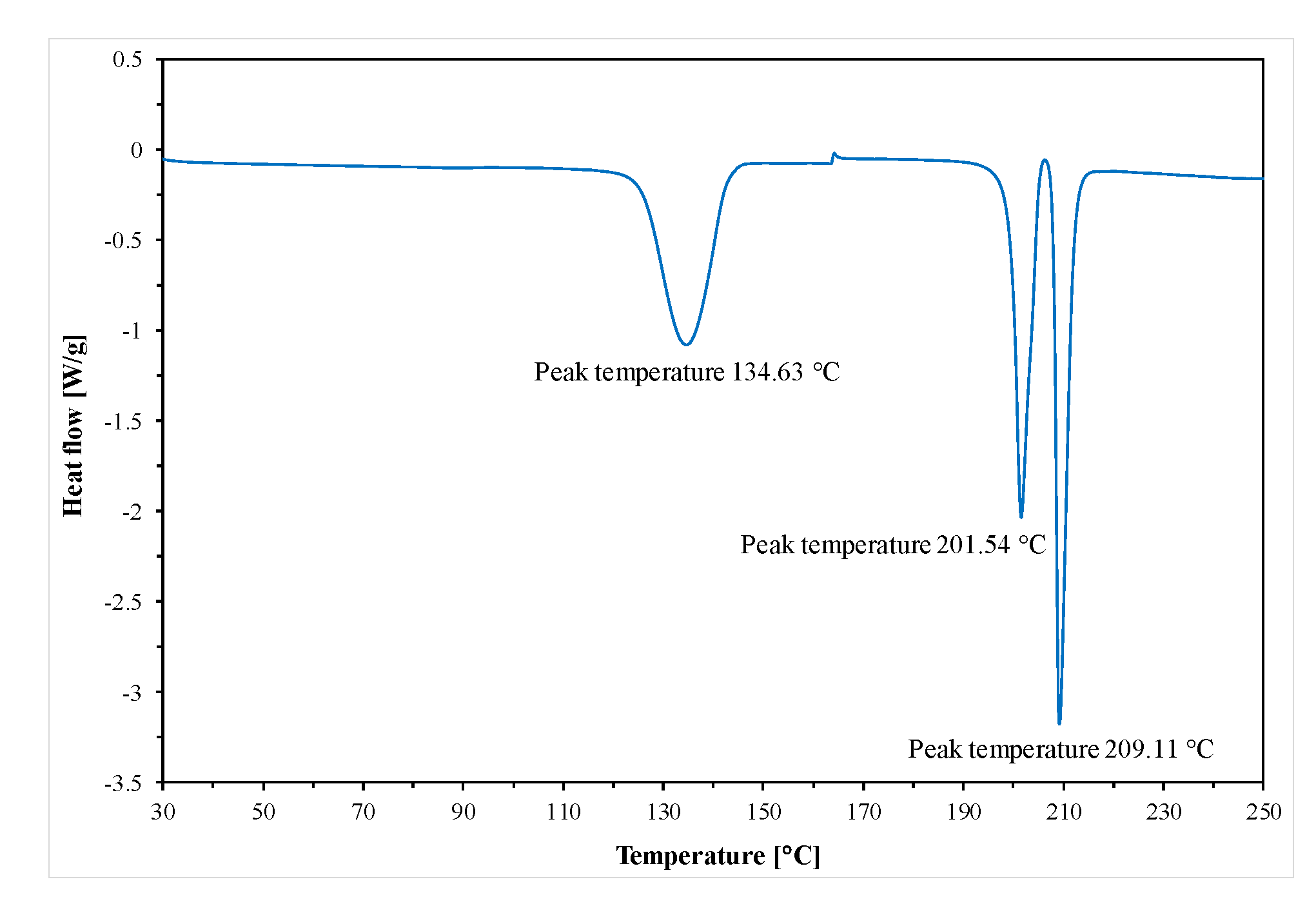
Thermal measurements were carried out using the standard differential scanning calorimetry (DSC) a TA 2920 from TA Instruments, Inc. (New Castle, DE, USA). This calorimeter is the heat-flux type and use a mechanical refrigerator to cool the sample. All experiments were performed in a nitrogen atmosphere with a constant flow rate of around 50 mL/min [
24]. The series of experimental heat flow rates were obtained by standard DSC at a heating rate of 10 deg/min after previous cooling also at 10 deg/min. The temperature and heat flow rate calibration in the DSC apparatus was performed using parameters of melting indium [T
m(onset) = 156.6 °C, ΔH
f = 28.45 J·g (3.281 kJ/mol)], [
24]. The masses of the samples used for the DSC measurements were 10–30 mg. The thermal data were collected from the first and second heating run after controlled cooling. By the standard DSC measurements, total heat flow rate was obtained for investigated samples.
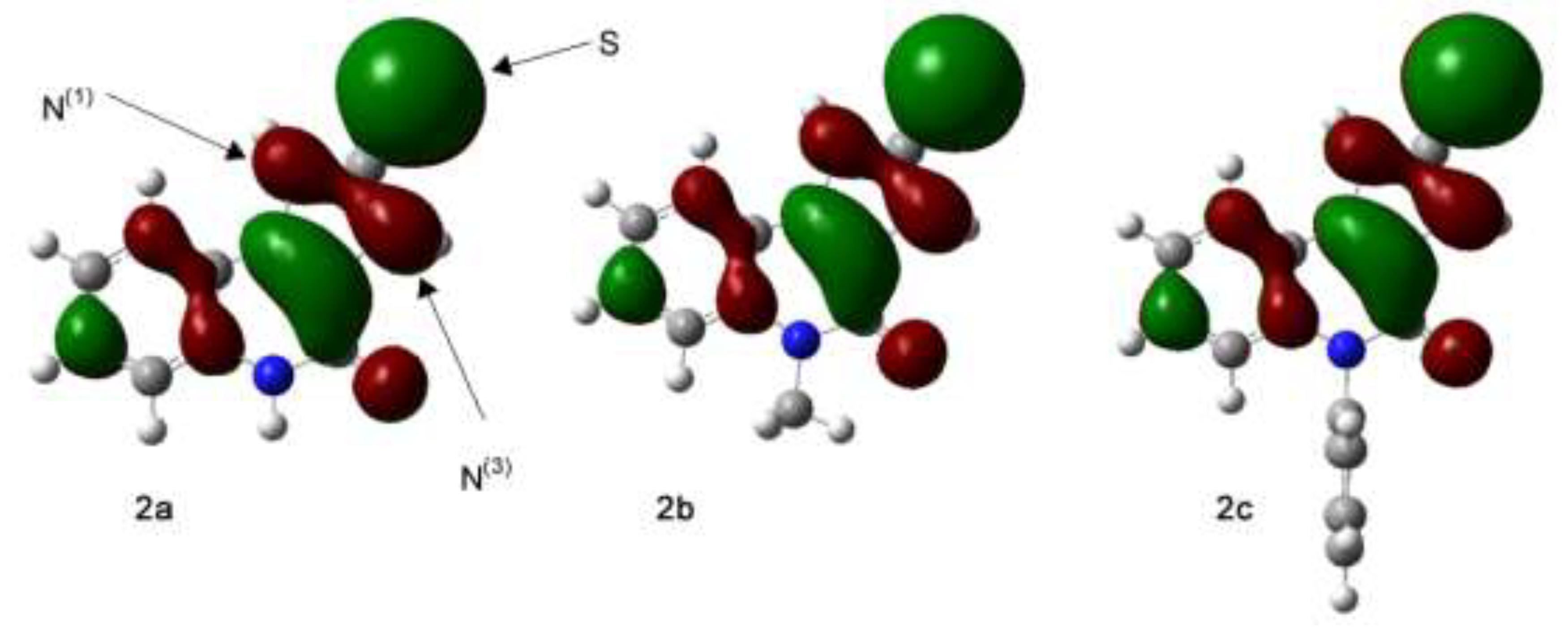
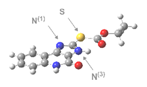
The molecular modelling was performed using a set of computational methods based on the electron density of the tested compounds in the stationary state, i.e., density functional theory DFT [
25], with use the Gaussian 09 application [
26]. The B3LYP functional was selected as the most suitable for the tasks associated with organic compounds. The quantum-mechanical calculations were performed using the 6-311++g(d,p) basis set [
27], with Grimme dispersion correction (D3 version) which considered intramolecular hydrogen bonds and electrostatic interactions [
28]. The charge value was 0 (zero) and spin state value was 1, because the calculated models of molecules were neutral, in single state. The values of the mole percentage were calculated on the basis of Gibbs free energy (enthalpy). Gibbs free energy was calculated at temperature 298.15 K. Frequency calculations were performed at the same level of theory as the geometry optimization to confirm whether the obtained structures were minima (no imaginary frequency) or transition states (only one imaginary frequency). Structure visualization was performed using GaussView [
29].