A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes
Abstract
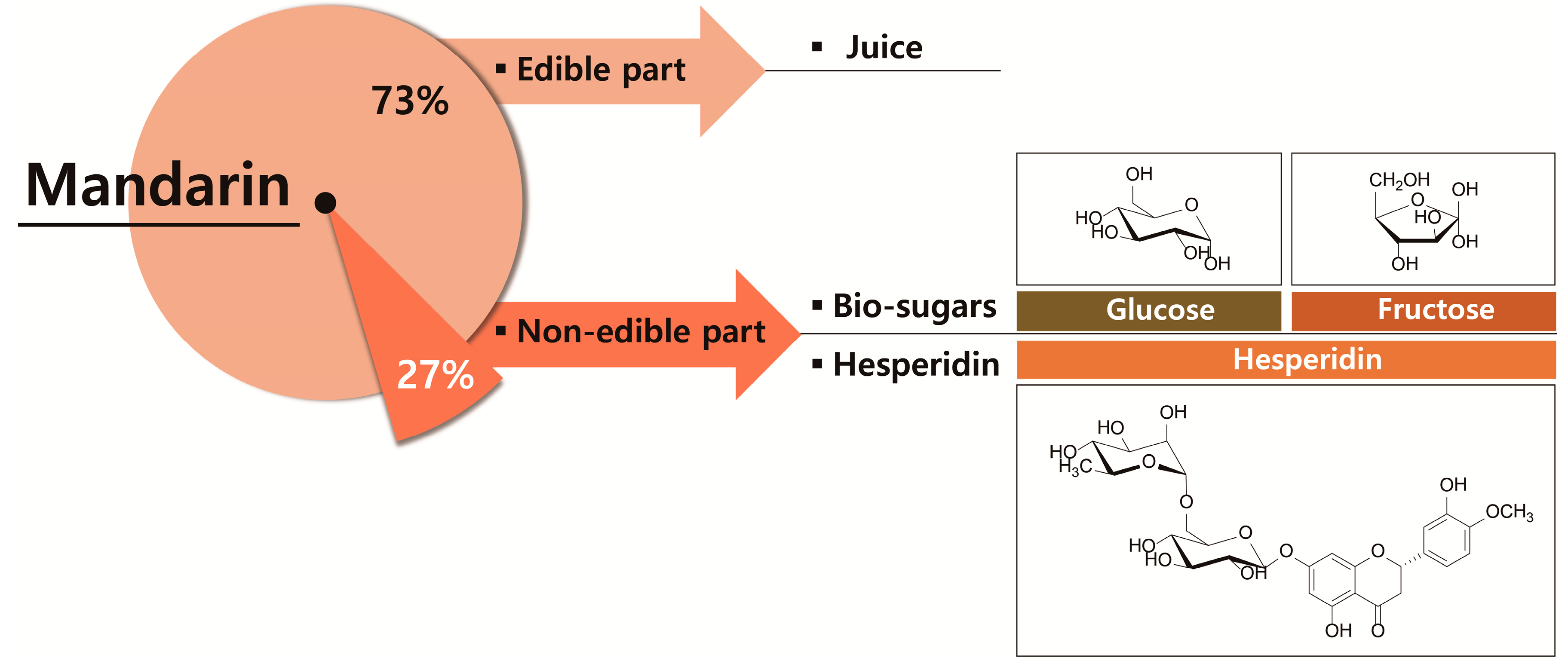
1. Introduction
2. Results
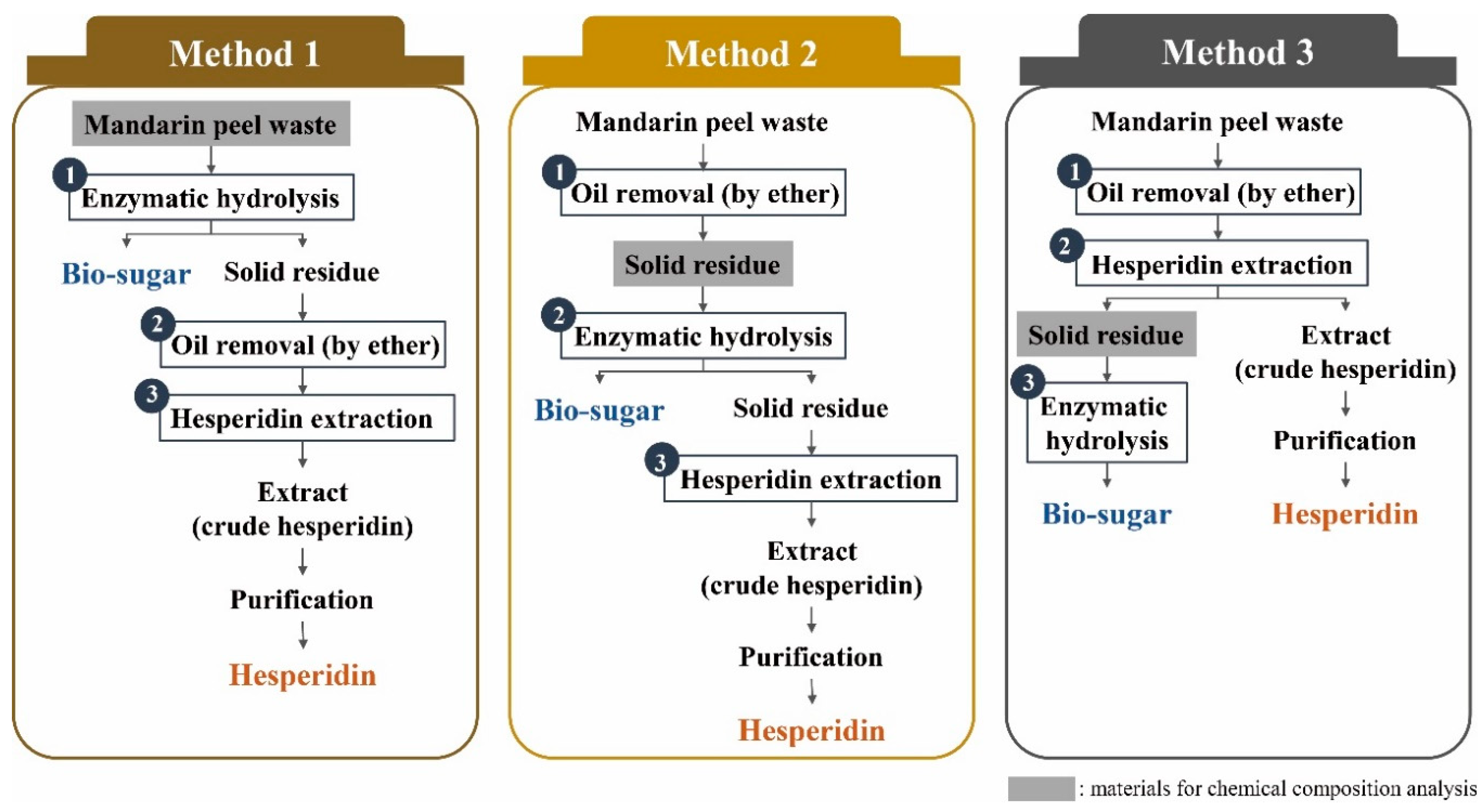
2.1. Design of Experimental Methods
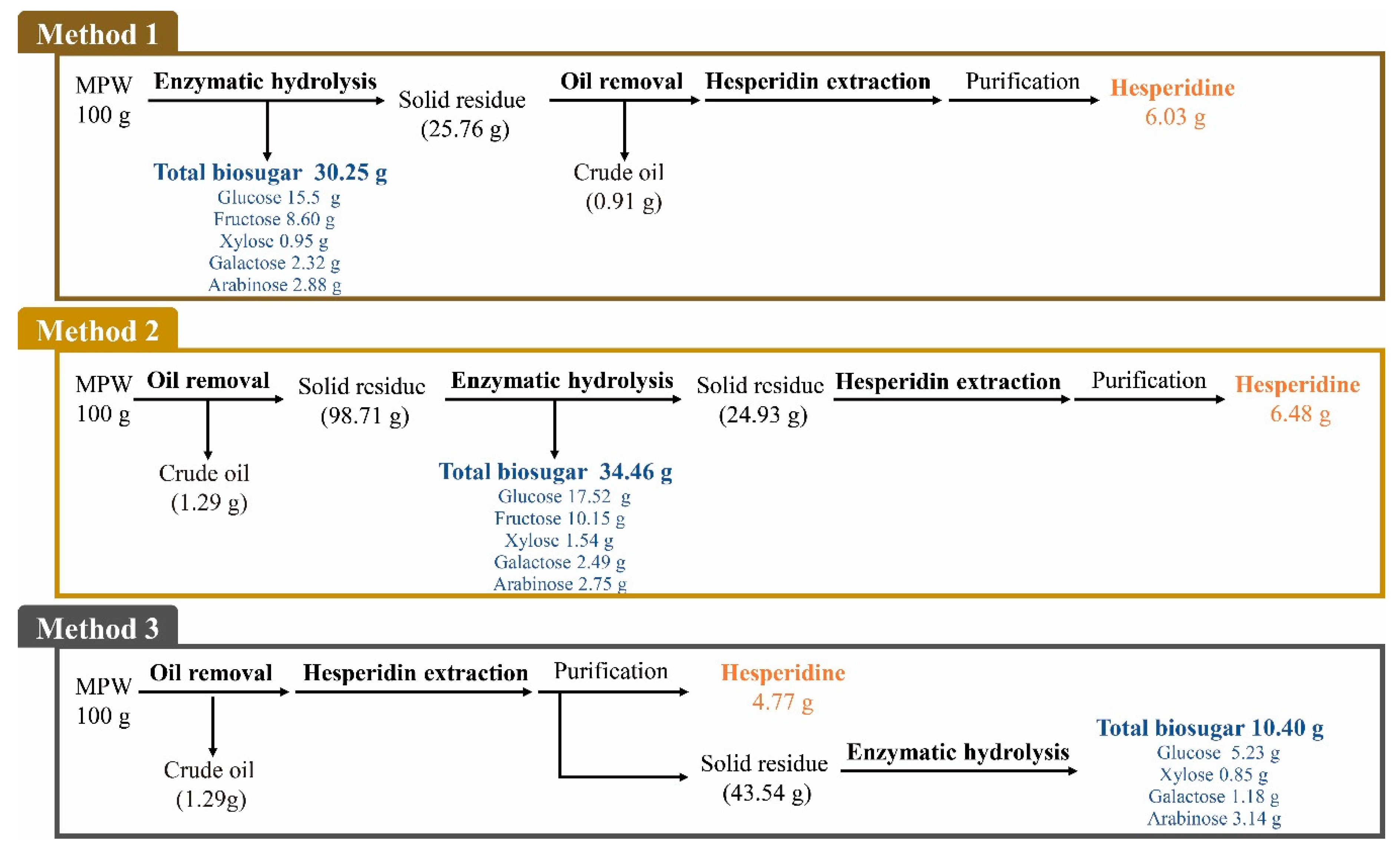
2.2. Chemical Composition of MPW after the Application of Different Treatment Methods
2.3. Optimization of Enzyme Loading
2.4. Biosugar Production via Enzymatic Hydrolysis
2.5. Isolation and Characterization of Hesperidin Obtained MPW
2.6. Overall Mass Balance
3. Materials and Methods
3.1. Materials
3.2. Carbohydrate Analysis
3.3. Optimization of Enzyme Loading
3.4. Enzymatic Hydrolysis
3.5. Removal of Oil Fraction from MPW
3.6. Methanol Extraction of Hesperidin
3.7. Purification of Hesperidin
3.8. GC/MS Analysis of Oil Fraction
3.9. Characterization of Hesperidin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murali Krishana, I.V.; Manickam, V. Environmental Management: Science and Engineering for Industry, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 1–4. [Google Scholar]
- Wunderlich, S.; Martinez, N.M. Conserving natural resources through food loss reduction: Production and consumption stages of the food supply chain. Int. Soil Water Conserv. Res. 2018, 6, 331–339. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Çalışkan Eleren, S.; Öziş Altınçekiç, Ş.; Altınçekiç, E. Biofuel Potential of Fruit Juice Industry Waste. J. Hazard. Toxic Radioact. Waste 2018, 22, 05018002. [Google Scholar] [CrossRef]
- De Feo, G.; De Gisi, S. Using MCDA and GIS for hazardous waste landfill siting considering land scarcity for waste disposal. Waste Manag. 2014, 34, 2225–2238. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, J.; Yin, C.; Li, G.; Jiang, Y.; Yang, S. Jiang Effect of Ozone Treatment on Flavonoid Accumulation of Satsuma Mandarin (Citrus unshiu Marc.) during Ambient Storage. Biomolecules 2019, 9, 821. [Google Scholar] [CrossRef]
- Anker, R.; Anker, M. Living Wages around the World: Manual for Measurement; Edward Elgar Publishing Inc.: Northampton, MA, USA, 2017. [Google Scholar]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Cao, R.; Yang, X.; Strappe, P.M.; Blanchard, C.L.; Zhou, Z. Natural products derived from tea on the solubility of hesperidin by LC-TOF/MS and NMR. Int. J. Food Prop. 2017, 20, S270–S278. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage. J. Photochem. Photobiol. B Boil. 2015, 148, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Farzaei, M.H.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Ali, M. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA. Preprints 2020, 2020030333. [Google Scholar] [CrossRef]
- Haslberger, A.; Jacob, U.; Hippe, B.; Karlic, H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: Mini review. Funct. Foods Health Dis. 2020, 10, 195. [Google Scholar] [CrossRef]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 2020030226. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Preprints 2020, 2020030214. [Google Scholar] [CrossRef]
- Mazzaferro, L.S.; Breccia, J.D. Quantification of hesperidin in citrus-based foods using a fungal diglycosidase. Food Chem. 2012, 134, 2338–2344. [Google Scholar] [CrossRef]
- Man, M.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef] [PubMed]
- Hesperidin Market 2020 Global Industry Share, Size, Revenue, Latest Trends, Business Boosting Strategies, CAGR Status, Growth Opportunities and Forecast 2026 by Market Reports World, Market Reports World. Available online: https://www.marketwatch.com (accessed on 29 July 2020).
- Manthey, J.A.; Grohmann, K. Concentrations of hesperidin and other orange peel flavonoids in citrus proceeding byproducts. J. Agric. Food Chem. 1996, 44, 811–814. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Bourne, R.A.; Smith, R.L.; Poliakoff, M. The 24 Principles of Green Engineering and Green Chemistry: “IMPROVEMENTS PRODUCTIVELY”. Green Chem. 2008, 10, 268. [Google Scholar] [CrossRef]
- Crovotto, G.; Binello, A.; Orio, L. Green extraction techniques for high-quality natural products. Agro Food Ind. Hi Tech 2011, 22, 57–59. [Google Scholar]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fallico, B.; Passerini, A.; Rapisarda, P.; Maccarone, E. Recovery of hesperidin from orange peel by concentration of extracts on styrene-divinylbenzene resin. J. Agric. Food Chem. 1999, 47, 4391–4397. [Google Scholar] [CrossRef]
- M’Hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N. Extraction Methods of Citrus Peel Phenolic Compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Cho, E.J.; Song, Y.; Oh, C.H.; Funada, R.; Bae, H.-J. Use of coffee flower as a novel resource for the production of bioactive compounds, melanoidins, and bio-sugars. Food Chem. 2019, 299, 125120. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Choi, Y.-S.; Bae, H.-J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crop. Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Hodge, D.B.; Andersson, C.; Berglund, K.A.; Rova, U. Detoxification requirements for bioconversion of softwood dilute acid hydrolyzates to succinic acid. Enzym. Microb. Technol. 2009, 44, 309–316. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kamal, S. Economical Bioconversion of Lignocellulosic Materials to Value-Added Products. J. Biotechnol. Biomater. 2012, 2, 1–2. [Google Scholar] [CrossRef]
- Ikan, R. Natural Products: A Laboratory Guide, 2nd ed.; Academic Press, Inc.: Cambridge, UK, 1991. [Google Scholar]
- Li, B.; Smith, B.; Hossain, M. Extraction of phenolics from citrus peels. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of citrus waste by switching in the production of nanocellulose. IET Nanobiotechnol. 2016, 10, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Galbe, M.; Zacchi, G. Pretreatment of Lignocellulosic Materials for Efficient Bioethanol Production. Adv. Biochem. Eng. Biotechnol. 2007, 108, 41–65. [Google Scholar] [CrossRef]
- Wingren, A.; Galbe, M.; Zacchi, G. Techno-Economic Evaluation of Producing Ethanol from Softwood: Comparison of SSF and SHF and Identification of Bottlenecks. Biotechnol. Prog. 2008, 19, 1109–1117. [Google Scholar] [CrossRef]
- Newman, R.H.; Vaidya, A.; Sohel, M.I.; Jack, M.W. Optimizing the enzyme loading and incubation time in enzymatic hydrolysis of lignocellulosic substrates. Bioresour. Technol. 2013, 129, 33–38. [Google Scholar] [CrossRef]
- Choi, I.S.; Cho, E.J.; Moon, J.-H.; Bae, H.-J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef]
- Choi, I.S.; Lee, Y.G.; Khanal, S.K.; Park, B.J.; Bae, H.-J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples. (Laboratory Analytical Procedure); National Renewable Energy Laboratory: Golden, CO, USA, 2006. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| (%) | Method I | Method II | Method III |
|---|---|---|---|
| Rhamnose | 2.7 ± 0.1 | 2.9 ± 0.5 | 2.0 ± 0.1 |
| Arabinose | 3.6 ± 0.0 | 3.6 ± 0.1 | 9.5 ± 1.2 |
| Xylose | 1.4 ± 0.1 | 1.4 ± 0.0 | 2.8 ± 0.3 |
| Mannose | 1.2 ± 0.1 | 1.2 ± 0.0 | 1.7 ± 0.1 |
| Galactose | 1.8 ± 0.0 | 1.9 ± 0.1 | 4.1 ± 0.4 |
| Glucose | 24.8 ± 1.0 | 25.8 ± 0.7 | 16.1 ± 1.0 |
| Total | 35.5 ± 1.3 | 36.8 ± 1.4 | 36.1 ± 2.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.J.; Lee, Y.G.; Chang, J.; Bae, H.-J. A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes. Molecules 2020, 25, 4286. https://doi.org/10.3390/molecules25184286
Cho EJ, Lee YG, Chang J, Bae H-J. A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes. Molecules. 2020; 25(18):4286. https://doi.org/10.3390/molecules25184286
Chicago/Turabian StyleCho, Eun Jin, Yoon Gyo Lee, Jihye Chang, and Hyeun-Jong Bae. 2020. "A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes" Molecules 25, no. 18: 4286. https://doi.org/10.3390/molecules25184286
APA StyleCho, E. J., Lee, Y. G., Chang, J., & Bae, H.-J. (2020). A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes. Molecules, 25(18), 4286. https://doi.org/10.3390/molecules25184286




