Optimization of High-Pressure Extraction Process of Antioxidant Compounds from Feteasca regala Leaves Using Response Surface Methodology
Abstract
1. Introduction
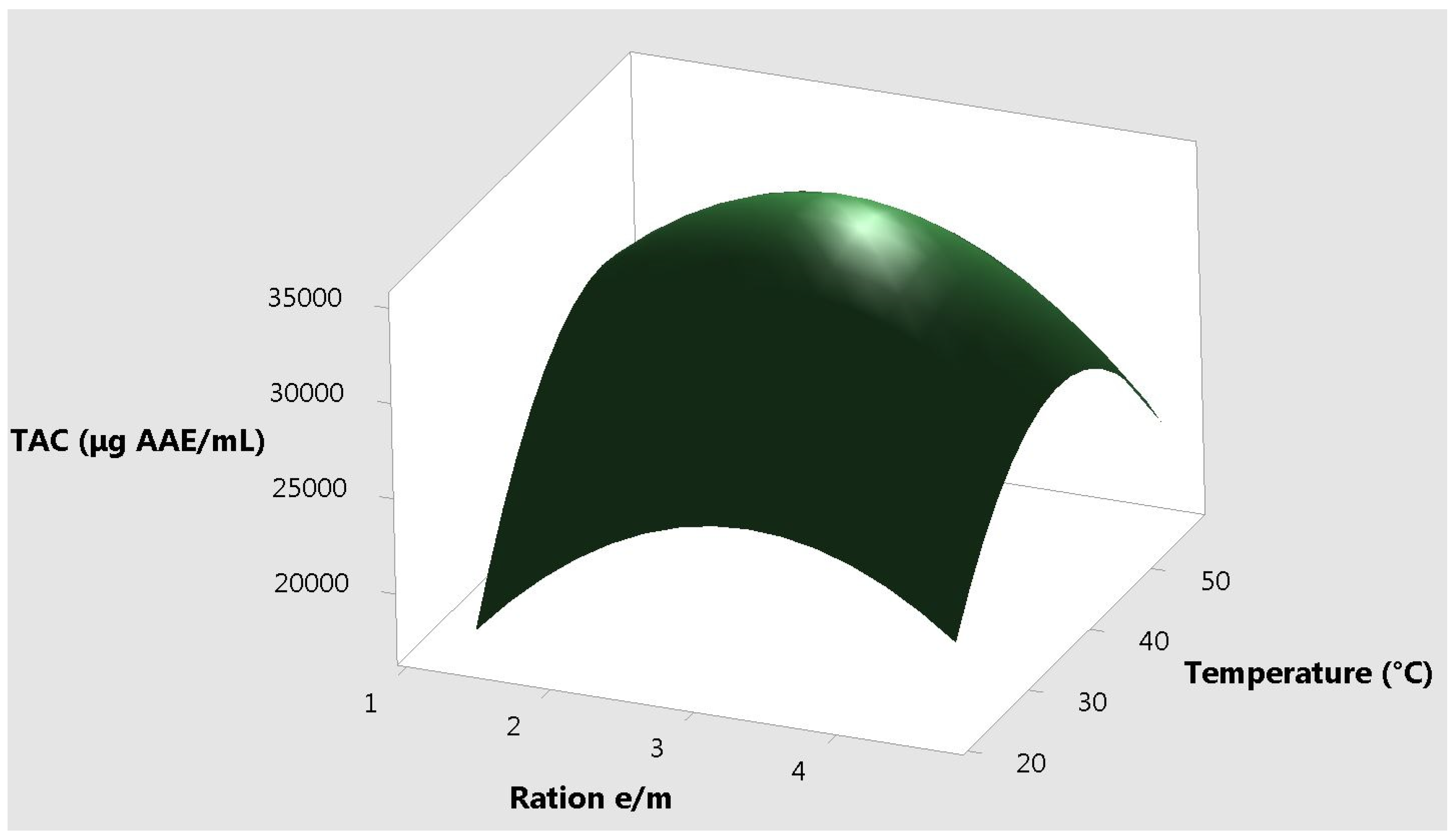
2. Results and Discussion
Verification of Experiments
3. Materials and Methods
3.1. Chemicals
3.2. Extraction Process
3.3. Determination of Total Antioxidant Capacity
3.4. Determination of Resveratrol Content
3.5. Experimental Design and Statistical Analysis
3.6. Model Verification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aradhya, M.; Dangl, G.; Prins, B.; Boursiquot, J.; Walker, M.; Meredith, C.; Simon, C. Genetic structure and differentiation in cultivated grape, Vitis vinifera L. Genet. Res. 2003, 81, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, L.; Gambuti, A.; De Cicco, P.; Ercolano, G.; Ianaro, A.; Tagliatela-Scafati, O.; Moio, L.; Forino, M. NMR-based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia 2018, 125, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Ricci, D.; Giacomini, E.; Carloni, S.; Azzolini, C.; Gordillo, B.; Jara-Palacios, M.J.; et al. Chemical composition and “in vitro” anti-inflammatory activity of Vitis vinifera L. (var. Sangiovese) tendrils extract. J. Funct. Foods 2016, 20, 291–302. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; SantAna, A.S.; Orlien, O. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Fiume, M.M. Safety Assessment of Vitis vinifera (Grape) Ingredients as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2012; pp. 1–29. [Google Scholar]
- Katalinic, V.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15. [Google Scholar]
- Dresch, R.R.; Dresch, M.K.; Guerreiro, A.F.; Biegelmeyer, R.; Holzschuh, M.H.; Rambo, D.F.; Henriques, A.T. Phenolic Compounds from the Leaves of Vitis labrusca and Vitis vinifera L. as a Source of Waste Byproducts: Development and Validation of LC Method and Antichemotactic Activity. Food Anal. Methods 2014, 7, 527–539. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodriguez, F.; Oliveira, M.B. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–291. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Crişan, D.; Crişan, M.; Moldovan, M.; Lupşor, M.; Badea, R. Ultrasonografic assessement of the cutaneous changes induced by topical flavonoid therapy. Clin. Cosmet. Investig. Dermatol. 2012, 5, 7–13. [Google Scholar]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Cornacchione, S.; Sadick, N.S.; Neveu, M.; Talbourdet, S.; Lazou, K.; Viron, C.; Renimel, I.; de Quéral, D.; Kurfurst, R.; Schnebert, S.; et al. In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract. J. Drugs Dermatol. 2007, 6, 8–13. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15677. [Google Scholar] [CrossRef]
- Yahya, N.A.; Attan, N.; Wahab, R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Bimakr, M.; Russly, A.R.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Park, N.Y.; Lee, G.D.; Jeong, Y.J.; Kwon, J.H. Optimization of extraction conditions for physicochemical properties of ethanol extracts from Chrysanthemum boreale. Korean J. Food Nutr. 1998, 27, 585–590. [Google Scholar]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Gan, C.Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Kwak, H.J.; Park, S.J.; Kim, Y.N.; Yoo, G.; Jeong, E.J.; Kim, S. Optimization of extraction conditions for enhancing estrogenic activity of Rheum undulatum Linne’ using response surface methodology. Sep. Sci. Technol. 2019, 55, 2080–2089. [Google Scholar] [CrossRef]
- Kwon, J.H.; Belanger, J.M.R.; Pare, J.R.J. Optimization of microwave-assisted extraction (MAP) for ginseng components by response surface methodology. J. Agric. Food Chem. 2003, 51, 1807–1810. [Google Scholar] [CrossRef]
- Minitab. Available online: https://support.minitab.com/en-us/minitab-express/1/help-and-how-to/modeling-statistics/anova/how-to/two-way-anova/interpret-the-results/all-statistics-and-graphs/ (accessed on 17 July 2020).
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Majeed, M.; Hussain, A.I.; Chatha, S.A.S.; Khosa, M.K.K.; Kamal, G.M.; Kamal, M.A.; Zhang, X.; Liu, M. Optimization protocol for the extraction of antioxidant components from Origanum vulgare leaves using response surface methodology. Saudi J. Biol. Sci. 2016, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.G.; Santosh, K.V.; Sharma, A.K. Optimization of extraction protocol of Parmelia perlata and its validation for protective effects against oxalate-induced renal injury in NRK-52E cells. J. Herb. Med. 2018, 12, 79–87. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Lee, W.C.; Yusof, S.; Hamid, N.S.A.; Baharin, B.S. Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM). J. Food Eng. 2006, 73, 55–63. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhiev, L.; Georgieva, S. Useful Bioactive Substances from Wastes: Recovery of Trans-Resveratrol from Grapevine Stems. Open Chem. Eng. J. 2016, 10, 4–9. [Google Scholar] [CrossRef][Green Version]
- Matloub, A. Optimization of polyphenol extraction from Vitis vinifera L. leaves, antioxidant activity and its correlation with amelioration effect on AlCl3-induced Alzheimer’s disease. Arch. Pharm. Sci. Ain Shams Univ. 2018, 2, 97–110. [Google Scholar] [CrossRef]
- Nabli, R.; Achour, S.; Jourdes, M.; Teissedre, P.-L.; Helal, A.N.; Ezzili, B. Anthocyanin composition and extraction from Grenache noir (Vitis vinifera L.) vine leaf using an experimental design. I-By ethanol or sulfur dioxide. J. Int. Sci. Vigne Vin 2012, 46, 4. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |
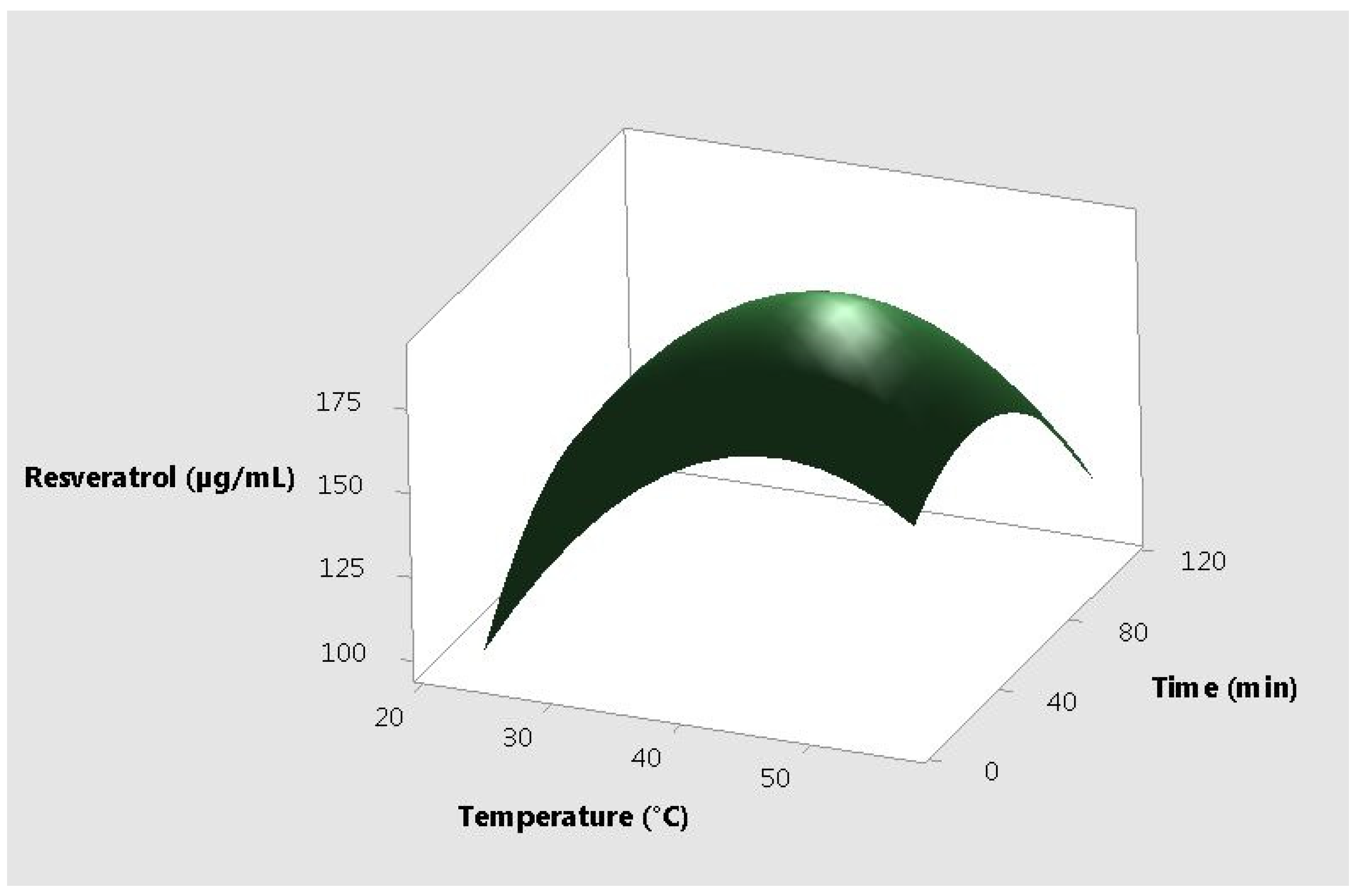
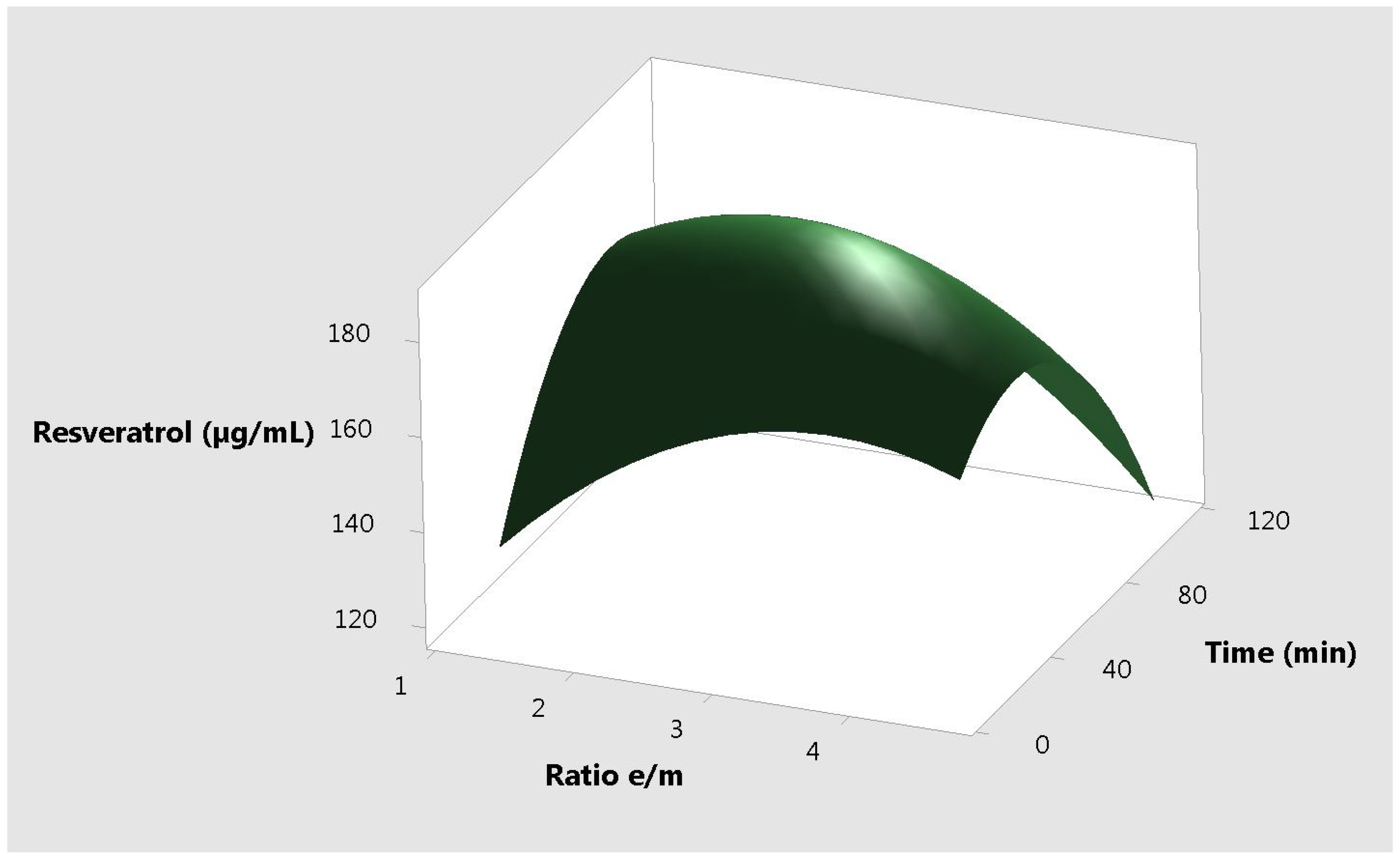
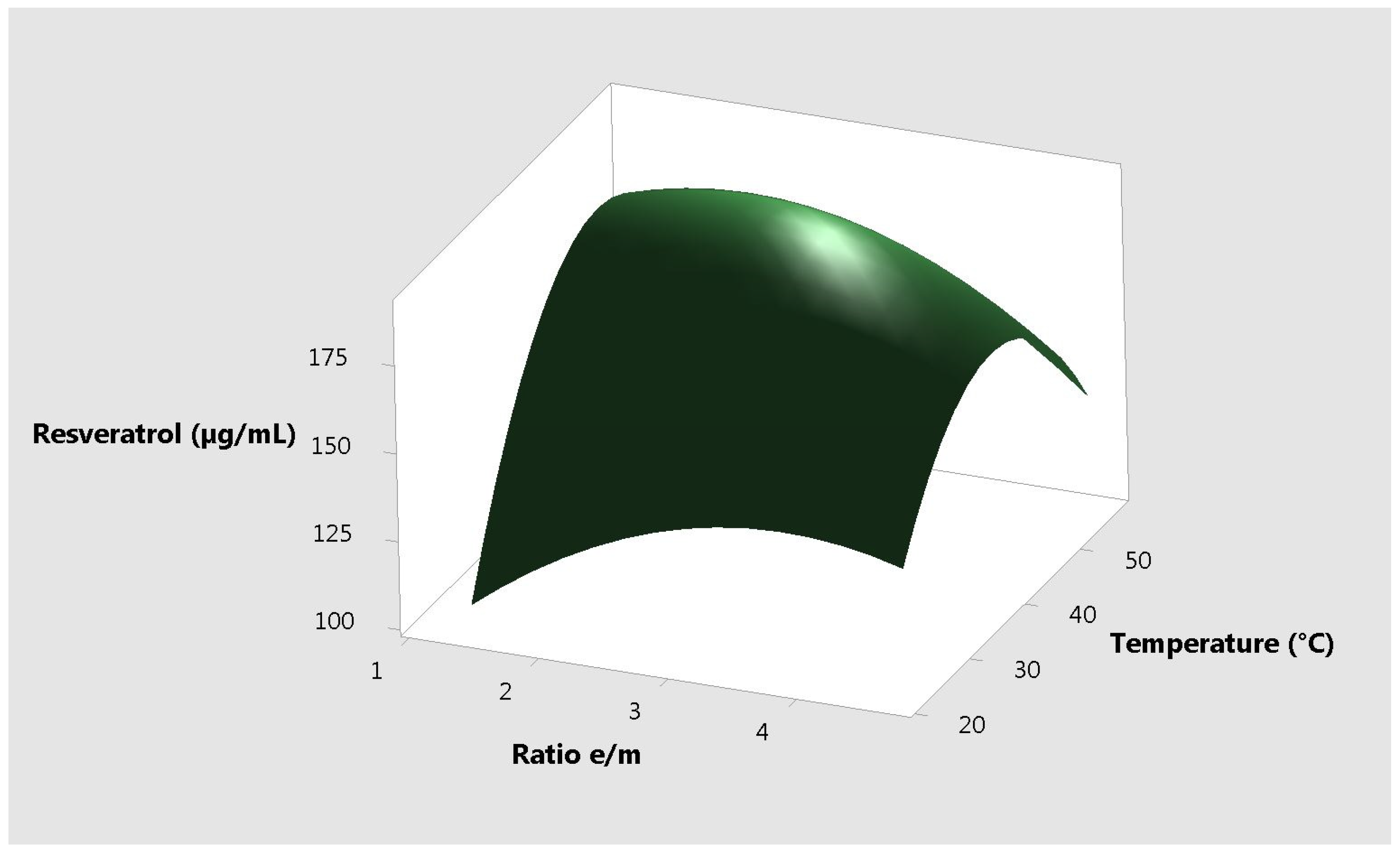
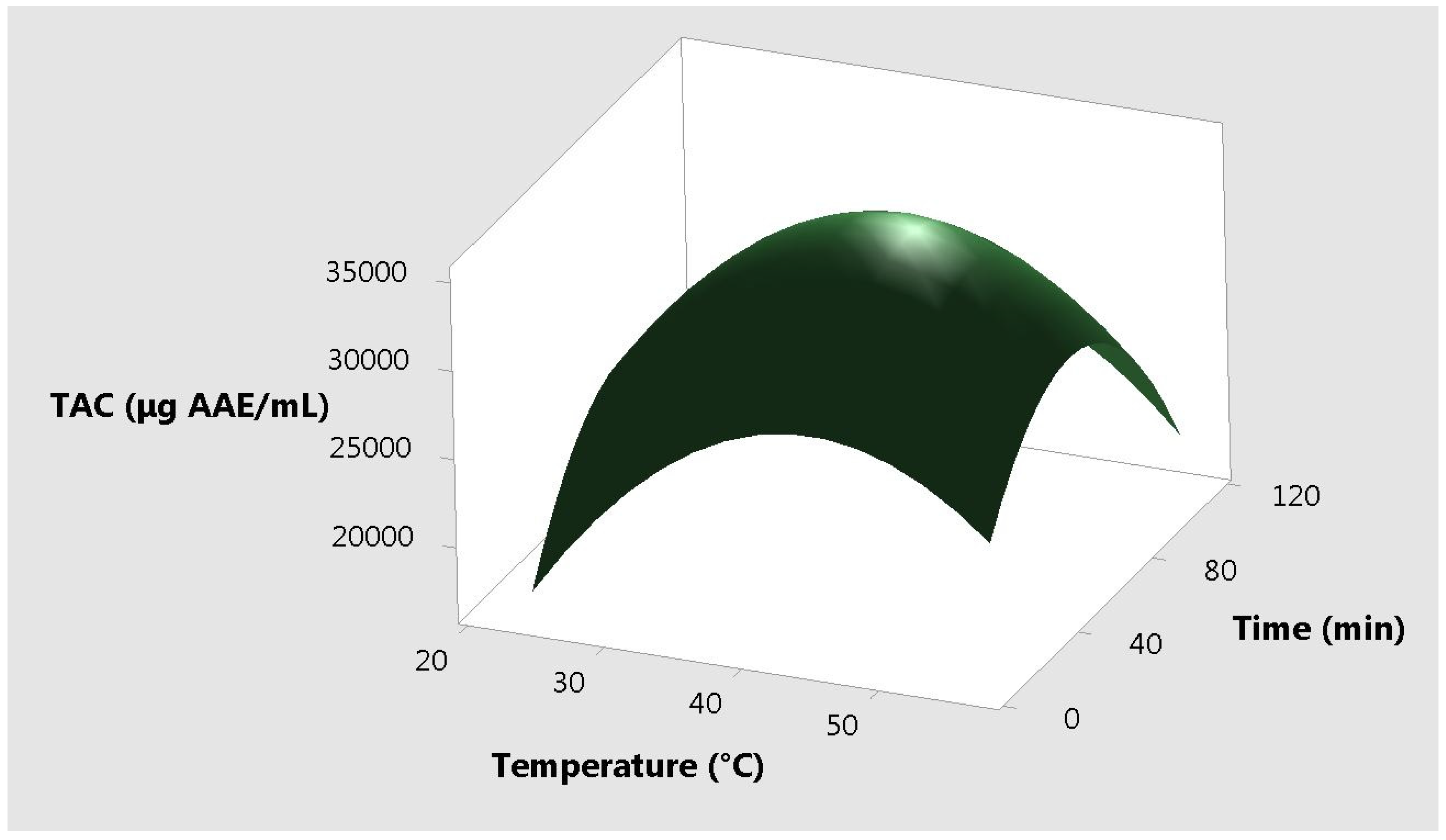

| Ratio of Ethanol to Plant Material | Temperature (°C) | Time (min) | Total Antioxidant Capacity (µg AAE /mL) | Resveratrol Content (µg/mL) |
|---|---|---|---|---|
| 4 | 30.00 | 90.00 | 25,331 | 152.1 |
| 3 | 40.00 | 60.00 | 34,793 | 190.1 |
| 2 | 50.00 | 30.00 | 28,719 | 172.4 |
| 3 | 40.00 | 60.00 | 34,726 | 188.5 |
| 2 | 30.00 | 90.00 | 26,397 | 158.7 |
| 3 | 40.00 | 60.00 | 33,978 | 175.3 |
| 3 | 40.00 | 110.45 | 25,408 | 155.2 |
| 2 | 30.00 | 30.00 | 24,947 | 141.8 |
| 4 | 50.00 | 90.00 | 24,825 | 139.1 |
| 3 | 40.00 | 19.55 | 28,819 | 167.8 |
| 3 | 40.00 | 60.00 | 34,896 | 181.5 |
| 3 | 23.18 | 60.00 | 24,097 | 124.6 |
| 4 | 30.00 | 30.00 | 26,781 | 160.8 |
| 4.5 | 40.00 | 60.00 | 29,937 | 174.4 |
| 3 | 56.82 | 60.00 | 31,093 | 177.6 |
| 1.2 | 40.00 | 60.00 | 28,674 | 169.4 |
| 4 | 50.00 | 30.00 | 28,103 | 165.7 |
| 3 | 40.00 | 60.00 | 34,394 | 194.9 |
| 2 | 50.00 | 90.00 | 28,805 | 167.1 |
| 3 | 40.00 | 60.00 | 34,735 | 190.5 |
| Source | Degrees of Freedom (DF) | Adjusted Sums of Squares (Adj SS) | Adjusted Mean Squares (Adj MS) | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 5675.70 | 630.63 | 7.77 | 0.002 |
| Linear | 3 | 1216.72 | 405.57 | 5.00 | 0.023 |
| Ratio e/m | 1 | 14.13 | 14.13 | 0.17 | 0.685 |
| Temperature | 1 | 1055.03 | 1055.03 | 13.00 | 0.005 |
| Time | 1 | 147.56 | 147.56 | 1.82 | 0.207 |
| Square | 3 | 3705.73 | 1235.24 | 15.23 | 0.000 |
| Ratio e/m ∗ Ratio e/m | 1 | 493.00 | 493.00 | 6.08 | 0.033 |
| Temperature ∗ Temperature | 1 | 2512.07 | 2512.07 | 30.96 | 0.000 |
| Time ∗ Time | 1 | 1307.69 | 1307.69 | 16.12 | 0.002 |
| 2-Way Interaction | 3 | 753.25 | 251.08 | 3.09 | 0.076 |
| Ratio e/m ∗ Temperature | 1 | 277.30 | 277.30 | 3.42 | 0.094 |
| Ratio e/m ∗ Time | 1 | 274.95 | 274.95 | 3.39 | 0.095 |
| Temperature ∗ Time | 1 | 201.00 | 201.00 | 2.48 | 0.147 |
| Error | 10 | 811.32 | 81.13 | ||
| Lack-of-Fit | 5 | 557.90 | 111.58 | 2.20 | 0.203 |
| Pure Error | 5 | 253.42 | 50.68 | ||
| Total | 19 | 6487.02 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 274,182,082 | 30,464,676 | 26.49 | 0.000 |
| Linear | 3 | 31,824,976 | 10,608,325 | 9.22 | 0.003 |
| Ratio e/m | 1 | 212,586 | 212,586 | 0.18 | 0.676 |
| Temperature | 1 | 25,775,042 | 25,775,042 | 22.41 | 0.001 |
| Time | 1 | 5,837,348 | 5,837,348 | 5.08 | 0.048 |
| Square | 3 | 232,582,223 | 77,527,408 | 67.42 | 0.000 |
| Ratio e/m ∗ Ratio e/m | 1 | 60,576,745 | 60,576,745 | 52.68 | 0.000 |
| Temperature ∗ Temperature | 1 | 101,583,872 | 101,583,872 | 88.34 | 0.000 |
| Time ∗ Time | 1 | 115,028,536 | 115,028,536 | 100.03 | 0.000 |
| 2-Way Interaction | 3 | 9,774,882 | 3,258,294 | 2.83 | 0.092 |
| Ratio e/m ∗ Temperature | 1 | 3,596,562 | 3,596,562 | 3.13 | 0.107 |
| Ratio e/m ∗ Time | 1 | 4,904,712 | 4,904,712 | 4.27 | 0.066 |
| Temperature ∗ Time | 1 | 1,273,608 | 1,273,608 | 1.11 | 0.317 |
| Error | 10 | 11,499,604 | 1,149,960 | ||
| Lack-of-Fit | 5 | 10,912,332 | 2,182,466 | 18.58 | 0.003 |
| Pure Error | 5 | 587,272 | 117,454 | ||
| Total | 19 | 285,681,686 |
| S | R-sq | R-sq (ad) | R-sq (pred) | |
|---|---|---|---|---|
| Resveratrol content | 9.00733 | 87.49% | 76.24% | 29.01% |
| Total antioxidant capacity | 1072.36 | 95.97% | 92.35% | 70.71% |
| Ratio e/m | Temperature (°C) | Time (min) | Expected Value for Total Antioxidant Capacity (µg AAE /mL) | Expected Value for Resveratrol Content (µg/mL) | Composite Desirability |
|---|---|---|---|---|---|
| 2.92 | 43.2 | 55.4 | 34,840 | 189 | 0.955 |
| Parameter | Predicted Value | Observed Value |
|---|---|---|
| Total antioxidant capacity (µg AAE /mL) | 34,839 | 34,623 ± 29.7 |
| Resveratrol content (µg/mL) | 189.0 | 182.4 ± 15.3 |
| Independent Variables | Factor Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ratio (e/m) | 2 | 3 | 4 |
| Temperature (°C) | 30 | 40 | 50 |
| Time (min) | 30 | 60 | 90 |
| Standard Order | Run Order | Ratio e/m | Temperature (°C) | Time (min) |
|---|---|---|---|---|
| 6 | 1 | 4 | 30.00 | 90.00 |
| 19 | 2 | 3 | 40.00 | 60.00 |
| 3 | 3 | 2 | 50.00 | 30.00 |
| 15 | 4 | 3 | 40.00 | 60.00 |
| 5 | 5 | 2 | 30.00 | 90.00 |
| 18 | 6 | 3 | 40.00 | 60.00 |
| 14 | 7 | 3 | 40.00 | 110.45 |
| 1 | 8 | 2 | 30.00 | 30.00 |
| 8 | 9 | 4 | 50.00 | 90.00 |
| 13 | 10 | 3 | 40.00 | 30.00 |
| 16 | 11 | 3 | 40.00 | 60.00 |
| 11 | 12 | 3 | 23.18 | 60.00 |
| 2 | 13 | 4 | 30.00 | 30.00 |
| 10 | 14 | 4.5 | 40.00 | 60.00 |
| 12 | 15 | 3 | 56.82 | 60.00 |
| 9 | 16 | 1.2 | 40.00 | 60.00 |
| 4 | 17 | 4 | 50.00 | 30.00 |
| 20 | 18 | 3 | 40.00 | 60.00 |
| 7 | 19 | 2 | 50.00 | 90.00 |
| 17 | 20 | 3 | 40.00 | 60.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becze, A.; Babalau-Fuss, V.L.; Varaticeanu, C.; Roman, C. Optimization of High-Pressure Extraction Process of Antioxidant Compounds from Feteasca regala Leaves Using Response Surface Methodology. Molecules 2020, 25, 4209. https://doi.org/10.3390/molecules25184209
Becze A, Babalau-Fuss VL, Varaticeanu C, Roman C. Optimization of High-Pressure Extraction Process of Antioxidant Compounds from Feteasca regala Leaves Using Response Surface Methodology. Molecules. 2020; 25(18):4209. https://doi.org/10.3390/molecules25184209
Chicago/Turabian StyleBecze, Anca, Vanda Liliana Babalau-Fuss, Cerasel Varaticeanu, and Cecilia Roman. 2020. "Optimization of High-Pressure Extraction Process of Antioxidant Compounds from Feteasca regala Leaves Using Response Surface Methodology" Molecules 25, no. 18: 4209. https://doi.org/10.3390/molecules25184209
APA StyleBecze, A., Babalau-Fuss, V. L., Varaticeanu, C., & Roman, C. (2020). Optimization of High-Pressure Extraction Process of Antioxidant Compounds from Feteasca regala Leaves Using Response Surface Methodology. Molecules, 25(18), 4209. https://doi.org/10.3390/molecules25184209






