β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity
Abstract
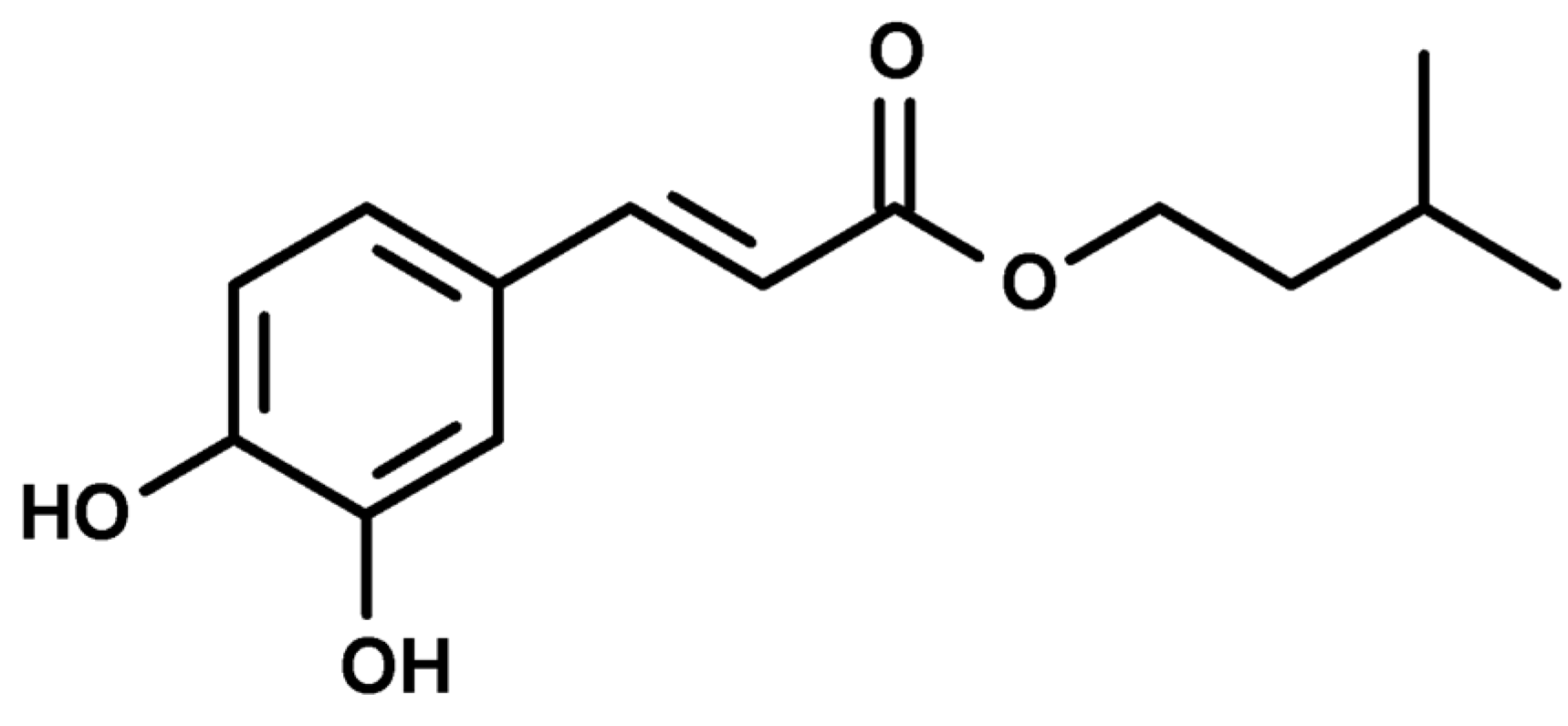
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. ICaf:β-CD Inclusion Complexes
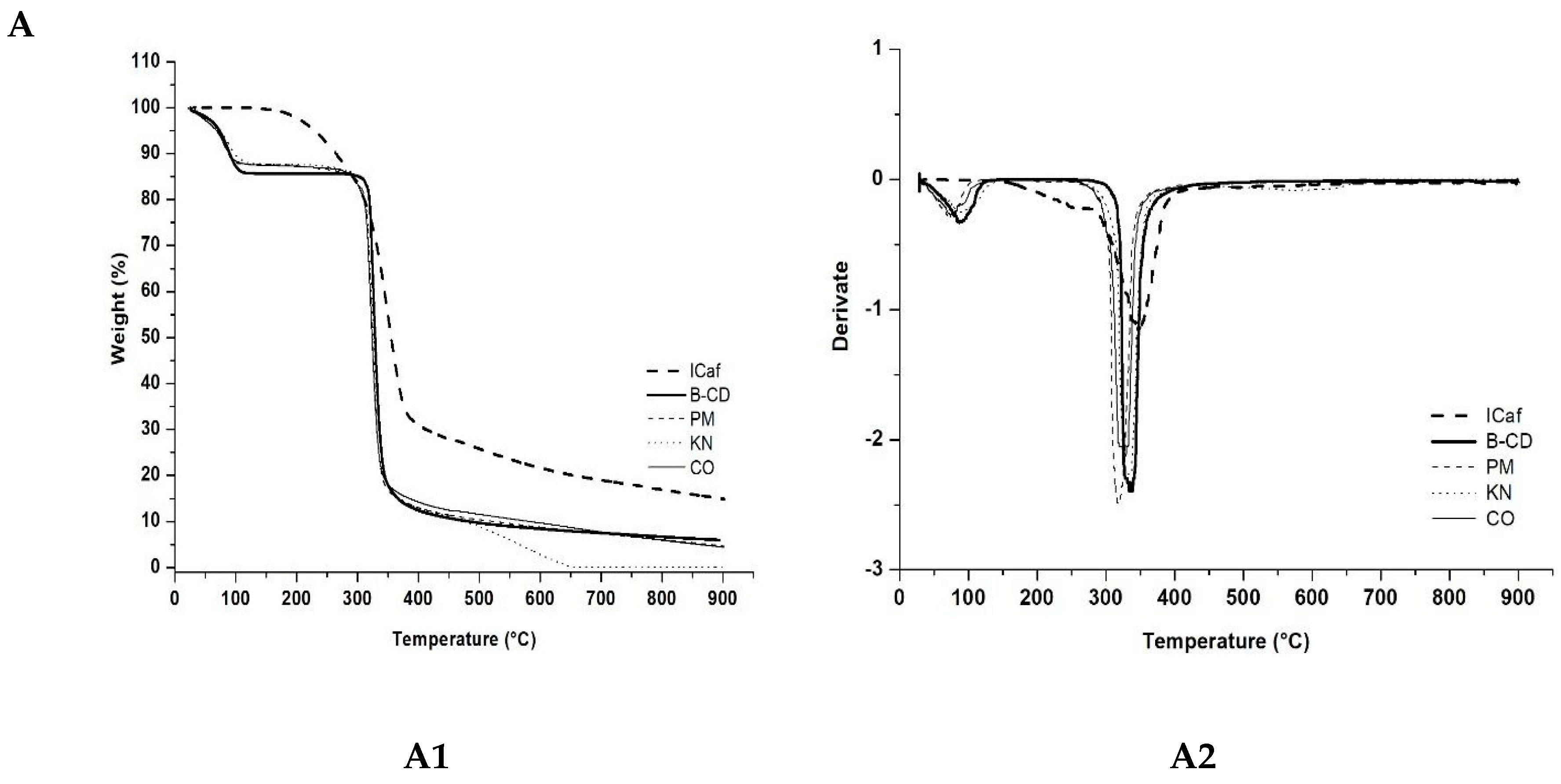
3.3. Thermal Analysis
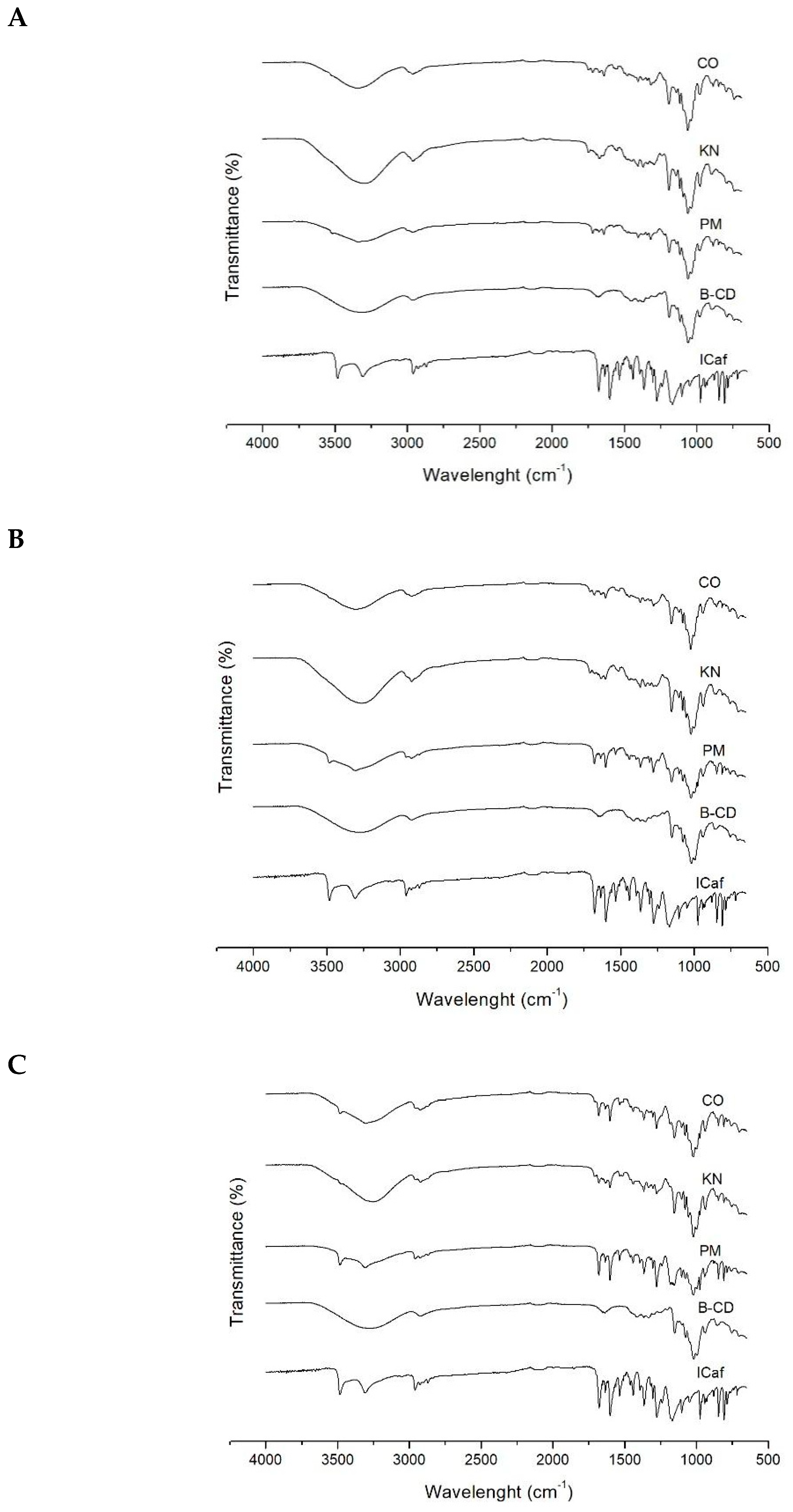
3.4. Fourier-Transform Infra-Red Analysis (FTIR)
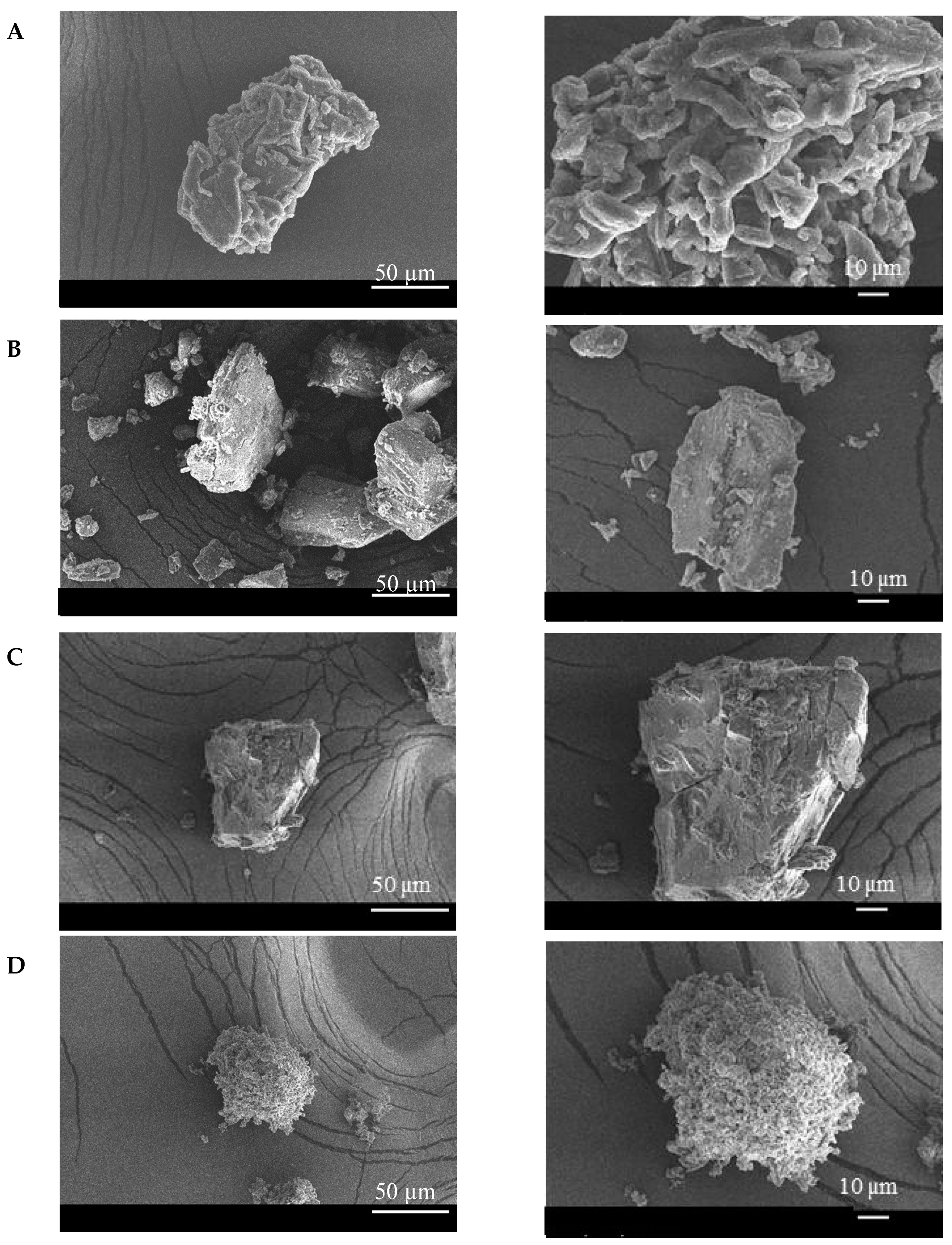
3.5. Scanning Electron Microscopy (SEM)
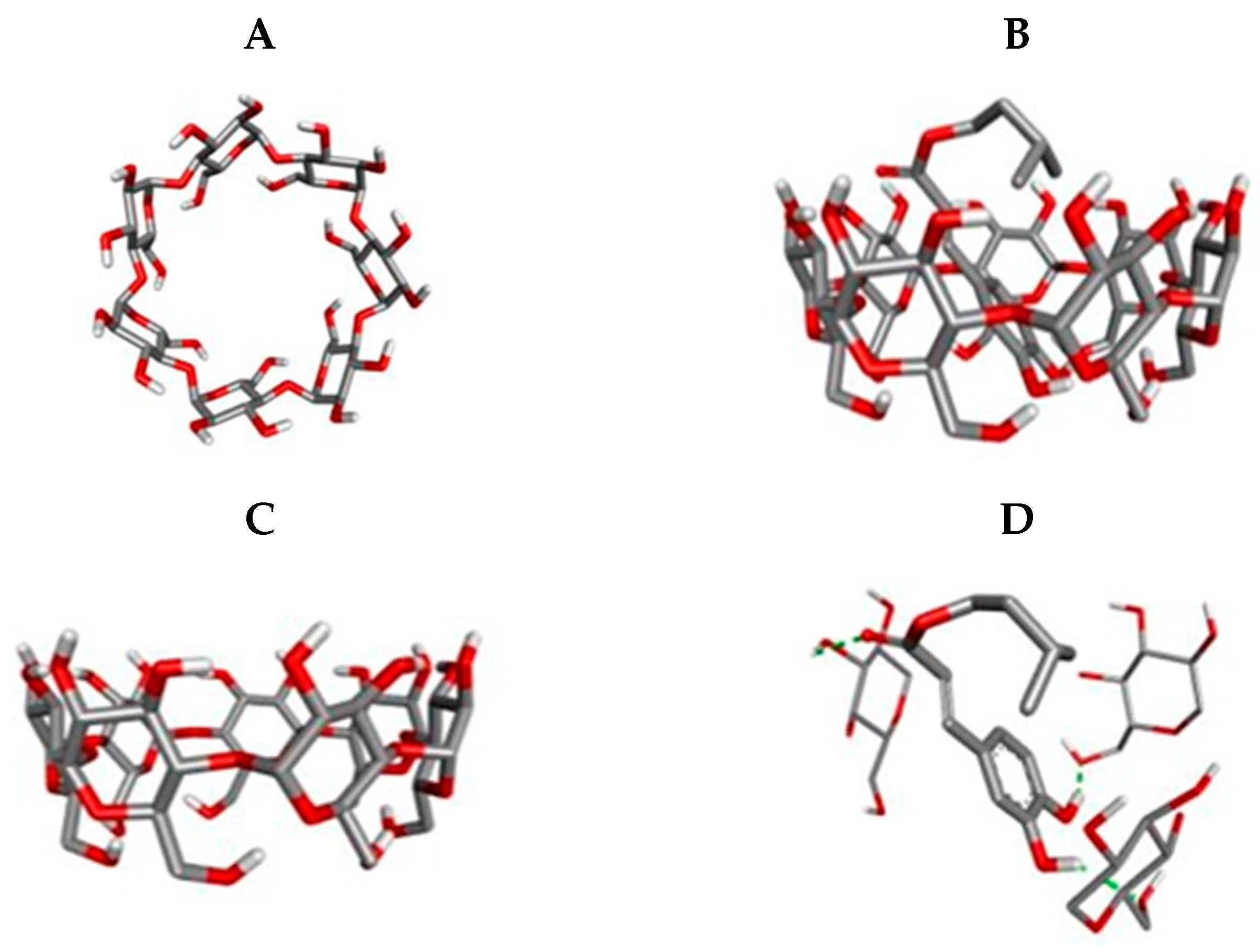
3.6. Molecular Docking
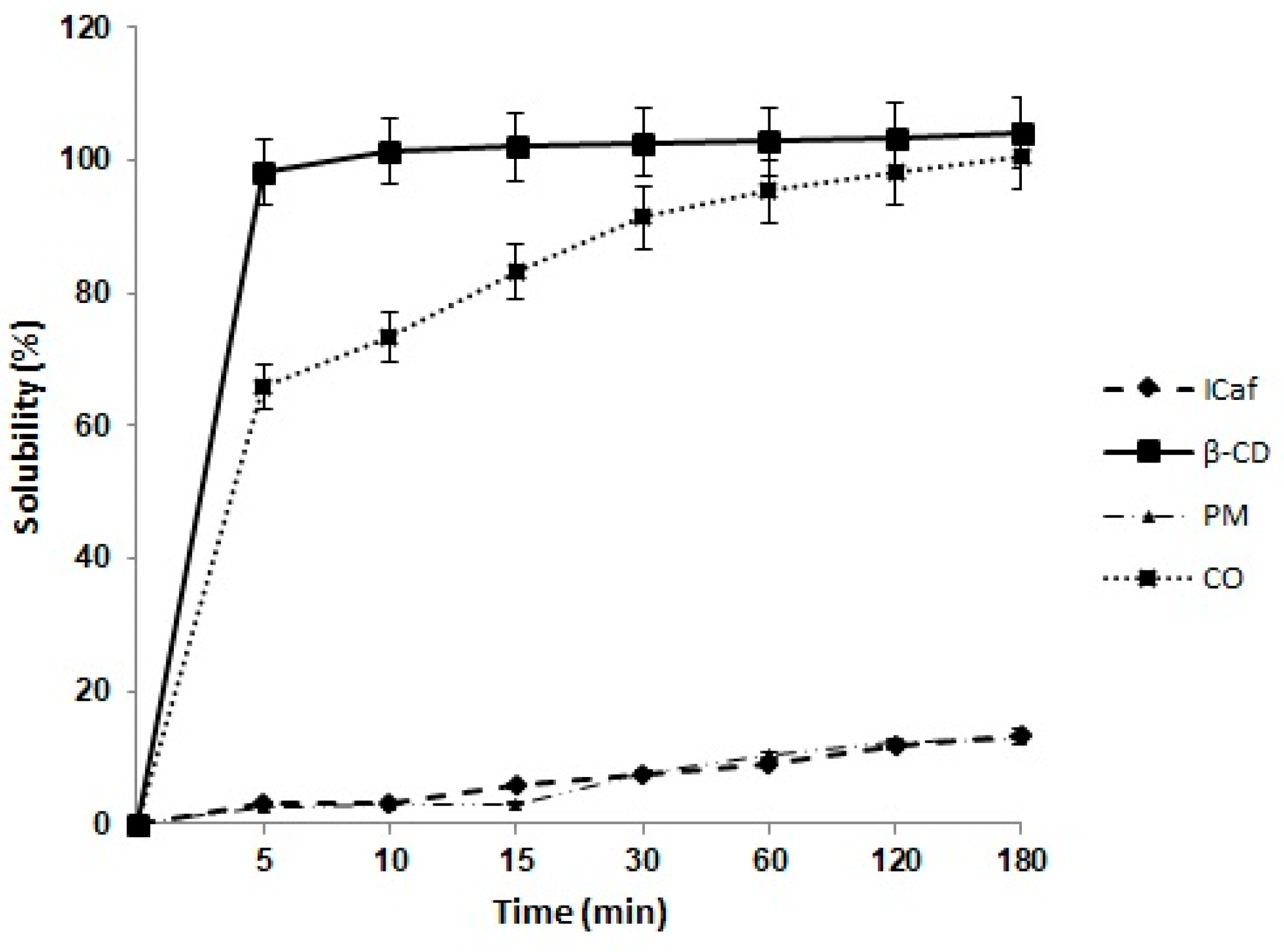
3.7. Dissolution Assay
3.8. Biological Assays
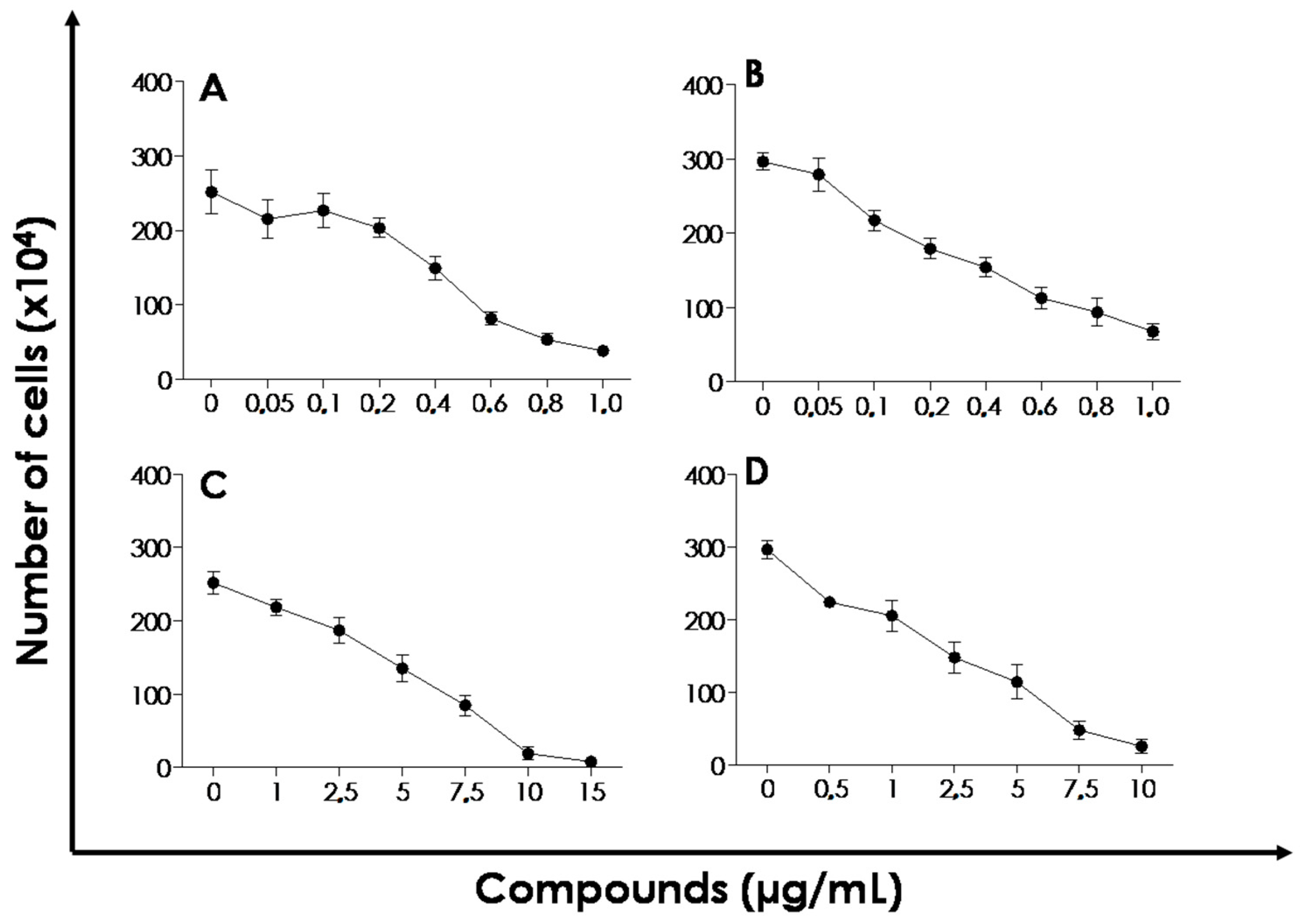
Effect of ICaf:β-CD on the Proliferation of L. amazonensis and L. chagasi
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elisama, A.C.; Silva, R.d.S.; Gabriela, C.d.C.; Aline, G.M.F.; Maria, L.d.C.B.; Andre, L.S.d.; Helena, C.C.; Viviane, L. Leishmaniasis: History, evolution of treatment and the need for new drugs. J. Curr. Biotechnol. 2014, 3, 279–288. [Google Scholar]
- Souto, E.B.; Dias-Ferreira, J.; Craveiro, S.A.; Severino, P.; Sanchez-Lopez, E.; Garcia, M.L.; Silva, A.M.; Souto, S.B.; Mahant, S. Therapeutic Interventions for Countering Leishmaniasis and Chagas′s Disease: From Traditional Sources to Nanotechnological Systems. Pathogens 2019, 8, 119. [Google Scholar] [CrossRef]
- Status of Endemicity of Visceral Leishmaniasis, Worldwide, 2018. Available online: https://www.who.int/leishmaniasis/burden/GHO_VL_2018.pdf (accessed on 30 June 2020).
- Abamor, E.S. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes in vitro. Asian Pac. J. Trop. Med. 2017, 10, 25–34. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discovery Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Neugart, S.; Schreiner, M.; Grune, T.; Rohn, S.; Ott, C. Amaranth′s 2-Caffeoylisocitric Acid—An Anti-Inflammatory Caffeic Acid Derivative That Impairs NF-κB Signaling in LPS-Challenged RAW 264.7 Macrophages. Nutrients 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Henah, M.B.; Taseen, G.; Ehtishamul, H. Anti-neoplastic and calcium modulatory action of caffeic acid phenethyl ester and dasatinib in C6 glial cells: A therapeutic perspective. CNS Neurol. Disorders-Drug Targets 2016, 15, 54–63. [Google Scholar]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antiviral Res. 2018, 160, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective effect of caffeic acid phenethyl ester in a mouse model of Alzheimer′s disease involves Nrf2/HO-1 pathway. Aging Dis. 2018, 9, 605. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Tolba, M.F.; Omar, H.A.; Azab, S.S.; Khalifa, A.E.; Abdel-Naim, A.B.; Abdel-Rahman, S.Z. Caffeic acid phenethyl ester: A review of its antioxidant activity, protective effects against ischemia-reperfusion injury and drug adverse reactions. Crit. Rev. Food Sci. Nutr. 2016, 56, 2183–2190. [Google Scholar] [CrossRef]
- Arasoğlu, T.; Derman, S. Assessment of the Antigenotoxic Activity of Poly (d, l-lactic-co-glycolic acid) Nanoparticles Loaded with Caffeic Acid Phenethyl Ester Using the Ames Salmonella/Microsome Assay. J. Agric. Food Chem. 2018, 66, 6196–6204. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.O.; Freire Pessoa, H.L.; Lira, A.B.; Castillo, Y.P.; de Sousa, D.P. Synthesis, Antibacterial Evaluation, and QSAR of Caffeic Acid Derivatives. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Marques, C.S.F.; Severino, P.; Fricks, A.T.; dos Santos, A.L.S.; Andrade, L.N.; Chaud, M.; Souto, E.B.; Pergentinho, D.; de Oliveira, S.S.C. Processo de Obtenção de Complexo de Inclusão de Cafeato de Isopentila em Ciclodextrina e Produto Obtido Para o Tratamento da Leishmaniose. Patent BR 10 2019 011166 6, 30.05.2019, 2020. (submitted). [Google Scholar]
- Angelina, A.; Catherine, R.-L.; Adam, B. Drug–Cyclodextrin Association Constants Determined by Surface Tension and Surface Pressure Measurements: I. Host—Guest Complexation of Water Soluble Drugs by Cyclodextrins: Polymyxin B—β Cyclodextrin System. J. Colloid Interface Sci. 1999, 212, 275–285. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Nuñez, G.A.; Espinoza-Gómez, H.; Flores-López, L.Z. A comparative study of the effect of α-, β-, and γ-cyclodextrins as stabilizing agents in the synthesis of silver nanoparticles using a green chemistry method. Mater. Sci. Eng. C 2014, 43, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.G.; Thrivikraman, G.; Menezes, P.P.; França, C.M.; Lima, B.S.; Carvalho, Y.M.; Souza, E.P.; Duarte, M.C.; Shanmugam, S.; Quintans-Júnior, L.J. Carvacrol/β-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Qian, H.; Tang, P.; Tang, Y.; Liu, Y.; Pu, H.; Zhang, M.; Zhao, L.; Li, H. Preparation, Characterization, and Properties of Inclusion Complexes of Balofloxacin with Cyclodextrins. AAPS Pharm. Sci. Technol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Galvão, J.G.; Cerpe, P.; Santos, D.A.; Gonsalves, J.K.; Santos, A.J.; Nunes, R.K.; Lira, A.A.; Alves, P.B.; La Corte, R.L.; Blank, A.F.; et al. Lippia gracilis essential oil in β-cyclodextrin inclusion complexes: An environmentally safe formulation to control Aedes aegypti larvae. Pest Manag. Sci. 2019, 75, 452–459. [Google Scholar] [CrossRef]
- Sbârcea, L.; Udrescu, L.; Ledeţi, I.; Szabadai, Z.; Fuliaş, A.; Sbârcea, C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril. J. Therm. Anal. Calorim. 2016, 123, 2377–2390. [Google Scholar] [CrossRef]
- Herrera, A.; Rodríguez, F.J.; Bruna, J.E.; Abarca, R.L.; Galotto, M.J.; Guarda, A.; Mascayano, C.; Sandoval-Yáñez, C.; Padula, M.; Felipe, F.R.S. Antifungal and physicochemical properties of inclusion complexes based on β-cyclodextrin and essential oil derivatives. Food Res. Int. 2019, 121, 127–135. [Google Scholar] [CrossRef]
- Sancho, M.I.; Andujar, S.; Porasso, R.D.; Enriz, R.D. Theoretical and experimental study of inclusion complexes of β-cyclodextrins with chalcone and 2′, 4′-dihydroxychalcone. J. Phys. Chem. B 2016, 120, 3000–3011. [Google Scholar] [CrossRef]
- Andrade, T.A.; Freitas, T.S.; Araújo, F.O.; Menezes, P.P.; Dória, G.A.A.; Rabelo, A.S.; Quintans-Júnior, L.J.; Santos, M.R.; Bezerra, D.P.; Serafini, M.R. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomed. Pharmacother. 2017, 89, 201–207. [Google Scholar] [CrossRef]
- Mohandoss, S.; Atchudan, R.; Edison, T.N.J.I.; Mandal, T.K.; Palanisamy, S.; You, S.; Napoleon, A.A.; Shim, J.-J.; Lee, Y.R. Enhanced solubility of guanosine by inclusion complexes with cyclodextrin derivatives: Preparation, characterization, and evaluation. Carbohydr. Polym. 2019, 224, 115166. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.B.; Burusco, K.K.; Jaime, C.; Venâncio, T.; de Carvalho, A.F.S.; Murgas, L.D.S.; Pinto, L.d.M.A. Complexes between methyltestosterone and β-cyclodextrin for application in aquaculture production. Carbohydr. Polym. 2018, 179, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.B.; Da Silva Júnior, W.F.; De Oliveira Pinheiro, J.G.; Da Fonseca, A.G.; Lemos, T.M.A.M.; De Oliveira Rocha, H.A.; De Azevedo, E.P.; Mendonça Junior, F.J.B.; Neves de Lima, Á.A. Characterization and Antiproliferative Activity of a Novel 2-Aminothiophene Derivative-β-Cyclodextrin Binary System. Molecules 2018, 23, 3130. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. J. Food Hydrocolloids 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Simsek, T.; Simsek, S.; Mayer, C.; Rasulev, B. Combined computational and experimental study on the inclusion complexes of β-cyclodextrin with selected food phenolic compounds. Struct. Chem. 2019, 30, 1395–1406. [Google Scholar] [CrossRef]
- da Silva Júnior, W.F.; Bezerra de Menezes, D.L.; de Oliveira, L.C.; Koester, L.S.; Oliveira de Almeida, P.D.; Lima, E.S.; de Azevedo, E.P.; da Veiga Júnior, V.F.; Neves de Lima, Á.A. Inclusion Complexes of β and HPβ-Cyclodextrin with α, β Amyrin and In Vitro Anti-Inflammatory Activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Bolattin, M.B.; Nandibewoor, S.T.; Joshi, S.D.; Dixit, S.R.; Chimatadar, S.A. Interaction of hydralazine with human serum albumin and effect of β-cyclodextrin on binding: Insights from spectroscopic and molecular docking techniques. Ind. Eng. Chem. Res. 2016, 55, 5454–5464. [Google Scholar] [CrossRef]
- Nurhidayah, E.; Ivansyah, A.; Martoprawiro, M.; Zulfikar, M. A Molecular docking study to predict enantioseparation of some chiral carboxylic acid derivatives by methyl-β-cyclodextrin. JPhCS 2018, 1013, 012203. [Google Scholar] [CrossRef]
- Saidman, E.; Chattah, A.K.; Aragón, L.; Sancho, M.; Camí, G.; Garnero, C.; Longhi, M. Inclusion complexes of β-cyclodextrin and polymorphs of mebendazole: Physicochemical characterization. Eur. J. Pharm. Sci. 2019, 127, 330–338. [Google Scholar] [CrossRef]
- da Câmara Rocha, J.; da Franca Rodrigues, K.A.; do Nascimento Néris, P.L.; da Silva, L.V.; Almeida, F.S.; Lima, V.S.; Peixoto, R.F.; da Câmara Rocha, J.; Veras, R.C.; de Medeiros, I.A. Biological activity of Morita-Baylis-Hillman adduct homodimers in L. infantum and L. amazonensis: Anti-Leishmania activity and cytotoxicity. Parasitol. Res. 2019, 118, 3067–3076. [Google Scholar] [CrossRef]
- Aditya, N.P.; Vathsala, P.G.; Vieira, V.; Murthy, R.S.; Souto, E.B. Advances in nanomedicines for malaria treatment. Adv. Colloid Interface Sci. 2013, 201–202, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discovery Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cutrone, G.; Casas-Solvas, J.M.; Vargas-Berenguel, A. Cyclodextrin-modified inorganic materials for the construction of nanocarriers. Int. J. pharm. 2017, 531, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiri, G.; Cran, M.J.; Smallridge, A.J.; Bigger, S.W. Optimisation of β-cyclodextrin inclusion complexes with natural antimicrobial agents: Thymol, carvacrol and linalool. J. Microencapsulation 2018, 35, 26–35. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Siqueira, L.A.; Zanini, M.S.; dos Santos Matos, A.P.; Quaresma, C.H.; da Silva, L.M.; de Andrade, S.F.; Severi, J.A.; Villanova, J.C.O. Physicochemical and in vitro biological evaluations of furazolidone-based β-cyclodextrin complexes in Leishmania amazonensis. Res. Vet. Sci. 2018, 119, 143–153. [Google Scholar] [CrossRef]
- Oliveira, M.G.; Brito, R.G.; Santos, P.L.; Araujo-Filho, H.G.; Quintans, J.S.; Menezes, P.P.; Serafini, M.R.; Carvalho, Y.M.; Silva, J.C.; Almeida, J.R. α-Terpineol, a monoterpene alcohol, complexed with β-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem. Biol. Interact. 2016, 254, 54–62. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- de Sousa Araújo, P.S.; de Oliveira, S.S.C.; d′Avila-Levy, C.M.; dos Santos, A.L.S.; Branquinha, M.H. Susceptibility of promastigotes and intracellular amastigotes from distinct Leishmania species to the calpain inhibitor MDL28170. Parasitol. Res. 2018, 117, 2085–2094. [Google Scholar] [CrossRef]
- Marinho, F.d.A.; Gonçalves, K.C.d.S.; Oliveira, S.S.d.; Oliveira, A.-C.d.S.C.d.; Bellio, M.; d′Avila-Levy, C.M.; Santos, A.L.S.d.; Branquinha, M.H. Miltefosine induces programmed cell death in Leishmania amazonensis promastigotes. Memorias Do Inst. Oswaldo Cruz 2011, 106, 507–509. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Andrade, H.M.; Melo, M.N.; Coelho, E.A.F.; Avelar, D.; Gazzinelli, R.T. Leishmaniose visceral canina: Novos antígenos para diagnóstico e vacinas. Gerais Rev. De Saúde Públ. Do SUS/MG 2017, 1, 49–50. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
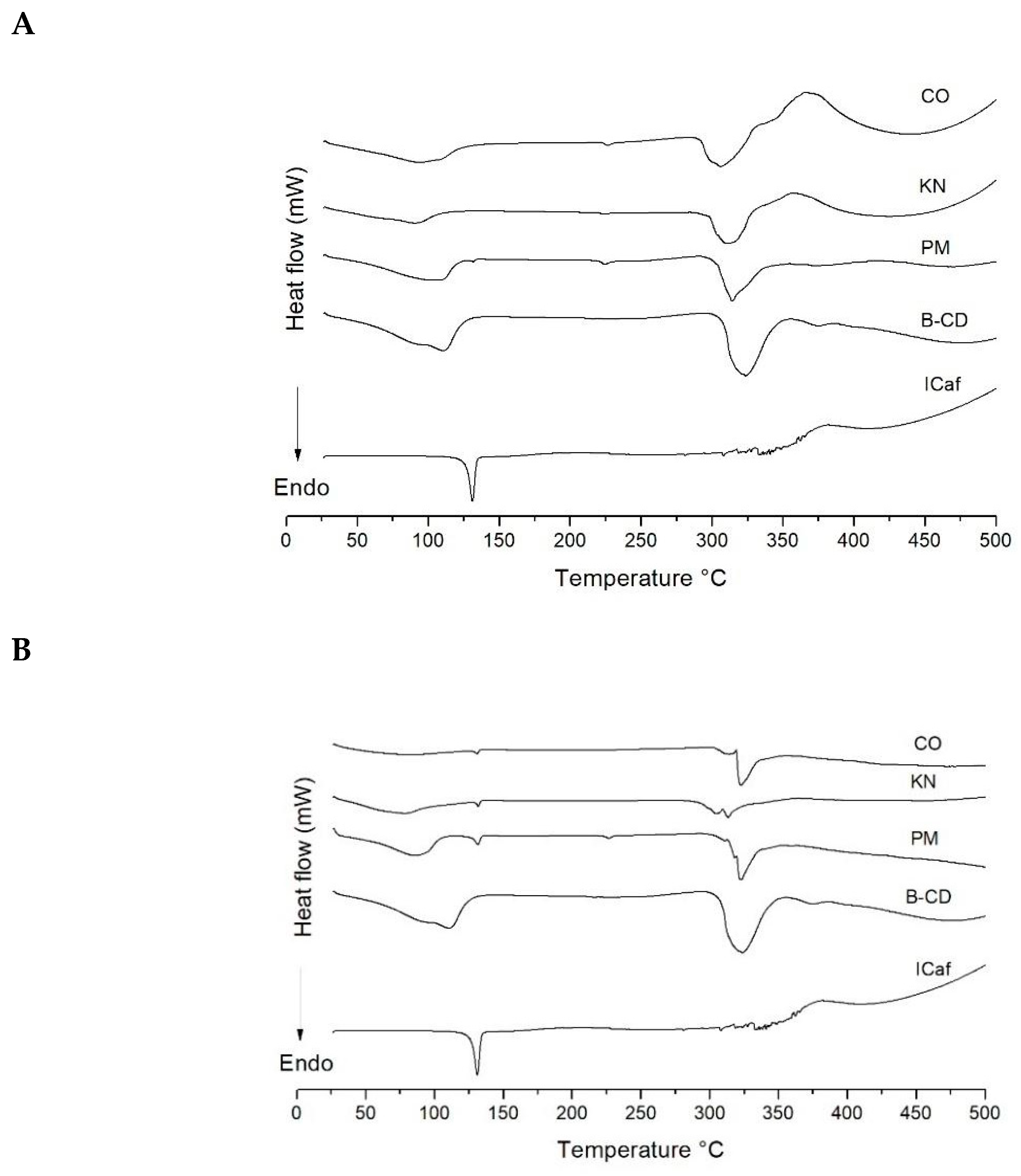
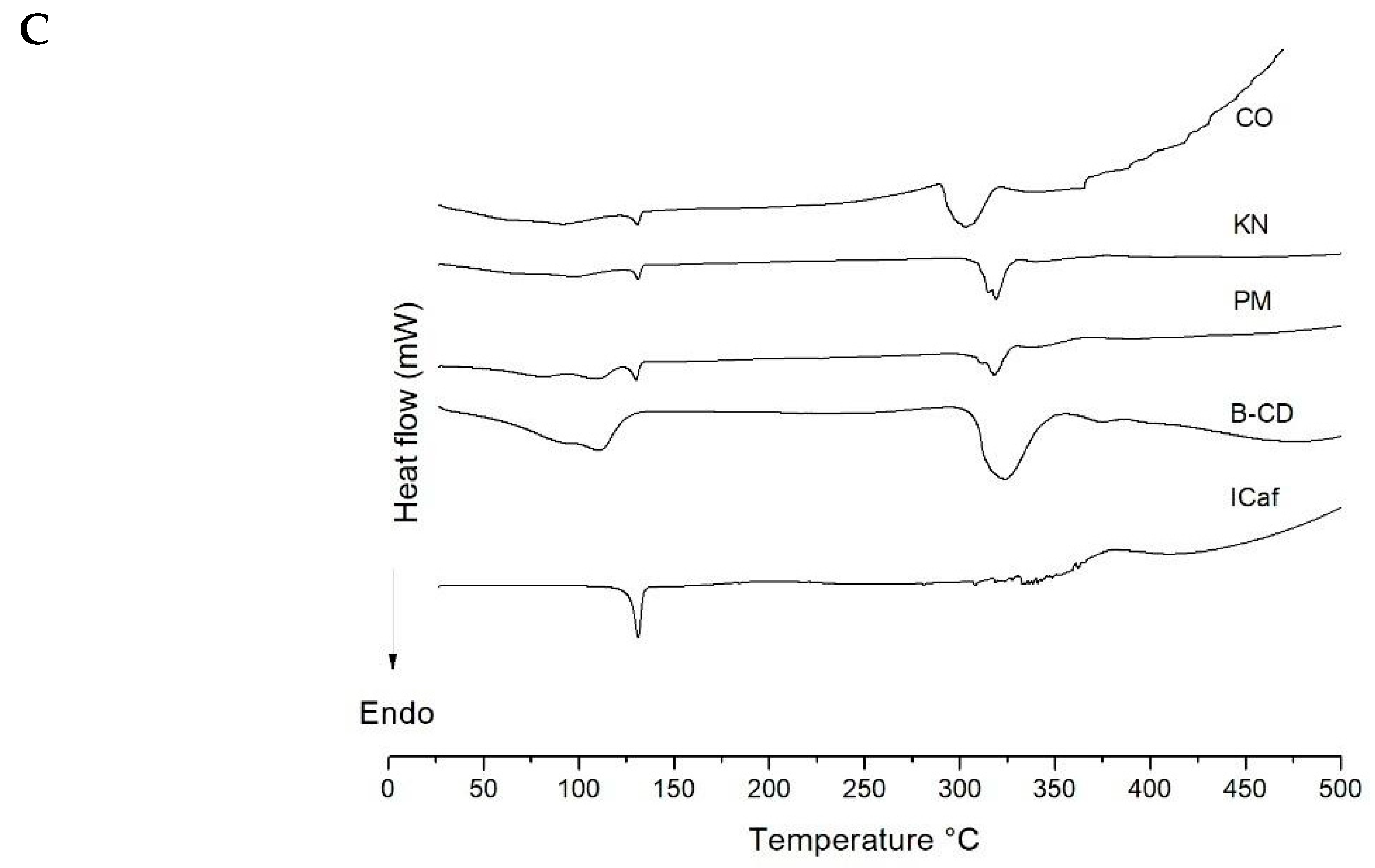

| Sample | Events | Tonset (°C) | Tendset (°C) | ∆H (J) |
|---|---|---|---|---|
| β-CD | 1 | 63.80 | 142.16 | 1.73 |
| 2 | 308.71 | 343.18 | 1.53 | |
| ICaf | 1 | 127.63 | 133.77 | 0.22 |
| PM 0.25:1 | 1 | 61.92 | 118.56 | 0.26 |
| 2 | 128.80 | 133.67 | 0.01 | |
| 3 | 220.79 | 228.54 | 0.02 | |
| 4 | 302.49 | 328.33 | 0.89 | |
| KN 0.25:1 | 1 | 96.04 | 106.75 | 0.32 |
| 2 | 298.69 | 326.48 | 1.10 | |
| CO 0.25:1 | 1 | 72.01 | 124.52 | 0.71 |
| 2 | 223.29 | 230.85 | 0.02 | |
| 3 | 292.41 | 327.90 | 1.56 | |
| PM 1:1 | 1 | 64.30 | 103.73 | 0.76 |
| 2 | 127.67 | 134.52 | 0.05 | |
| 3 | 319.40 | 332.30 | 0.51 | |
| KN 1:1 | 1 | 43.22 | 93.39 | 0.58 |
| 2 | 130.14 | 133.54 | 0.01 | |
| 3 | 309.49 | 320.60 | 0.48 | |
| CO 1:1 | 1 | 129.19 | 132.79 | 0.01 |
| 2 | 318.53 | 320.40 | 0.51 | |
| PM 2:1 | 1 | 128.11 | 132.68 | 0.09 |
| 2 | 313.51 | 325.19 | 0.27 | |
| KN 2:1 | 1 | 130.08 | 133.17 | 0.04 |
| 2 | 311.19 | 326.05 | 0.38 | |
| CO 2:1 | 1 | 126.87 | 133.22 | 0.04 |
| 2 | 291.04 | 322.76 | 0.64 |
| Sample | ∆m1 (%) 30–170 °C | ∆m2 (%) 170–280 °C | ∆m3 (%) 280–400 °C | ∆m4 (%) 400–900 °C |
|---|---|---|---|---|
| ICaf | 0.72 | 11.99 | 56.34 | 16.05 |
| β-CD | 13.57 | 0.09 | 73.15 | 13.19 |
| PM 0.25:1 | 12.40 | 1.93 | 72.68 | 8.34 |
| KN 0.25:1 | 11.72 | 1.43 | 73.29 | 12.87 |
| CO 0.25:1 | 12.00 | 1.29 | 71.83 | 9.81 |
| PM 1:1 | 10.9 | 3.74 | 65.28 | 9.85 |
| KN 1:1 | 10.62 | 5.58 | 61.61 | 9.47 |
| CO 1:1 | 6.48 | 6.49 | 70.31 | 9.74 |
| PM 2:1 | 9.81 | 6.05 | 66.04 | 11.85 |
| KN 2:1 | 8.26 | 7.23 | 62.85 | 11.99 |
| CO 2:1 | 7.73 | 8.30 | 65.21 | 11.66 |
| Compound | Affinity (kcal/mol) | Atoms de Interaction | Type of Interaction | From | For | Distance (Å) |
|---|---|---|---|---|---|---|
| Isopentyl caffeate | −4.9 | O | Hydrogen bond | β-CD | ICaf | 2.51 |
| H | ||||||
| ICaf | β-CD | 2222 | ||||
| H | 2.05 | |||||
| 2.15 |
| Compounds | IC50 (µM) L. amazonensis | IC50 (µM) L. chagasi |
|---|---|---|
| ICaf | 1.56 (1.35–1.73) | 1.71 (1.46–1.88) |
| ICaf:β-CD (CO, 1:1) | 2.74 (2.33–2.91) | 1.95 (1.64–2.11) |
| β-CD | 88.10 (67.21–95.6) | 88.10 (67.21–95.6) |
| Miltefosine | 6.69 (5.74–8.04) | 6.38 (4.85–7.94) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.S.F.; Barreto, N.S.; Oliveira, S.S.C.d.; Santos, A.L.S.; Branquinha, M.H.; Sousa, D.P.d.; Castro, M.; Andrade, L.N.; Pereira, M.M.; Silva, C.F.d.; et al. β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules 2020, 25, 4181. https://doi.org/10.3390/molecules25184181
Marques CSF, Barreto NS, Oliveira SSCd, Santos ALS, Branquinha MH, Sousa DPd, Castro M, Andrade LN, Pereira MM, Silva CFd, et al. β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules. 2020; 25(18):4181. https://doi.org/10.3390/molecules25184181
Chicago/Turabian StyleMarques, Carine S. F., Nathalia S. Barreto, Simone S. C. de Oliveira, André L. S. Santos, Marta H. Branquinha, Damião P. de Sousa, Mayara Castro, Luciana N. Andrade, Matheus M. Pereira, Classius F. da Silva, and et al. 2020. "β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity" Molecules 25, no. 18: 4181. https://doi.org/10.3390/molecules25184181
APA StyleMarques, C. S. F., Barreto, N. S., Oliveira, S. S. C. d., Santos, A. L. S., Branquinha, M. H., Sousa, D. P. d., Castro, M., Andrade, L. N., Pereira, M. M., Silva, C. F. d., Chaud, M. V., Jain, S., Fricks, A. T., Souto, E. B., & Severino, P. (2020). β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules, 25(18), 4181. https://doi.org/10.3390/molecules25184181












