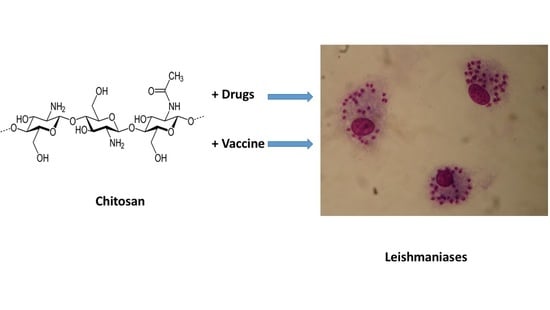
Chitosan Contribution to Therapeutic and Vaccinal Approaches for the Control of Leishmaniasis
Abstract
1. Introduction
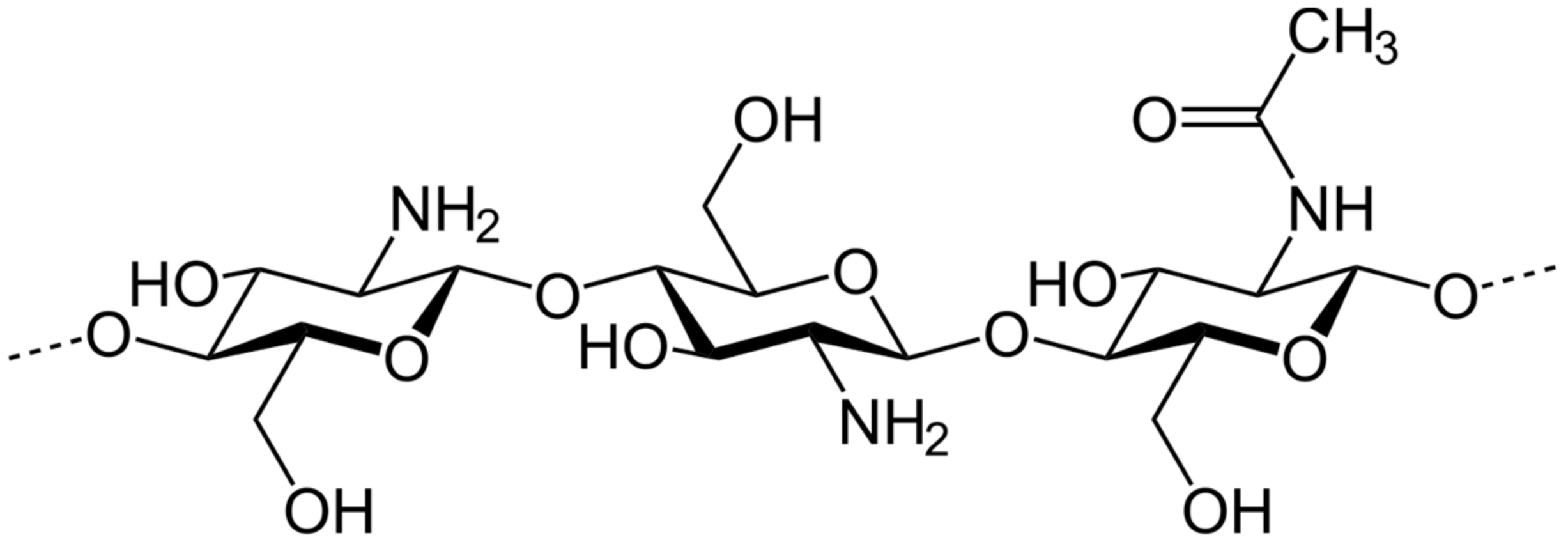
2. Chitosan: Nature and Different Uses
3. Activity of Chitosan and Its Derivatives on Leishmania Parasites
3.1. In Vitro Antileishmanial Activity of Chitosan and Its Derivatives
3.2. In Vivo Antileishmanial Activity of Chitosan on Cutaneous Leishmaniasis BALB/c Mice Model
3.3. Pilot Clinical Study of Chitosan Efficacy on Cutaneous Leishmaniasis Lesions in Patients
4. Chitosan-Based Drug Loaded Formulations for the Chemotherapy of Cutaneous Leishmaniasis
4.1. Amphotericin B-Chitosan Nanoformulations
4.2. Paromomycin-Chitosan Nanoformulations
4.3. Meglumine Antimoniate-Chitosan Nanoformulations
4.4. Rifampicin Loaded Nanotransfersomes (NTs) Incorporated in Chitosan Gel
4.5. Curcumin-Chitosan Nanoformulations
4.6. β-. Lapachone-Chitosan
4.7. Betulinic Acid-Chitosan
4.8. Ursolic Acid-Chitosan
4.9. S-Nitroso-Mercaptosuccinic Acid–Loaded Chitosan Nanoparticles
4.10. Chitosan Polymer as a Booster for Drug Efficacy
4.11. Drug Combined Formulations
4.11.1. Amphotericin B-Miltefosine-Chitosan Lipid Nanoparticles
4.11.2. Paromomycin-Selenium-Chitosan Hydrogel
4.11.3. Antimony-Titanium-Chitosan
5. Immunomodulatory Effect of Chitosan
6. Chitosan and Chitin for Antileishmanial Vaccines
6.1. Leishmania Antigens Encapsulated in Chitosan Nanoparticles
6.2. Leishmania Superoxide Dismutase Loaded Chitosan Nanoparticles
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Valero, N.N.H.; Uriarte, M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: A systematic review. Parasitol. Res. 2020, 119, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy: Challenges and opportunities. Clin. Microbiol. Infect. 2011, 17, 1478–1483. [Google Scholar] [CrossRef]
- Annang, F.; Pérez-Moreno, G.; García-Hernández, R.; Cordon-Obras, C.; Martín, J.; Tormo, J.R.; Rodríguez, L.; de Pedro, N.; Gómez-Pérez, V.; Valente, M.; et al. High-throughput screening platform for natural product-based drug discovery against three neglected tropical diseases: Human African trypanosomiasis, leishmaniasis, and Chagas disease. J. Biomol. Screen. 2015, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pomel, S.; Mao, W.; Ha-Duong, T.; Cavé, C.; Loiseau, P.M. GDP-Mannose Pyrophosphorylase: A biologically validated target for drug development against leishmaniasis. Front. Cell. Infect. Microbiol. 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, R.; Bou Youness, H.; Lachaud, L.; Bastien, P.; Masquefa, C.; Bonnet, P.A.; El Hajj, H.; Khalifeh, I. EAPB0503: An imiquimod analog with potent in vitro activity against cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica. PLoS Negl. Trop. Dis. 2018, 12, e0006854. [Google Scholar] [CrossRef] [PubMed]
- Intakhan, N.; Chanmol, W.; Somboon, P.; Bates, M.D.; Yardley, V.; Bates, P.A.; Jariyapan, N. Antileishmanial activity and synergistic effects of amphotericin B deoxycholate with allicin and andrographolide against Leishmania martiniquensis in vitro. Pathogens 2020, 9, 49. [Google Scholar] [CrossRef]
- Téllez, J.; Echeverry, M.C.; Romero, I.; Guatibonza, A.; Santos Ramos, G.; Borges, A.C.; Frézard, F.; Demicheli, C. Use of liposomal nanoformulations in antileishmania therapy: Challenges and perspectives. J. Liposome Res. 2020, 31, 1–33. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/chitosan and its derivatives: Fundamental problems and practical approaches. Biochemistry 2020, 85 (Suppl. 1), S154–S176. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Pereira, A.E.S.; de Morais Ribeiro, L.N.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R.; et al. Carvacrol and linalool co-loaded in b-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef] [PubMed]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Eweis, M.; Elkholy, S.S.; Elsabee, M.Z. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugarbeet pathogens. Int. J. Biol. Macromol. 2006, 38, 1–8. [Google Scholar] [CrossRef]
- Grice, I.D.; Mariottini, G.L. Glycans with antiviral activity from marine organisms. Results Probl. Cell Differ. 2018, 65, 439–475. [Google Scholar] [CrossRef]
- Braz, E.M.A.; Silva, S.C.C.C.E.; da Silva, D.A.; Carvalho, F.A.A.; Barreto, H.M.; Santos Júnior, L.S.; da Silva Filho, E.C. Modified chitosan-based bioactive material for antimicrobial application: Synthesis and characterization. Int. J. Biol. Macromol. 2018, 117, 640–647. [Google Scholar] [CrossRef]
- Pierre, G.; Salah, R.; Gardarin, C.; Traikia, M.; Petit, E.; Delort, A.M.; Mameri, N.; Moulti-Mati, F.; Michaud, P. Enzymatic degradation and bioactivity evaluation of C-6 oxidized chitosan. Int. J. Biol. Macromol. 2013, 60, 383–392. [Google Scholar] [CrossRef]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaish, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as novel penetration enhancers for transdermal and dermal drug delivery systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Esboei, B.R.; Mohebali, M.; Mousavi, P.; Fakhar, M.; Akhoundi, B. Potent antileishmanial activity of chitosan against Iranian strain of Leishmania major (MRHO/IR/75/ER): In vitro and in vivo assay. J. Vector Borne Dis. 2018, 55, 111–115. [Google Scholar] [CrossRef]
- Riezk, A.; Raynes, J.G.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of chitosan and its derivatives against Leishmania major and Leishmania mexicana in vitro. Antimicrob. Agents Chemother. 2020, 64, e01772-19. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez D’Alaya, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Esmaeilzadeh, S.; Zarei, M.; Ahmadi, F. Potential application of nanochitosan film as a therapeutic agent against cutaneous leishmaniasis caused by L. major. Parasitol. Res. 2015, 114, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Abdollahimajd, F.; Moravvej, H.; Dadkhahfar, S.; Mahdavi, H.; Mohebali, M.; Mirzadeh, H. Chitosan-based biocompatible dressing for treatment of recalcitrant lesions of cutaneous leishmaniasis: A pilot clinical study. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zadeh Mehrizi, T.; Ardestani, M.S.; Molla Hoseini, M.H.; Khamesipour, A.; Mosaffa, N.; Ramezani, A. Novel nano-sized chitosan amphotericin B formulation with considerable improvement against Leishmania major. Nanomedicine 2018, 13, 3129–3147. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Franca, J.R.; Fuscaldi, L.L.; Santos, M.L.; Duarte, M.C.; Lage, P.S.; Martins, V.T.; Costa, L.E.; Fernandes, S.O.; Cardoso, V.N.; et al. An optimized nanoparticle delivery system based on chitosan and chondroitin sulfate molecules reduces the toxicity of amphotericin B and is effective in treating tegumentary leishmaniasis. Int. J. Nanomed. 2014, 9, 5341–5353. [Google Scholar] [CrossRef][Green Version]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 1309–1330. [Google Scholar] [CrossRef]
- Gupta, P.K.; Jaiswal, A.K.; Asthana, S.; Verma, A.; Kumar, V.; Shukla, P.; Dwivedi, P.; Dube, A.; Mishra, P.R. Self assembled ionically sodium alginate cross-linked amphotericin B encapsulated glycol chitosan stearate nanoparticles: Applicability in better chemotherapy and non-toxic delivery in visceral leishmaniasis. Pharm. Res. 2015, 32, 1727–1740. [Google Scholar] [CrossRef]
- Shahnaz, G.; Edagwa, B.J.; McMillan, J.E.; Akhtar, S.; Raza, A.; Qureshi, N.; Yasinzai, M.; Gendelman, H.E. Development of mannose-achored thiolated amphotericin B nanocarriers for treatment of visceral leishmaniasis. Nanomedicine 2017, 12, 99–115. [Google Scholar] [CrossRef]
- Sarwar, H.S.; Sohail, M.F.; Saljoughian, N.; Rehman, A.U.; Akhtar, S.; Nadhman, A.; Yasinzai, M.; Gendelman, H.E.; Satoskar, A.R.; Shahnaz, G. Design of mannosylated oral amphotericin B nanoformulation: Efficacy and safety in visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 521–531. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef]
- Qu, X.; Khutoryanskiy, V.V.; Stewart, A.; Rahman, S.; Papahadjopoulos-Sternberg, B.; Dufes, C.; McCarthy, D.; Wilson, C.G.; Lyons, R.; Carter, K.; et al. Carbohydrate-based micelle clusters which enchance hydrophobic drug bioavailability by up to 1 order of magnitude. Biomacromolecules 2006, 7, 3452–3459. [Google Scholar] [CrossRef]
- Siew, A.; Le, H.; Thiovolet, M.; Gellert, P.; Schatzlein, A.; Uchegbu, I. Enchanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol. Pharm. 2012, 9, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dwivedi, P.; Khatik, R.; Jaiswal, A.K.; Dube, A.; Shukla, P.; Mishra, P.R. Development of 4-sulfated N-acetyl galactosamine anchored chitosan nanoparticles: A dual strategy for effective management of leishmaniasis. Colloids Surf. B Biointerfaces 2015, 136, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zadeh Mehrizi, T.; Khamesipour, A.; Shafiee Ardestani, M.; Ebrahimi Shahmabadi, H.; Haji Molla Hoseini, M.; Mosaffa, N.; Ramezani, A. Comparative analysis between four model nanoformulations of amphotericin B-chitosan, amphotericin B-dendrimer, betulinic acid-chitosan and betulinic acid-dendrimer for treatment of Leishmania major: Real-time PCR assay plus. Int. J. Nanomed. 2019, 14, 7593–7607. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Paromomycin-loaded mannosylated chitosan nanoparticles: Synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019, 197, 105072. [Google Scholar] [CrossRef]
- Afzal, I.; Sarwar, H.S.; Sohail, M.F.; Varikuti, S.; Jahan, S.; Akhtar, S.; Yasinzai, M.; Satoskar, A.R.; Shanhaz, G. Mannosylated thiolated paromomycin-loaded PLGA nanoparticles for the oral therapy of visceral leishmaniasis. Nanomedicine 2019, 14, 387–406. [Google Scholar] [CrossRef]
- Sarwar, H.S.; Ashraf, S.; Akhtar, S.; Sohail, M.F.; Hussain, S.Z.; Rafay, M.; Yasinzai, M.; Hussain, I.; Shahnaz, G. Mannosylated thiolated polyethyleneimine nanoparticles or the enhanced efficacy of antimonial drugs against leishmaniasis. Nanomedicine 2018, 13, 25–41. [Google Scholar] [CrossRef]
- Pujals, G.; Sune-Negre, J.M.; Perez, P.; Garcia, E.; Portus, M.; Tico, J.R.; Minarro, M.; Carrio, J. In vitro evaluation of the effectiveness and cytotoxicity of meglumine antimoniate microspheres produced by spray drying against Leishmania infantum. Parasitol. Res. 2008, 102, 1243–1247. [Google Scholar] [CrossRef]
- Do Valle, T.Z.; Oliveira Neto, M.P.; Schubach, A.; Lagrange, P.H.; Da Costa, S.C. New World tegumentar leishmaniasis: Chemotherapeutic activity of rifampicin in humans and experimental murine model. Pathol. Biol. 1995, 43, 618–621. [Google Scholar]
- Livshin, R.; Weinrauch, L.; Even-Paz, Z.; El-On, J. Efficacy of rifampicin and isoniazid in cutaneous leishmaniasis. Int. J. Dermatol. 1987, 26, 55–59. [Google Scholar] [CrossRef]
- Kochar, D.K.; Saini, G.; Kochar, S.K.; Sirohi, P.; Bumb, R.A.; Mehta, R.D.; Purohit, S.K. A double blind, randomised placebo controlled trial of rifampicin with omeprazole in the treatment of human cutaneous leishmaniasis. J. Vector Borne Dis. 2006, 43, 161–167. [Google Scholar] [PubMed]
- Rabia, S.; Khaleeq, N.; Batool, S.; Dar, M.J.; Kim, D.W.; Din, F.U.; Khan, G.M. Rifampicin-loaded nanotransferosomal gel for treatment of cutaneous leishmaniasis: Passive targeting via topical route. Nanomedicine 2020, 15, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. 2019, 14, 117–122. [Google Scholar] [CrossRef]
- Sarkar, A.; De, R.; Mukhopadhyay, A.K. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated disease. World J. Gastroenterol. 2016, 22, 2738–2746. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Kumar, A.; Singh, S.K. Chalcone and curcumin derivatives: A way ahead for malarial treatment. Mini Rev. Med. Chem. 2013, 13, 2116–2133. [Google Scholar] [CrossRef]
- Das, R.; Roy, A.; Dutta, N.; Majumder, H.K. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 2008, 13, 867–882. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Jacquet, P.; Azas, N.; Casanova, M. A novel hydrolase with a pro-death activity from the protozoan parasite Leishmania major. Cell Death Discov. 2019, 5, 99. [Google Scholar] [CrossRef]
- Chaubey, P.; Mishra, B.; Mudavath, S.L.; Patel, R.R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani. Int. J. Biol. Macromol. 2018, 111, 109–120. [Google Scholar] [CrossRef]
- De Almeida, E.R. Preclinical and clinical studies of lapachol and beta-lapachone. Open Nat. Prod. J. 2009, 2, 42–47. [Google Scholar] [CrossRef]
- Guimaraes, T.T.; Pinto, M.C.F.R.; Lanza, J.S.; Melo, M.N.; Monte-Neto, R.L.; Melo, I.M.M.; Diogo, E.B.T.; Ferreira, V.F.; Camara, C.A.; Valença, W.O.; et al. Potent naphtoquinones against antimony-sensitive and -resistant Leishmania parasites: Synthesis of a novel α- and nor-α-lapachone-based 1,2,3-triazoles by copper-catalyzed azide-alkyne cycloaddition. Eur. J. Med. Chem. 2013, 63, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Chau, Y.P.; Lu, K.S.; Kung, H.N. β-lapachone accelerates the recovery of burn-wound skin. Histol. Histopathol. 2011, 26, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.N.; Yang, M.J.; Chang, C.F.; Chau, Y.P.; Lu, K.S. In vitro and in vivo wound healing-promoting activities of beta-lapachone. Am. J. Physiol. Cell. Physiol. 2008, 295, C931–C934. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Schwartz, J.; Larrea, E.; Conde, I.; Font, M.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Assessement of β-lapachone loaded in lecithin-chitosan nanoparticles for the topical treatment of cutaneous leishmaniasis in L. major infected BALB/c mice. Nanomedicine 2015, 11, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Meira, C.S.; Barbosa-Filho, J.M.; Lanfredi-Rangel, A.; Guimaraes, E.T.; Moreira, D.R.; Soares, M.B. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016, 166, 108–115. [Google Scholar] [CrossRef]
- Dominguez-Carmona, D.B.; Escalante-Erosa, F.; Garcia-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D.; Chan-Bacab, J.; Gimenez-Turba, A.; Pena-Rodriguez, L.M. Antiprotozoal activity of Betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mukherjee, T.; Sengupta, S.; Chowdhury, S.R.; Mukhopadhyay, S.; Majumder, H.K. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Mol. Pharmacol. 2011, 80, 694–703. [Google Scholar] [CrossRef]
- Zadeh Mehrizi, T.; Shafiee Ardestani, M.; Haji Molla Hoseini, M.; Khamesipour, A.; Mosaffa, N.; Ramezani, A. Novel nanosized chitosan-betulinic acic against resistant Leishmania major and girts clinical observation of such parasite in kidney. Sci. Rep. 2018, 8, 11759. [Google Scholar] [CrossRef]
- Bilbao-Ramos, P.; Serrano, D.R.; Ruiz Saldana, H.K.; Torrado, J.J.; Bolas-Fernandez, F.; Dea-Avuela, M.A. Evaluating the potential of ursolic acid as bioproduct for cutaneous and visceral leishmaniasis. Molecules 2020, 25, 1394. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; De, A.K.; Bera, T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosanoligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017, 102, 996–1008. [Google Scholar] [CrossRef]
- Cabral, F.V.; Pelegrino, M.T.; Sauter, I.P.; Seabra, A.B.; Cortez, M.; Ribeiro, M.S. Nitric oxide-loaded chitosan nanoparticles as an innovative antileishmanial platform. Nitric Oxide 2019, 93, 25–33. [Google Scholar] [CrossRef]
- Grisin, T.; Bories, C.; Bombardi, M.; Loiseau, P.M.; Rouffiac, V.; Solgadi, A.; Mallet, J.M.; Ponchel, G.; Bouchemal, K. Supramolecular chitosan micro-platelets synergistically enchance anti-Candida albicans activity of amphotericin B using an immunocompetent murine model. Pharm. Res. 2017, 34, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Grisin, T.; Bories, C.; Loiseau, P.M.; Bouchemal, K. Cyclodextrin-mediated self-associating chitosan micro-platelets acts as a drug booster against Candida glabrata mucosal infection in immunocompetent mice. Int. J. Pharm. 2017, 519, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Malli, S.; Pomel, S.; Dennemont, I.; Loiseau, P.M.; Bouchemal, K. Combination of amphotericin B and chitosan platelets for the treatment of experimental cutaneous leishmaniasis: Histological and immunohistochemical examinations. J. Drug Deliv. Sci. Technol. 2019, 50, 34–41. [Google Scholar] [CrossRef]
- Malli, S.; Pomel, S.; Ayadi, Y.; Delomenie, C.; Da Costa, A.; Loiseau, P.M.; Bouchemal, K. Topically applied chitosan-coated poly(isobutylcyanoacrylate) nanoparticles are active against cutaneous leishmaniasis by accelerating lesion healing and reducing the parasitic load. ACS Appl. Biomater. 2019, 2, 2573–2586. [Google Scholar] [CrossRef]
- Pradines, B.; Lievin-Le Moal, V.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Cell line-dependent cytotoxicity of poly(isobutylcyanoacrylate) nanoparticles coated with chitosan and thiolated chitosan: Insights from cultured human epithelial Hela, Caco2/TC7 and HT29/MTX cells. Int. J. Pharm. 2015, 491, 17–20. [Google Scholar] [CrossRef]
- Tripathi, P.; Jaiswal, A.K.; Dube, A.; Mishra, P.R. Hexadecylphosphocholine (Miltefosine) stabilized chitosan modified ampholipospheres as prototype co-delivery vehicle for enhanced killing of L. donovani. Int. J. Biol. Macromol. 2017, 105 Pt 1, 625–637. [Google Scholar] [CrossRef]
- Schwartz, J.; Moreno, E.; Fernández, C.; Navarro-Blasco, I.; Nguewa, P.A.; Palop, J.A.; Irache, J.M.; Sanmartín, C.; Espuelas, S. Topical treatment of L. major infected BALB/c mice with a novel diselenide chitosan hydrogel formulation. Eur. J. Pharm. Sci. 2014, 62, 309–316. [Google Scholar] [CrossRef]
- Varshosaz, J.; Arbabi, B.; Pestehchian, N.; Saberi, S.; Delavari, M. Chitosan-titanium dioxide-glucantime nanoassemblies effects on promastigote and amastigote of Leishmania major. Int. J. Biol. Macromol. 2018, 107, 212–221. [Google Scholar] [CrossRef]
- Hoseini, M.H.; Moradi, M.; Alimohammadian, M.H.; Shahgoli, V.K.; Darabi, H.; Rostami, A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol. Int. 2016, 65, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Pawar, V.K.; Jaiswal, A.K.; Singh, Y.; Srikanth, C.H.; Chaurasia, M.; Bora, H.K.; Raval, K.; Meher, J.G.; Gayen, J.R.; et al. Chitosan coated PluronicF127 micelles for effective delivery of Amphotericin B in experimental visceral leishmaniasis. Int. J. Biol. Macromol. 2017, 105 Pt 1, 1220–1231. [Google Scholar] [CrossRef]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Pawar, V.K.; Dube, A.; Chourasia, M.K. Immunoadjuvant chemotherapy of visceral leishmaniasis in hamsters using amphotericin B-encapsulated nanoemulsion template-based chitosan nanocapsules. Antimicrob. Agents Chemother. 2013, 57, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A Third Generation Vaccine for Human Visceral Leishmaniasis and Post Kala Azar Dermal Leishmaniasis: First-in-human Trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Riteau, N.; Sher, A. Chitosan: An adjuvant with an unanticipated STING. Immunity 2016, 44, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Hojatizade, M.; Soleymani, M.; Tafaghodi, M.; Badiee, A.; Chavoshian, O.; Jaafari, M.R. Chitosan nanoparticles loaded with whole and soluble Leishmania antigens, and evaluation of their immunogenicity in a mouse model of leishmaniasis. Iran J. Immunol. 2018, 15, 281–293. [Google Scholar] [CrossRef]
- Danesh-Bahreini, M.A.; Shokri, J.; Samiei, A.; Kamali-Sarvestani, E.; Barzegar-Jalali, M.; Mohammadi-Samani, S. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842. [Google Scholar] [CrossRef]
- Rogers, M.E.; Hajmová, M.; Joshi, M.B.; Sadlova, J.; Dwyer, D.M.; Volf, P.; Bates, P.A. Leishmania chitinase facilitates colonization of sandfly vectors and enhances transmission to mice. Cell Microbiol. 2008, 10, 1363–1372. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczesna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan and chito-oligosaccarides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiseau, P.M.; Pomel, S.; Croft, S.L. Chitosan Contribution to Therapeutic and Vaccinal Approaches for the Control of Leishmaniasis. Molecules 2020, 25, 4123. https://doi.org/10.3390/molecules25184123
Loiseau PM, Pomel S, Croft SL. Chitosan Contribution to Therapeutic and Vaccinal Approaches for the Control of Leishmaniasis. Molecules. 2020; 25(18):4123. https://doi.org/10.3390/molecules25184123
Chicago/Turabian StyleLoiseau, Philippe M., Sébastien Pomel, and Simon L. Croft. 2020. "Chitosan Contribution to Therapeutic and Vaccinal Approaches for the Control of Leishmaniasis" Molecules 25, no. 18: 4123. https://doi.org/10.3390/molecules25184123
APA StyleLoiseau, P. M., Pomel, S., & Croft, S. L. (2020). Chitosan Contribution to Therapeutic and Vaccinal Approaches for the Control of Leishmaniasis. Molecules, 25(18), 4123. https://doi.org/10.3390/molecules25184123