Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides
Abstract
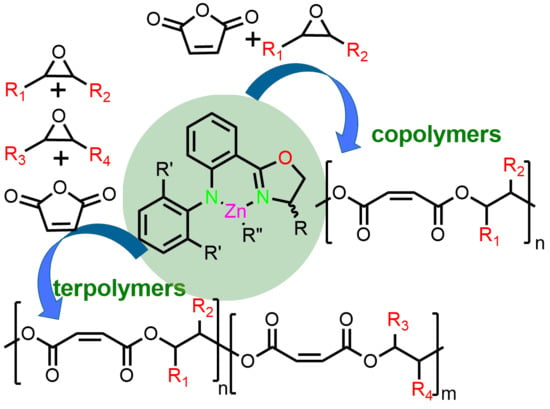
1. Introduction
2. Results and Discussions
2.1. Optimizing Reactions with MA/CHO
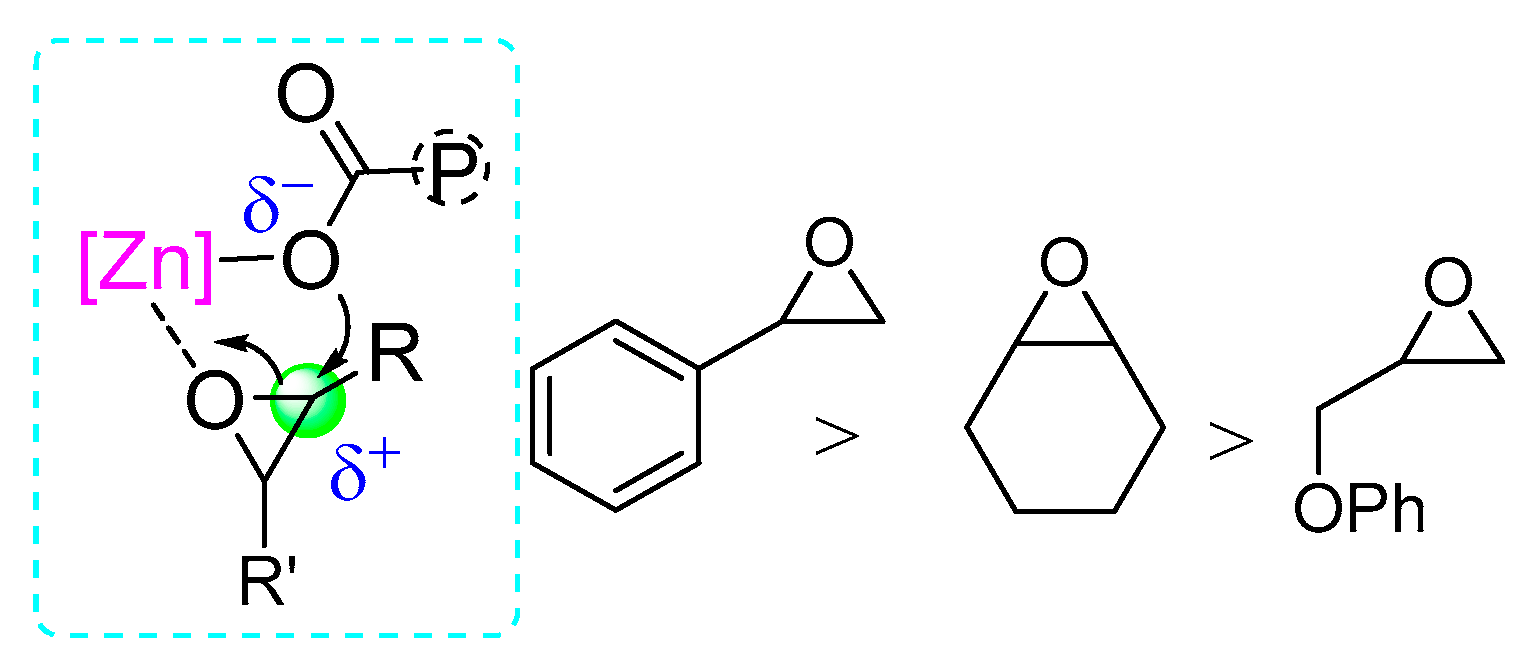
2.2. Comparison of Epoxides in ROCOP with MA
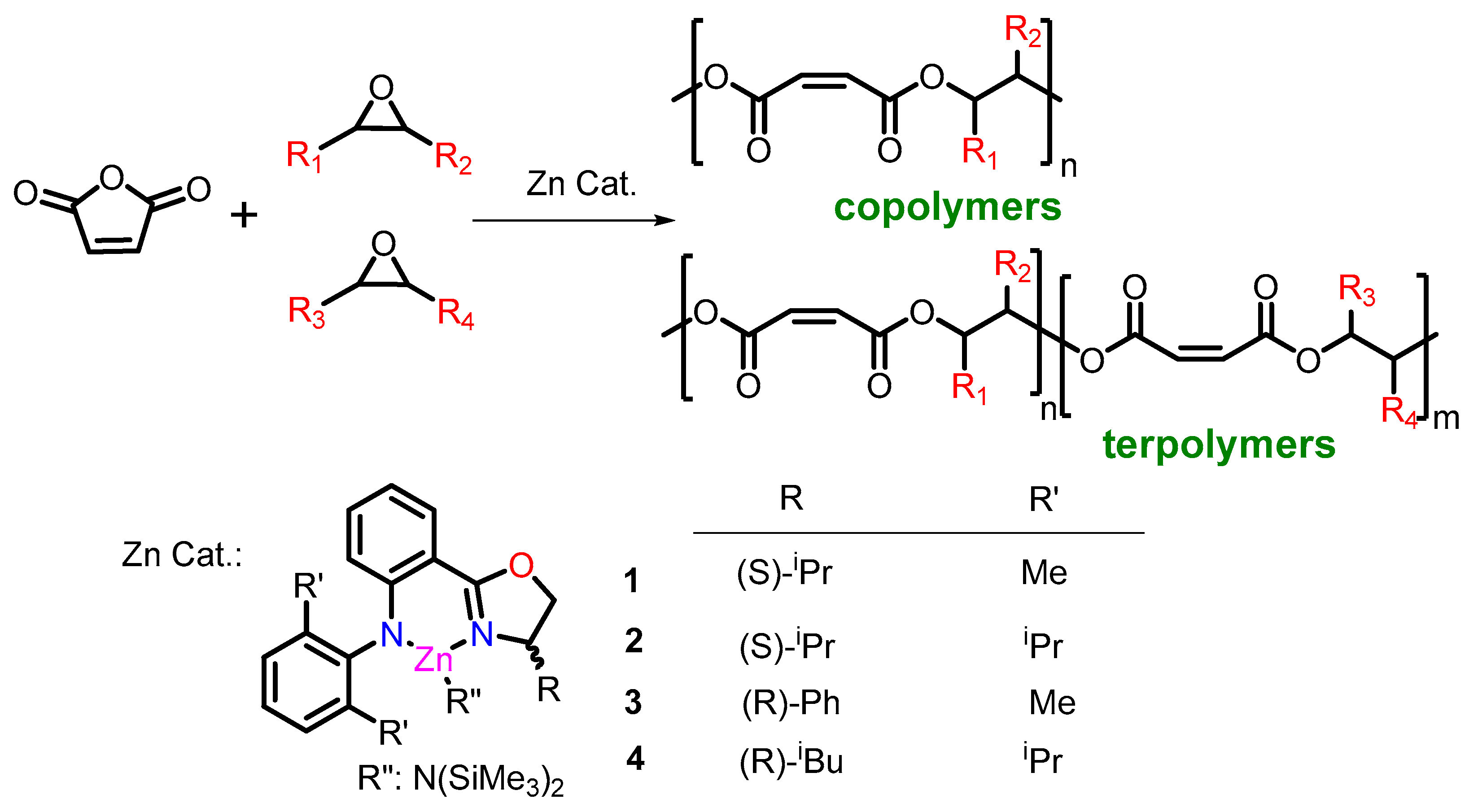
2.3. Effect of Catalysts and Anhydrides in ROCOP
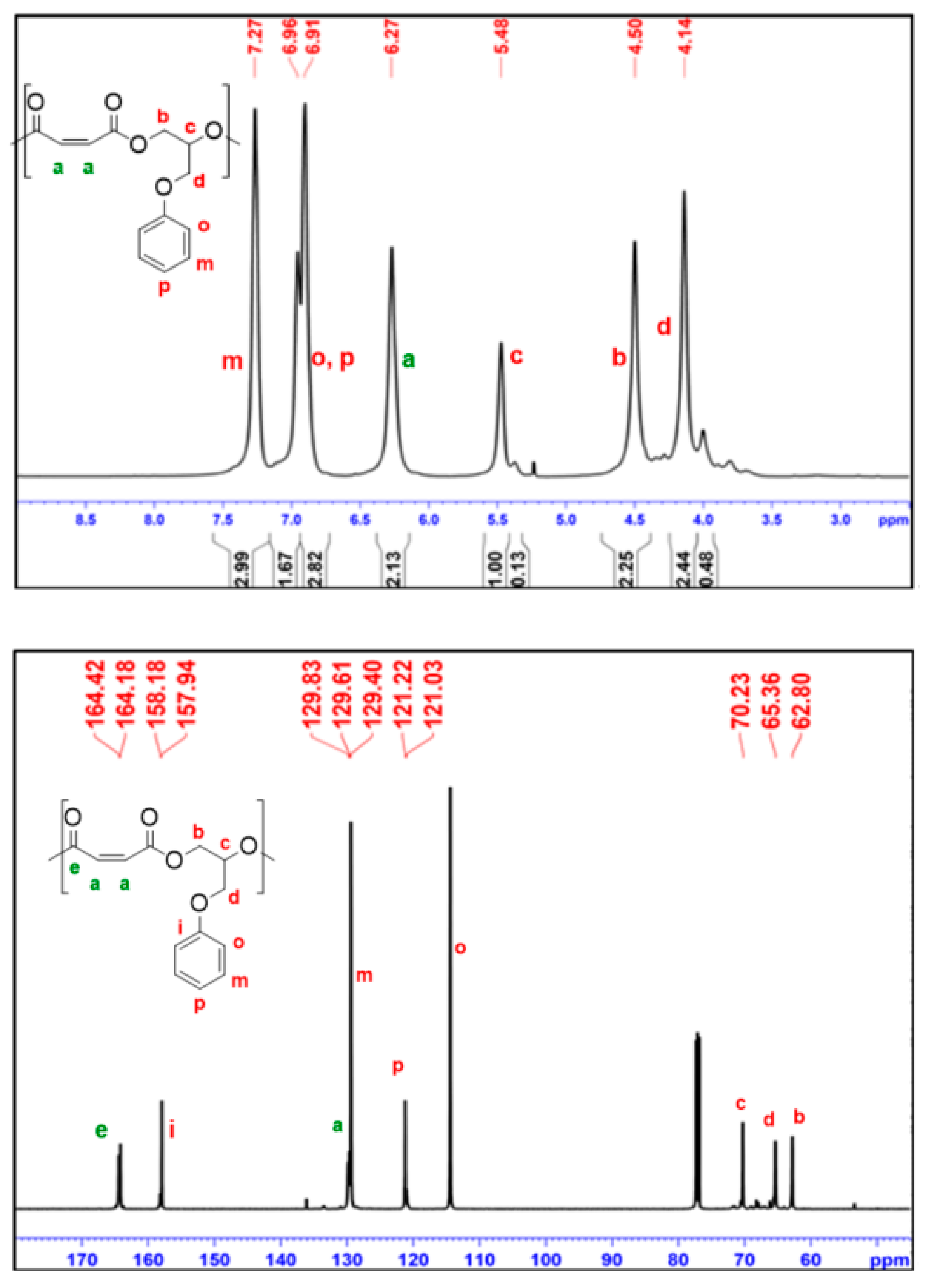
2.4. Terpolymerization of MA and Two Epoxides
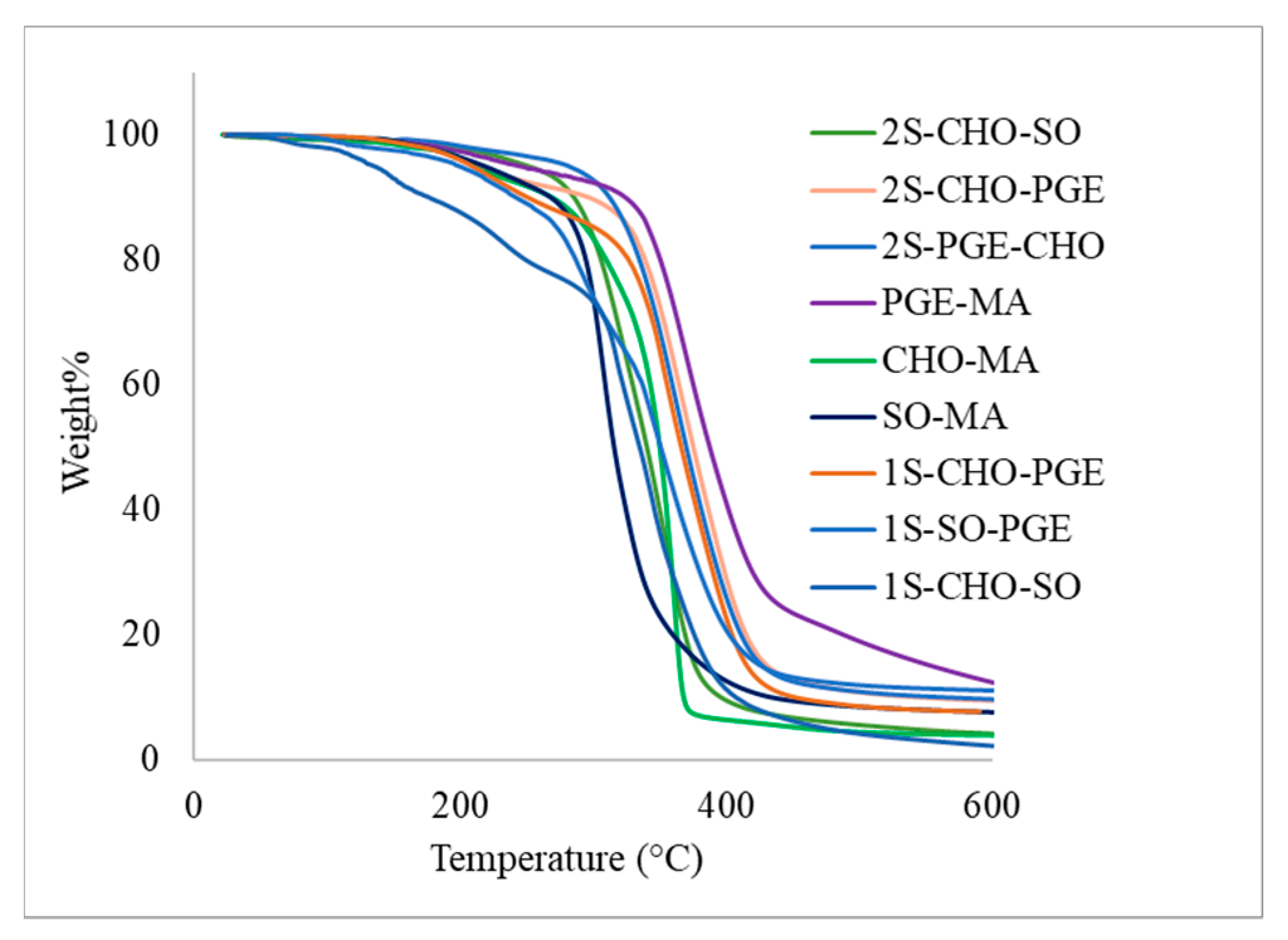
2.5. Thermal Properties of Copolymers and Terpolymers
3. Materials and Methods
3.1. General Procedure for Copolymerization and Terpolymerization
3.2. A Specific Example of Maleic Anhydride and Cyclohexene Oxide Using Cat-1
3.3. Examples of Terpolymerization using Cat-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Liu, Z.; Pumbeger, M.; Giraldo, C.V.; Ruesink, T.; Lu, L.; Yaszemski, M.J. A stimuli-responsive hydrogel for doxorubicin delivery. Biomaterials 2010, 31, 8051–8062. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, K.S.; Lee, S.H.; Kim, J.Y.; Lee, B.K.; Cho, D.W. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials 2011, 32, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [PubMed]
- Lutson, J.; Vass, F. Anionic copolymerization of cyclic ethers with cyclic anhydrides. Adv. Polym. Sci. 1984, 56, 91–133. [Google Scholar]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Robert, C.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, 586. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Syntheses of Biodegradable and biocompatible polymers by means of bismuth catalysts. Chem. Rev. 2009, 109, 5579–5594. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kakimoto, M.; Imai, Y. Novel synthesis of polyesters by palladium-catalyzed polycondensation of aromatic dibromides, bisphenols, or aliphatic diols with carbon monoxide. Macromolecules 1989, 22, 2593–2596. [Google Scholar] [CrossRef]
- Childers, M.I.; Longo, J.M.; Van Zee, N.J.; LaPointe, A.M.; Coates, G.W. Stereoselective epoxide polymerization and copolymerization. Chem. Rev. 2014, 114, 8129–8152. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, Y.; Carpentier, J.-F. Discrete cationic complexes for ring-opening polymerization catalysis of cyclic esters and epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.J.-L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of biodegradable polymers from renewable resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Sanford, M.J.; Carrodeguas, L.P.; Van Zee, N.J.; Kleij, A.W.; Coates, G.W. Alternating Copolymerization of propylene oxide and cyclohexene oxide with tricyclic anhydrides: Access to partially renewable aliphatic polyesters with high glass transition temperatures. Macromolecules 2016, 49, 6394–6400. [Google Scholar] [CrossRef]
- Carrodeguas, L.P.; Martín, C.; Kleij, A.W. Semiaromatic polyesters derived from renewable terpene oxides with high glass transitions. Macromolecules 2017, 50, 5337–5345. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Sainia, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef]
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Jeske, R.C.; DiCiccio, A.M.; Coates, G.W. Alternating copolymerization of epoxides and cyclic anhydrides: An improved route to aliphatic polyesters. J. Am. Chem. Soc. 2007, 129, 11330–11331. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.-Z.; Lu, H.-W.; Wang, H.-B.; Lu, X.-B. Making various degradable polymers from epoxides using a versatile dinuclear chromium catalyst. Macromolecules 2018, 51, 771–778. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Yang, M.; Liu, B.; Dong, X.; Theato, P. Highly cis/trans-stereoselective (ONSO)CrCl-catalyzed ring-opening copolymerization of norbornene anhydrides and epoxides. Macromolecules 2016, 49, 6232–6239. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Coates, G.W. Ring-opening copolymerization of maleic anhydride with epoxides: A chain-growth approach to unsaturated polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef]
- Baumgartner, R.; Song, Z.; Zhang, Y.; Cheng, J. Functional polyesters derived from alternating copolymerization of norbornene anhydride and epoxides. Polym. Chem. 2015, 6, 3586–3590. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Longo, J.M.; Rodríguez-Calero, G.G.; Coates, G.W. Development of highly active and regioselective catalysts for the copolymerization of epoxides with cyclic anhydrides: An unanticipated effect of electronic variation. J. Am. Chem. Soc. 2016, 138, 7107–7113. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, A.J.; Piccioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Wan, Z.-Q.; Longo, J.M.; Liang, L.-X.; Chen, H.-Y.; Hou, G.-J.; Yang, S.; Zang, W.-P.; Coates, G.W.; Lu, X.-B. Comprehensive understanding of polyester stereocomplexation. J. Am. Chem. Soc. 2019, 141, 14780–14787. [Google Scholar] [CrossRef]
- Nejad, E.H.; Paoniasari, A.; van Melis, C.G.W.; Koning, C.E.; Duchateau, R. Catalytic ring-opening copolymerization of limonene oxide and phthalic anhydride: Toward partially renewable polyesters. Macromolecules 2013, 46, 631–637. [Google Scholar] [CrossRef]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1, 4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Selective polymerization catalysis: Controlling the metal chain end group to prepare block copolyesters. J. Am. Chem. Soc. 2015, 137, 12179–12182. [Google Scholar] [CrossRef]
- Abbina, S.; Chidara, V.; Du, G. Ring opening copolymerization of styrene oxide and cyclic anhydrides using highly effective zinc amido-oxazolinate catalysts. ChemCatChem 2017, 9, 1343–1348. [Google Scholar] [CrossRef]
- Ellis, W.C.; Jung, Y.; Mulzer, M.; Di Girolamo, R.; Lobkovsky, E.B.; Coates, G.W. Copolymerization of CO2 and meso epoxides using enantioselective β-diiminate catalysts: A route to highly isotactic polycarbonates. Chem. Sci. 2014, 5, 4004–4011. [Google Scholar] [CrossRef]
- Liu, D.-F.; Wu, L.-Y.; Feng, W.-X.; Zhang, X.-M.; Wu, J.; Zhu, L.-Q.; Fan, D.-D.; Lu, X.-Q.; Shi, Q. Ring-opening copolymerization of CHO and MA catalyzed by mononuclear [Zn(L2)(H2O)] or trinuclear [Zn3(L2)2(OAc)2] complex based on the asymmetrical bis-Schiff-base ligand precursor. J. Mol. Catal. A Chem. 2014, 382, 136–145. [Google Scholar] [CrossRef]
- Brooks, B.A.; Lidston, C.A.; Coates, G.W. Mechanism-inspired design of bifunctional catalysts for the alternating ring-opening copolymerization of epoxides and cyclic anhydrides. J. Am. Chem. Soc. 2019, 141, 12760–12769. [Google Scholar] [CrossRef]
- Isnard, F.; Santulli, F.; Cozzolino, M.; Lamberti, M.; Pellecchia, C.; Mazzeo, M. Tetracoordinate aluminum complexes bearing phenoxy-based ligands as catalysts for epoxide/anhydride copolymerization: Some mechanistic insights. Catal. Sci. Technol. 2019, 9, 3090–3098. [Google Scholar] [CrossRef]
- Takenouchi, S.; Takasu, A.; Inai, Y.; Hirabayashi, T. Effects of geometrical difference of unsaturated aliphatic polyesters on their biodegradability ii. isomerization of poly(maleic anhydride-co-propylene oxide) in the presence of morpholine. Polym. J. 2002, 34, 36–42. [Google Scholar] [CrossRef]
- Saini, P.K.; Romain, C.; Zhu, Y.; Williams, C.K. Di-magnesium and zinc catalysts for the copolymerization of phthalic anhydride and cyclohexene oxide. Polym. Chem. 2014, 5, 6068–6075. [Google Scholar] [CrossRef]
- . Li, H.; Luo, H.; Zhao, J.; Zhang, G. Well-defined and structurally diverse aromatic alternating polyesters synthesized by simple phosphazene catalysis. Macromolecules 2018, 51, 2247–2257. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Zhang, G. Self-buffering organocatalysis tailoring alternating polyester. ACS Macro Lett. 2017, 6, 1094–1098. [Google Scholar] [CrossRef]
- Hu, L.-F.; Zhang, C.-J.; Wu, H.-L.; Yang, J.-L.; Liu, B.; Duan, H.-Y.; Zhang, X.-H. Highly active organic lewis pairs for the copolymerization of epoxides with cyclic anhydrides: Metal-free access to well-defined aliphatic polyesters. Macromolecules 2018, 51, 3126–3134. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Chen, X.-L.; Wang, B.; Pan, L.; Li, Y.-S. Metal-free, regioselective and stereoregular alternating copolymerization of monosubstituted epoxides and tricyclic anhydrides. Green Chem. 2018, 20, 3963–3973. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Asymmetric alternating copolymerization of meso-epoxides and cyclic anhydrides: Efficient access to enantiopure polyesters. J. Am. Chem. Soc. 2016, 138, 11493–11496. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure–property relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Chidara, V.K.; Bian, S.; Ugrinov, A.; Du, G. Synthesis of chiral C2-symmetric bimetallic zinc complexes of amido-oxazolinates and their application in copolymerization of co2 and cyclohexene oxide. ChemistrySelect 2016, 1, 3175–3183. [Google Scholar] [CrossRef]
- Abbina, S.; Du, G. Zinc-catalyzed highly isoselective ring opening polymerization of rac-lactide. ACS Macro Lett. 2014, 3, 689–692. [Google Scholar] [CrossRef]
- Shaik, M.; Peterson, J.; Du, G. Cyclic and linear polyhydroxylbutyrates from ring-opening polymerization of β-butyrolactone with amido-oxazolinate zinc catalysts. Macromolecules 2019, 52, 157–166. [Google Scholar] [CrossRef]
- Enthaler, S. Rise of the zinc age in homogeneous catalysis? ACS Catal. 2013, 3, 150–158. [Google Scholar] [CrossRef]
- Alonso-Fagundez, N.; Granados, M.L.; Mariscal, R.; Ojeda, M. Selective conversion of furfural to maleic anhydride and furan with Vox/Al2O3 catalysts. ChemSusChem 2012, 5, 1984–1990. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- Isnard, F.; Carratù, M.; Lamberti, M.; Venditto, V.; Mazzeo, M. Copolymerization of cyclic esters, epoxides and anhydrides: Evidence of the dual role of the monomers in the reaction mixture. Catal. Sci. Technol. 2018, 8, 5034–5043. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiter, M.; Adams, F.; Vagin, S.; Rieger, B. CO2-controlled one-pot synthesis of AB, ABA block, and statistical terpolymers from β-butyrolactone, epoxides, and CO2. J. Am. Chem. Soc. 2017, 139, 6787–6790. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.-Y.; Hu, L.-F.; Zhang, X.-H.; Du, B.-Y.; Xu, J.-T. Carbon dioxide-based copolymers with various architectures. Prog. Polym. Sci. 2018, 82, 120–157. [Google Scholar] [CrossRef]
- Li, H.; Luo, H.; Zhao, J.; Zhang, G. Sequence-selective terpolymerization from monomer mixtures using a simple organocatalyst. ACS Macro Lett. 2018, 7, 1420–1425. [Google Scholar] [CrossRef]
- Jeske, R.C.; Rowley, J.; Coates, G.W. Pre-rate-determining selectivity in the terpolymerization of epoxides, cyclic anhydrides, and CO2: A one-step route to diblock copolymers. Angew. Chem. Int. Ed. 2008, 47, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, C.; Zhang, T.; Xu, X.; Duan, R.; Luo, Y.; Sun, Z.; Pang, X.; Chen, X. One-pot synthesis of diblock polyesters by catalytic terpolymerization of lactide, epoxides, and anhydrides. Macromolecules 2019, 52, 3462–3470. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiter, M.; Mock, J.; Rieger, B. Terpolymerization of β-butyrolactone, epoxides, and CO2: Chemoselective CO2-switch and its impact on kinetics and material properties. Macromolecules 2019, 52, 8476–8483. [Google Scholar] [CrossRef]
- Abbina, S.; Du, G. Chiral Amido-Oxazolinate Zinc Complexes for asymmetric alternating copolymerization of CO2 and cyclohexene oxide. Organometallics 2012, 31, 7394–7403. [Google Scholar] [CrossRef]
- Binda, P.I.; Abbina, S.; Du, G. Modular synthesis of chiral β-diketiminato-type ligands containing 2-oxazoline moiety via Palladium-catalyzed amination. Synthesis 2011, 2609–2618. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 and polyesters are available from the authors. |
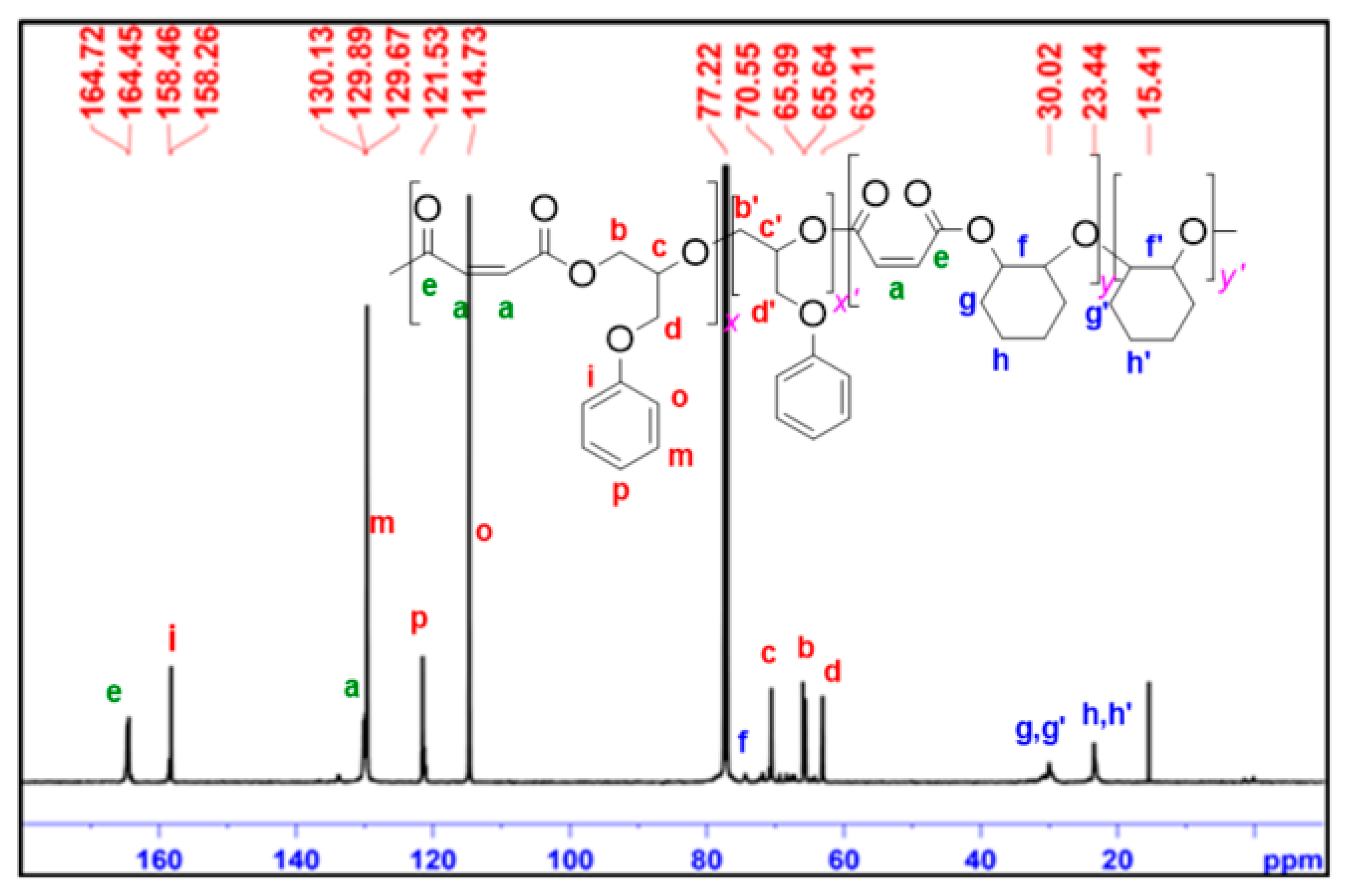
| Entry | [cat]:[CHO]:[MA] | Time | Convn [b] | Ester (%) [b] | Mn[c] (kg/mol) | Đ [c] |
|---|---|---|---|---|---|---|
| 1 | 0:100:100 | 24 | 0 | - | - | - |
| 2 | 1:100:100 | 16 | 100 | 53 | 5.9 | 1.29 |
| 3 [d] | 1:100:100 | 24 | 100 | 73 | 2.7 | 1.43 |
| 4 | 1:200:200 | 9 | 100 | 61 | 2.3 | 1.20 |
| 5 [e] | 1:200:200 | 16 | 96 | 65 | 1.2 | 1.19 |
| 6 [f] | 1:200:200 | 0.92 | 88 | 14 | 3.8 | 1.82 |
| 7 | 1:200:400 | 1 | 100 | 46 | 0.78 | 1.12 |
| 8 | 1:1000:1000 | 1.5 | 100 | 34 | 1.2 | 1.28 |
| Entry | Epoxides | Time (h) | Convn (%) | Ester (%) | Mn (kg mol−1) | Đ |
|---|---|---|---|---|---|---|
| 1 | SO | 0.33 | 100 | 76 | 2.6 | 1.13 |
| 2 | CHO | 9 | 100 | 61 | 2.3 | 1.20 |
| 3 | PGE | 48 | 100 | 95 | 4.8 | 2.05 |
| Entry | Cat | Anhydride | Time (h) | Convn (%) [b] | Ester (%) [b] | Mn[c] (kg mol−1) | Đ [c] |
|---|---|---|---|---|---|---|---|
| 1 | 1 | MA | 9 | 100 | 61 | 2.3 | 1.20 |
| 2 | 2 | MA | 3.5 | 100 | 44 | 2.6 | 1.30 |
| 3 | 3 | MA | 2 | 100 | 37 | 5.3 | 1.30 |
| 4 | 4 | MA | 2 | 100 | 40 | 2.9 | 1.34 |
| 5 | 1 | PA | 3 | 93 | 37 | 1.5 | 1.17 |
| 6 | 2 | PA | 5.5 | 97 | 40 | 1.8 | 1.24 |
| 7 | 3 | PA | 3 | 100 | 41 | 1.9 | 1.15 |
| 8 | 4 | PA | 4.5 | 97 | 44 | 1.8 | 1.20 |
| 9 | 1 | SA | 9 | 98 | 60 | 3.7 | 1.24 |
| 10 | 2 | SA | 5.5 | 78 | 74 | 4.2 | 1.30 |
| 11 | 3 | SA | 9 | 91 | 55 | 4.2 | 1.15 |
| 12 | 4 | SA | 11 | 98 | 67 | 3.3 | 1.14 |
| Run | Step 1 | Step 2 | Ester Ratio [b] | Mn[c] (kg mol−1) | Đ [c] |
|---|---|---|---|---|---|
| 1 | CHO/MA | PGE/MA | CHO:PGE = 1.0:2.3 | 4.8 | 2.21 |
| 2 | PGE/MA | CHO/MA | CHO:PGE = 1.0:2.7 | 10.3 | 1.88 |
| 3 | SO/MA | CHO/MA | CHO:SO = 1.0:3.8 | 3.9 | 1.41 |
| 4 | CHO/PGE/MA | CHO:PGE = 1.0:1.7 | 4.3 | 1.88 | |
| 5 | CHO/SO/MA | SO:CHO = 1.0:0.94 | 2.0 | 1.34 | |
| 6 | SO/PGE/MA | SO:PGE = 1.0:1.0 | 1.9 | 1.40 |
| Entry | Polymer | Tg | T−5% | T−50% |
|---|---|---|---|---|
| Table 2, entry 1 | p(SO-MA) | 39 | 256 | 314 |
| Table 2, entry 2 | p(CHO-MA) | 63 | 211 | 349 |
| Table 2, entry 3 | p(PGE-MA) | 30 | 244 | 387 |
| Table 4, entry 1 | p(CHO-PGE-MA) | 41 | 213 | 375 |
| Table 4, entry 2 | p(CHO-PGE-MA) | 44 | 281 | 370 |
| Table 4, entry 3 | p(SO-CHO-MA) | 61 | 219 | 352 |
| Table 4, entry 4 | p(CHO-PGE-MA) | 43 | 208 | 366 |
| Table 4, entry 5 | p(CHO-SO-MA) | 59 | 156 | 316 |
| Table 4, entry 6 | p(SO-PGE-MA) | 24 | 199 | 350 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, M.; Chidara, V.K.; Abbina, S.; Du, G. Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides. Molecules 2020, 25, 4044. https://doi.org/10.3390/molecules25184044
Shaik M, Chidara VK, Abbina S, Du G. Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides. Molecules. 2020; 25(18):4044. https://doi.org/10.3390/molecules25184044
Chicago/Turabian StyleShaik, Muneer, Vamshi K. Chidara, Srinivas Abbina, and Guodong Du. 2020. "Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides" Molecules 25, no. 18: 4044. https://doi.org/10.3390/molecules25184044
APA StyleShaik, M., Chidara, V. K., Abbina, S., & Du, G. (2020). Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides. Molecules, 25(18), 4044. https://doi.org/10.3390/molecules25184044