Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies
Abstract
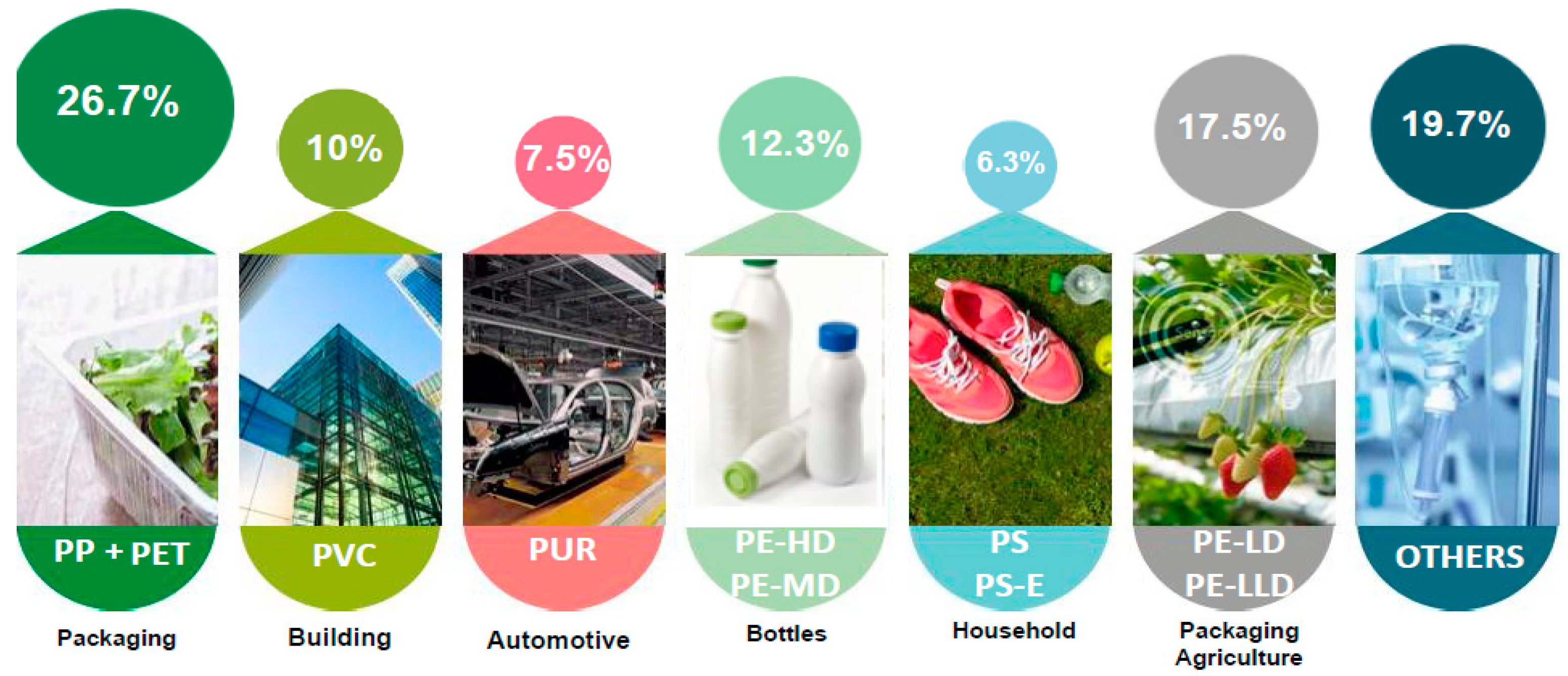
1. Background: Introducing Plastics and Microplastics
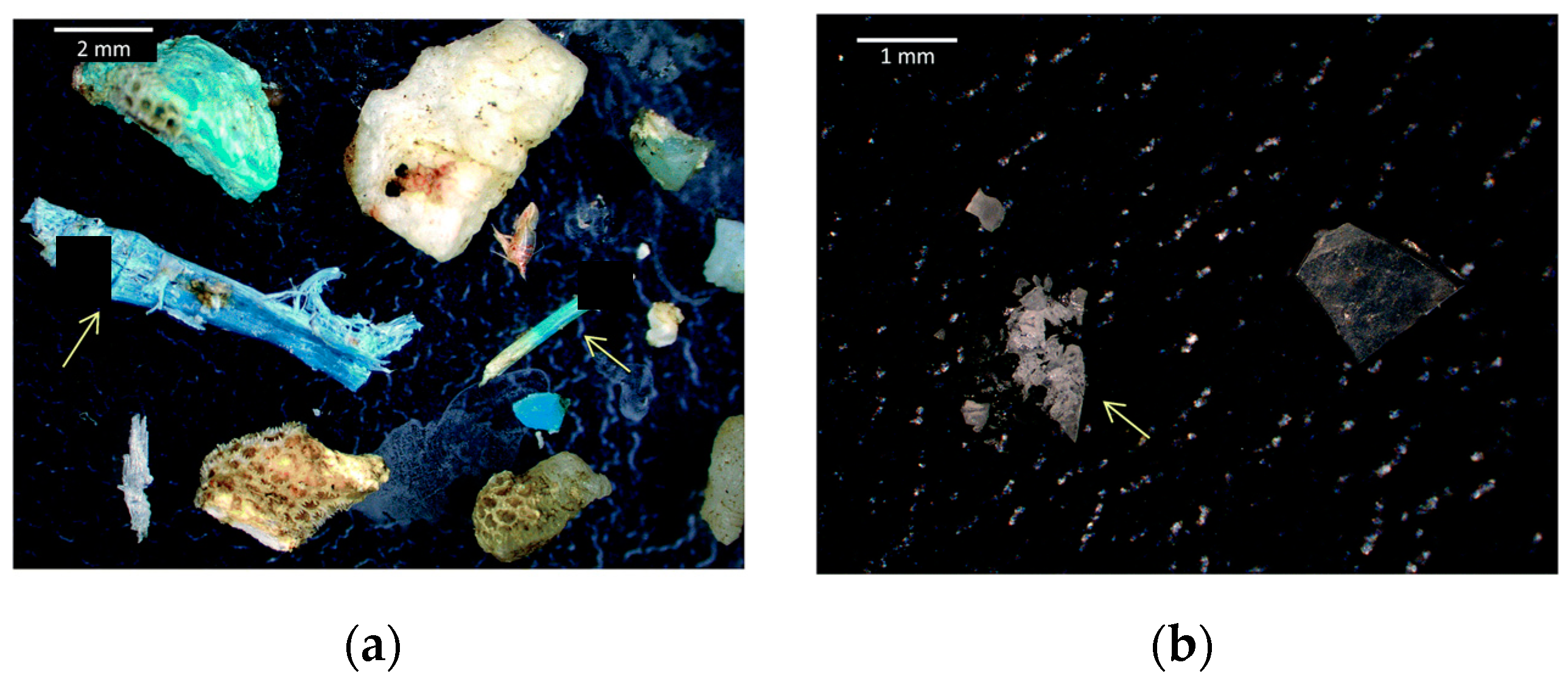
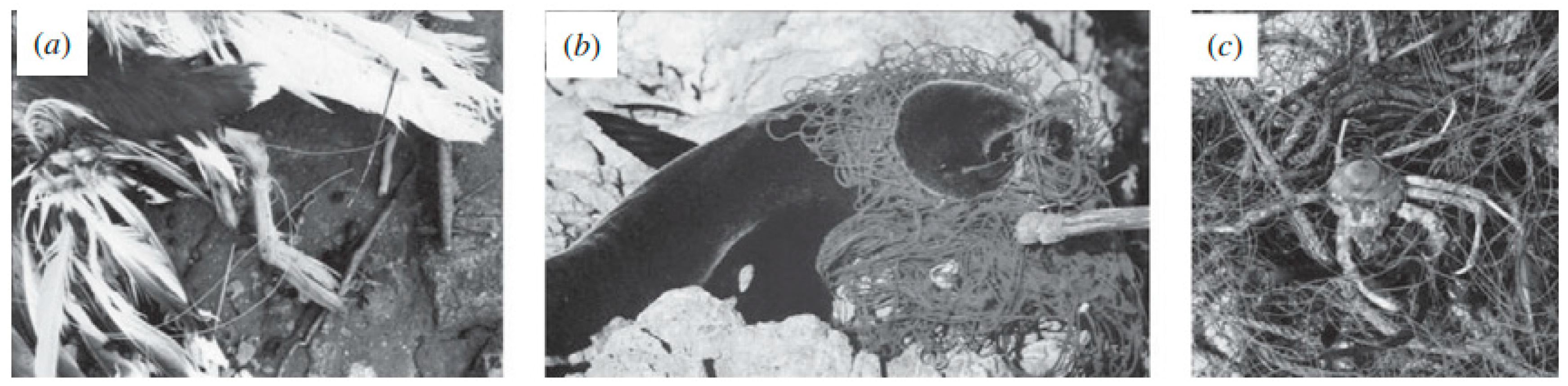
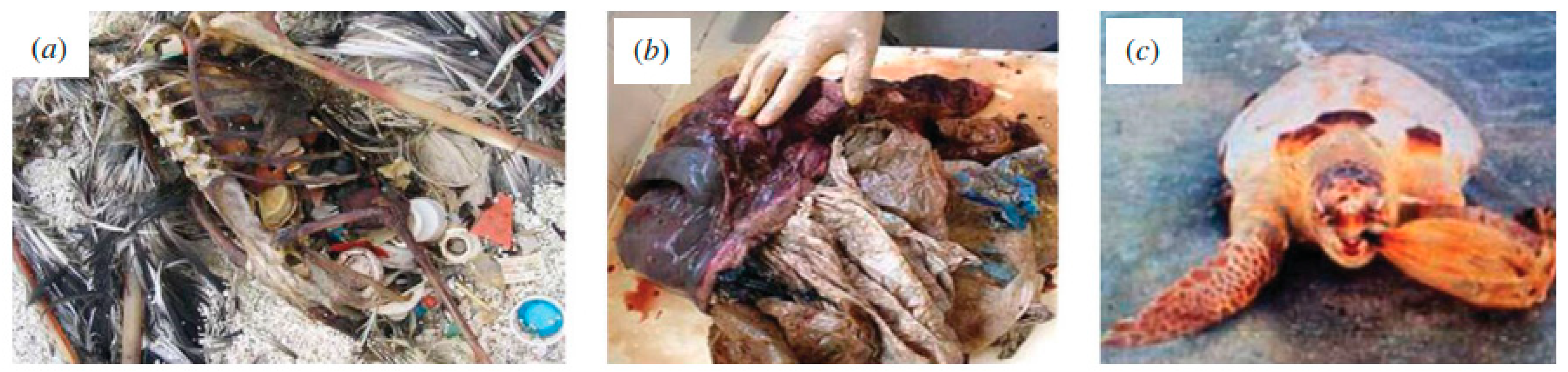
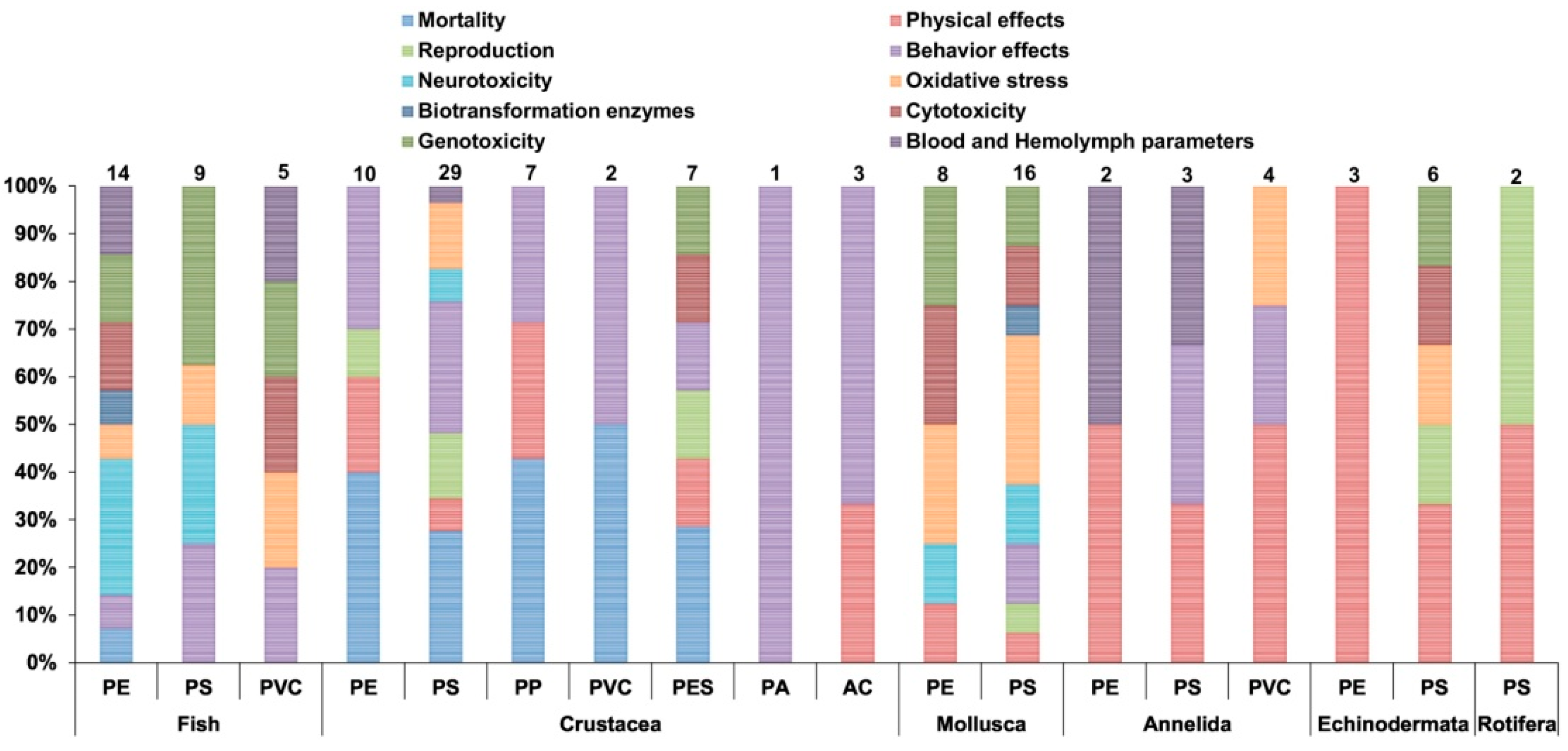
2. Plastics, Microplastics and Their Impact in the Ecosystems
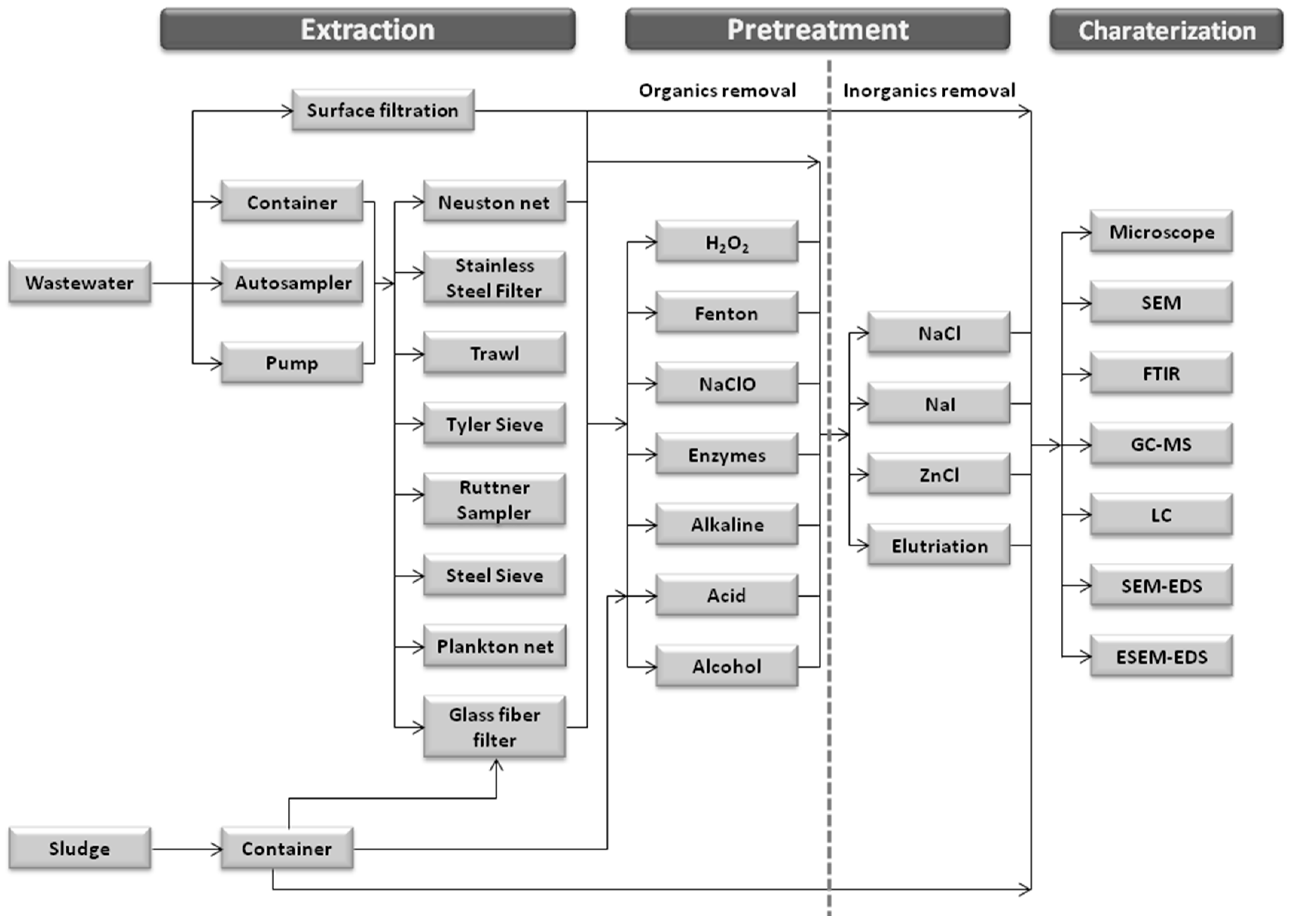
3. Detection of Microplastics in Wastewater Plants
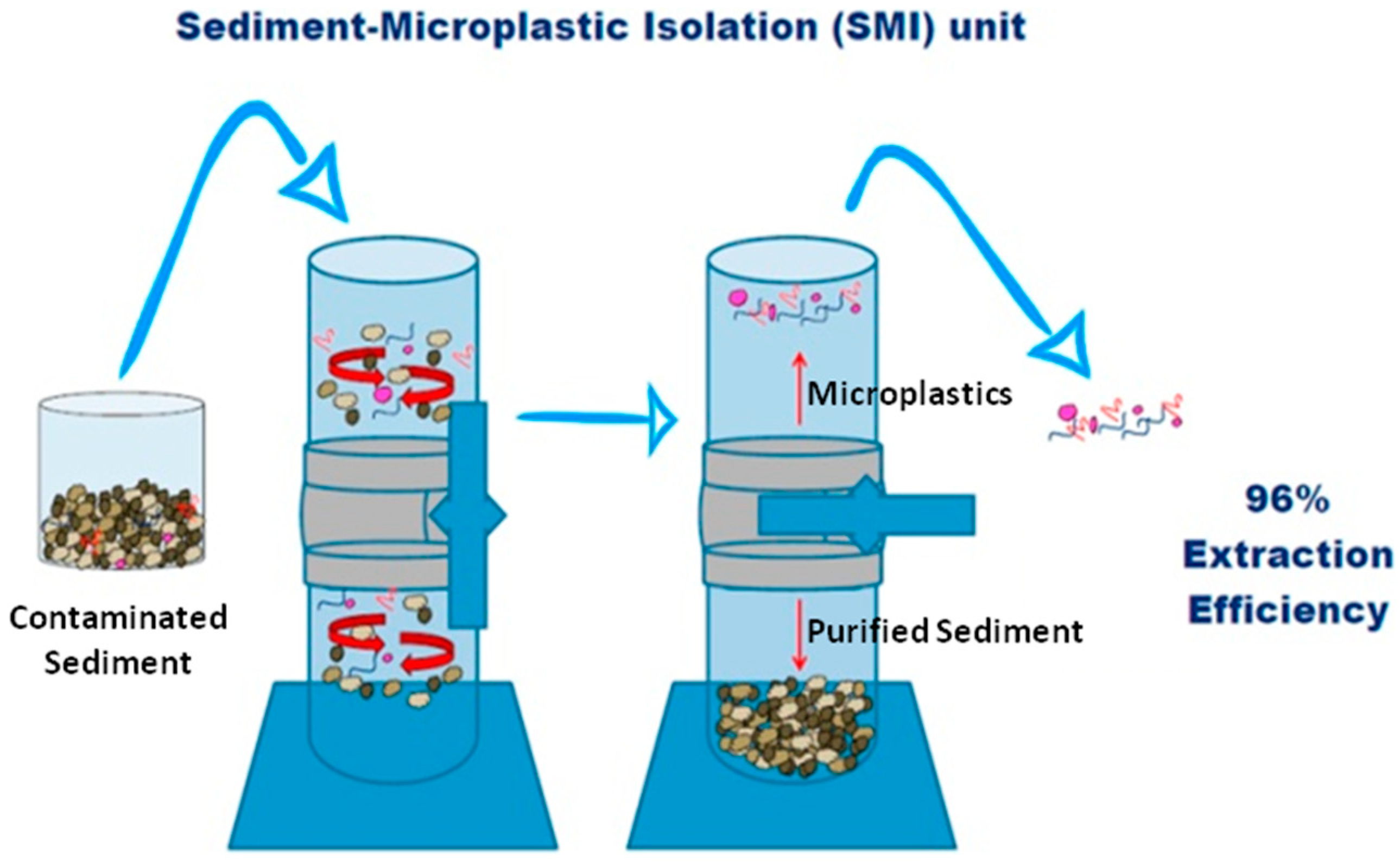
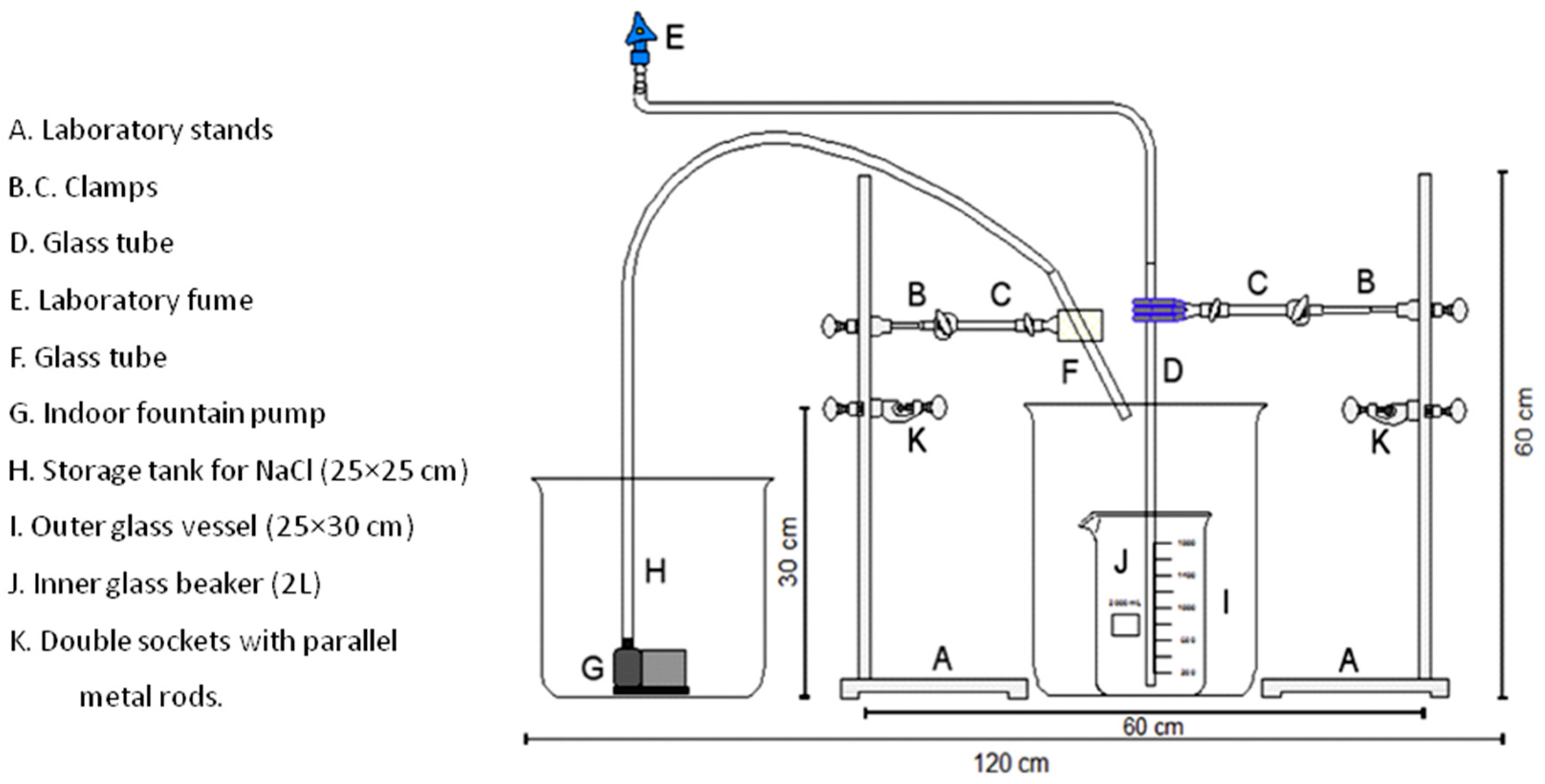
4. Removal of Microplastics: From Laboratory to Large Scale
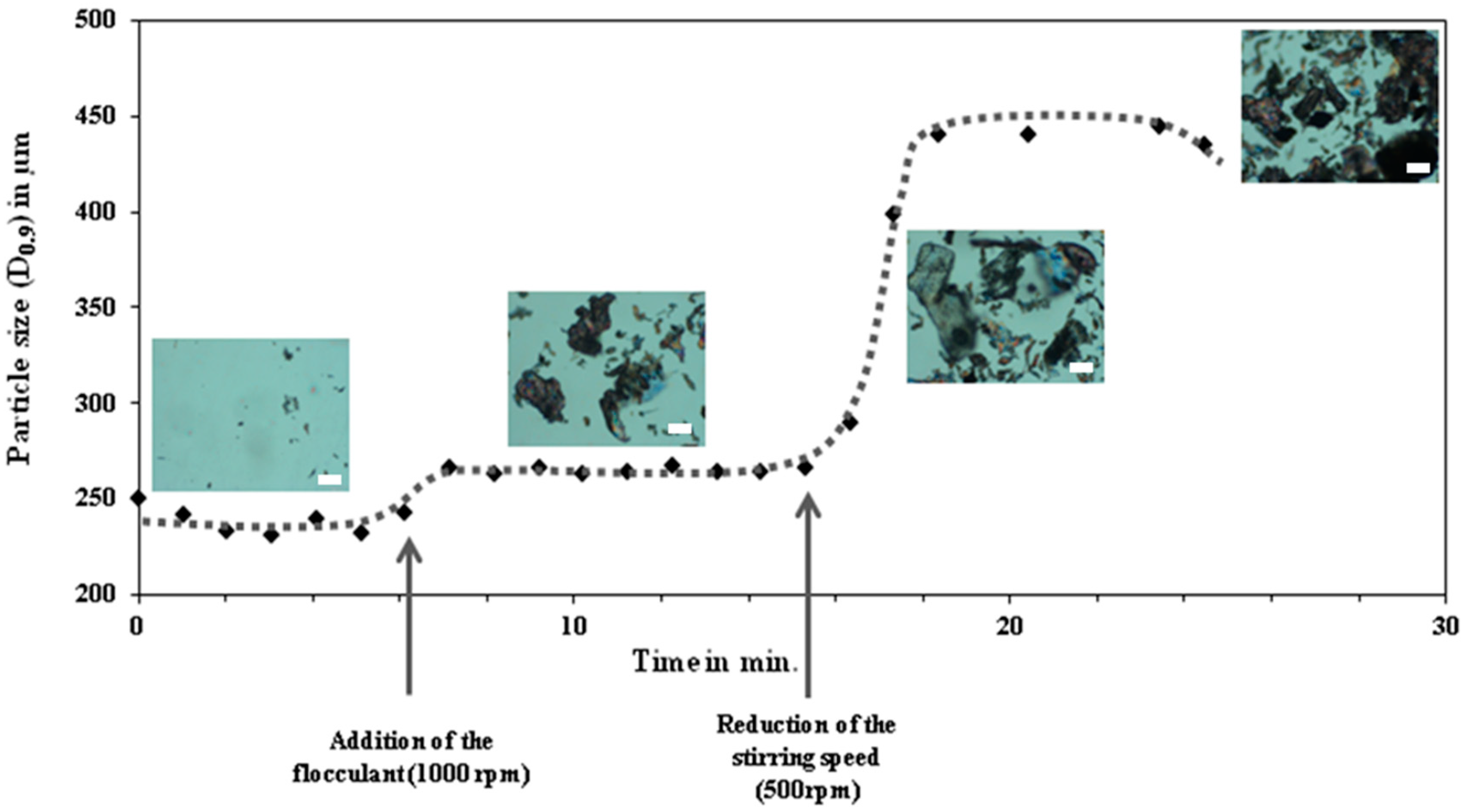
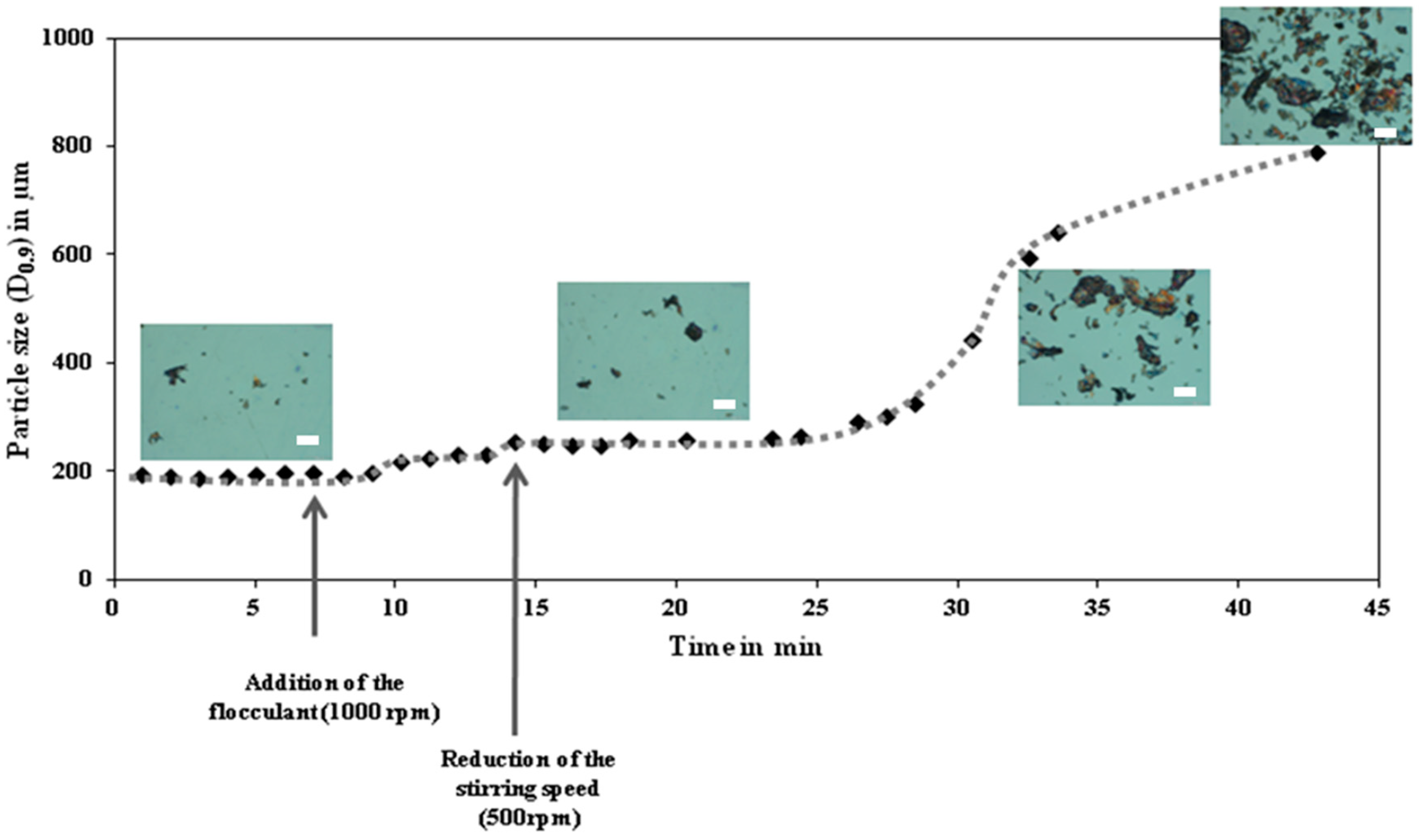
5. Flocculation and Its Relevance on Particles Separation: Background
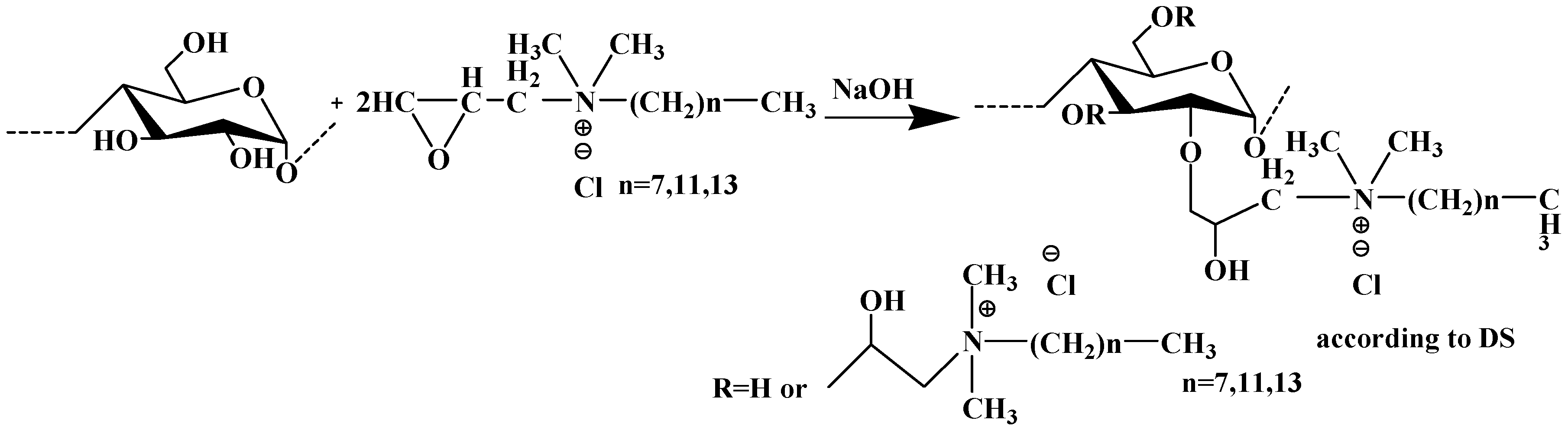
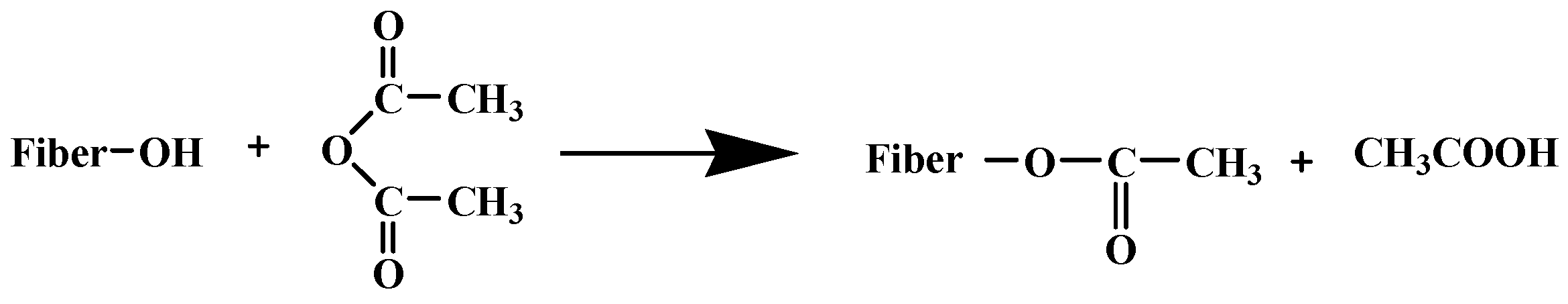
6. Bio-Based Solutions for Microplastics Removal: Current Trends and Future Perspectives
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Academies, S. A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Rima, F.J.G.S. Setting the facts straight on plastics. Available online: https://www.weforum.org/agenda/2019/10/plastics-what-are-they-explainer/ (accessed on 4 February 2020).
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Guardian, T. A million bottles a minute: World’s plastic binge ‘as dangerous as climate change’. Available online: https://www.theguardian.com/environment/2017/jun/28/a-million-a-minute-worlds-plastic-bottle-binge-as-dangerous-as-climate-change (accessed on 7 December 2019).
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in drinking water treatmen—Current knowledge and research needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plants. J. Hazard. Mater. 2019, 367, 504–512. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kwon, O.Y.; Lee, K.-W.; Song, Y.K.; Shim, W.J. Marine neustonic microplastics around the southeastern coast of Korea. Mar. Pollut. Bull. 2015, 96, 304–312. [Google Scholar] [CrossRef]
- Heo, N.W.; Hong, S.H.; Han, G.M.; Hong, S.; Lee, J.; Song, Y.K.; Jang, M.; Shim, W.J. Distribution of small plastic debris in cross-section and high strandline on Heungnam beach, South Korea. Ocean Sci. J. 2013, 48, 225–233. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Allsopp, M.; Walters, A.; Santillo, D.G. Plastic Debris in the World’s Oceans; GreenPeace: Amsterdam, the Netherlands, 2006; p. 44. [Google Scholar]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Wang, Z.-M.; Ghosal, S.; Rochman, C.; Gassel, M.; Wall, S. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal. Methods 2017, 9, 1479–1490. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Hamid, S.H.; Amin, M.B.; Maadhah, A.G. Handbook of Polymer Degradation; M. Dekker: New York, NY, USA, 1992. [Google Scholar]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc. R. Soc. B: Biol. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In YOUMARES 9—The Oceans: Our Research, Our Future, Proceedings of the 2018 Conference for YOUng MArine RESearcher, Oldenburg, Germany; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 101–120. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Rowe, D.; Thompson, R.; Galloway, T. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Björkner, B. Plastic Materials. In Textbook of Contact Dermatitis; Rycroft, R.J.G., Menné, T., Frosch, P.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 539–572. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.v.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.M.; Kuriyama, S.N.; Taylor, J.A.; Saal, F.S.v. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and Nano-plastics and Human Health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. [Google Scholar] [CrossRef]
- Koelmans, B.; Pahl, S.; Backhaus, T.; Bessa, F.; Calster, G.V.; Contzen, N.; Cronin, R.; Galloway, T.; Hart, A.; Henderson, L.; et al. The Best Available Evidence Suggests that Microplastics and Nanoplastics Do not Pose a Widespread Risk to Humans or the Environment, except in Small Pockets. But that Evidence is Limited, and the Situation Could Change if Pollution Continues at the Current Rate; European Comission: Berlin, Germany, 2019; p. 176. [Google Scholar]
- Bujnicki, J.; Dykstra, P.; Fortunato, E.; Grobert, N.; Heuer, R.-D.; Keskitalo, C.; Nurse, P. Environmental and Health Risks of Microplastic Pollution; European Commission: Brussels, Belgium, 2019; p. 64. [Google Scholar]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Kalčíková, G.; Alič, B.; Skalar, T.; Bundschuh, M.; Gotvajn, A. Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188. [Google Scholar] [CrossRef]
- Morrison, C.T.D. Invisibles. Available online: https://orbmedia.org/stories/Invisibles_plastics (accessed on 3 December 2019).
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Lots, F.; Behrens, P.; Vijver, M.; Horton, A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Olsen, Y.; Mitchell, R.; Davis, A.; Rowland, S.; John, A.; McGonigle, D.F.; Russell, A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- O’Brine, T.; Thompson, R.O.; Brine, T. Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 2010, 60, 2279–2283. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kunz, A.; Shim, W.; Walther, B. Microplastic contamination of table salts from Taiwan, including a global review. Sci. Rep. 2019, 9, s41598¨Cs019. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
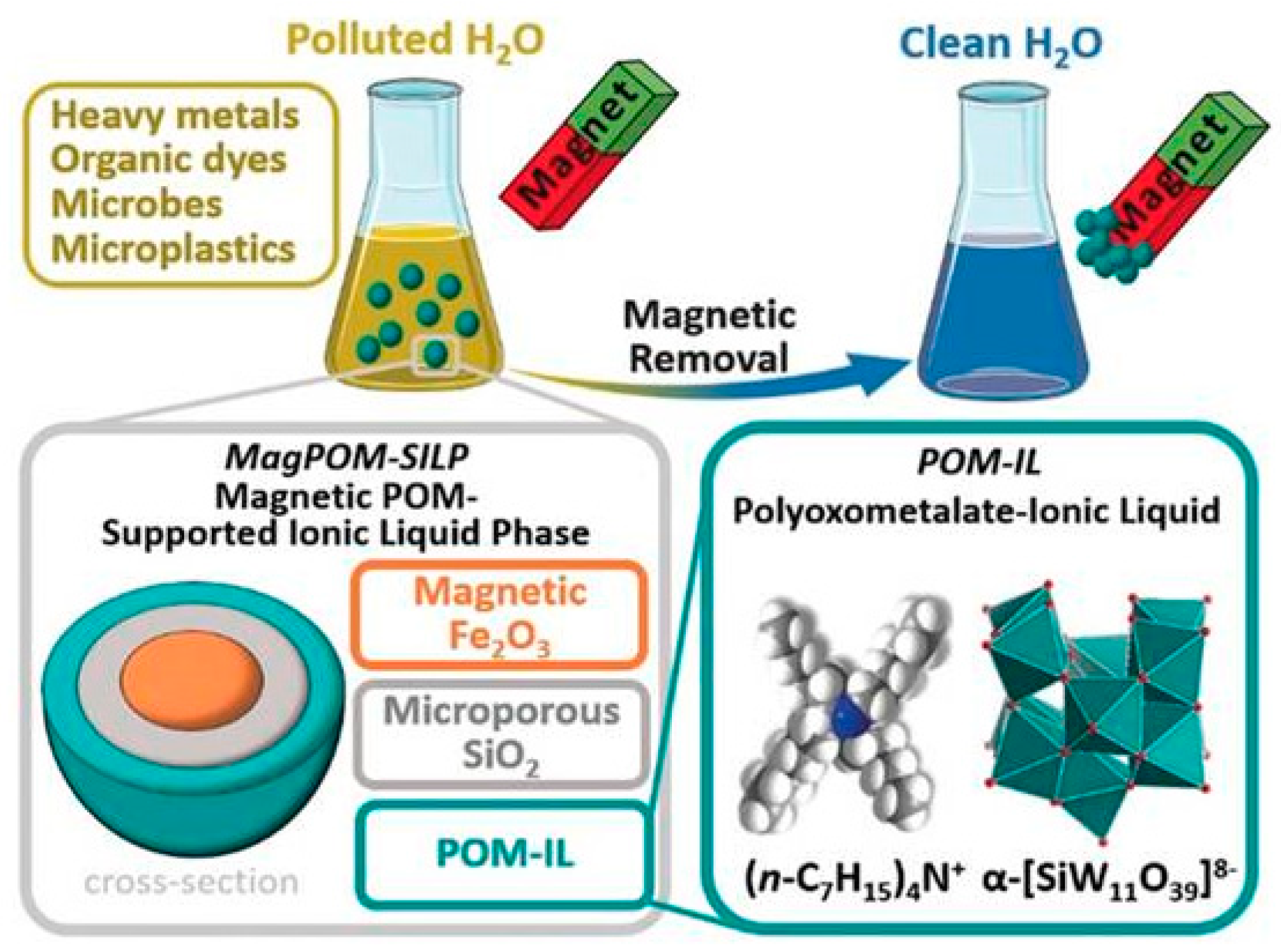
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angewandte Chemie Int. Ed. 2020, 59. [Google Scholar] [CrossRef] [PubMed]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, C.; Huang, Z. Sedimentation of nanoplastics from water with Ca/Al dual flocculants: Characterization, interface reaction, effects of pH and ion ratios. Chemosphere 2020, 252, 126450. [Google Scholar] [CrossRef]
- Suopajärvi, T. Functionalized Nanocelluloses in Wastewater Treatment Applications. Acta Univ. Ouluensis C 2015, 526. [Google Scholar]
- Wei, Y.; Cheng, F.; Zheng, H. Synthesis and flocculating properties of cationic starch derivatives. Carbohydr. Polym. 2008, 74, 673–679. [Google Scholar] [CrossRef]
- Merayo, N.; Balea, A.; de la Fuente, E.; Blanco, Á.; Negro, C. Interactions between cellulose nanofibers and retention systems in flocculation of recycled fibers. Cellulose 2017, 24, 677–692. [Google Scholar] [CrossRef]
- Cadotte, M.; Tellier, M.-E.; Blanco, A.; Fuente, E.; van de Ven, T.G.M.; Paris, J. Flocculation, Retention and Drainage in Papermaking: A Comparative Study of Polymeric Additives. Can. J. Chem. Eng. 2007, 85, 240–248. [Google Scholar] [CrossRef]
- Blanco, A.; Negro, C.; Fuente, E.; Tijero, J. Effect of Shearing Forces and Flocculant Overdose on Filler Flocculation Mechanisms and Floc Properties. Ind. Eng. Chem. Res. 2005, 44, 9105–9112. [Google Scholar] [CrossRef]
- Lourenço, A.D.S. Development of novel flocculants to treat oily waters using health-friendly processes. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2018. [Google Scholar]
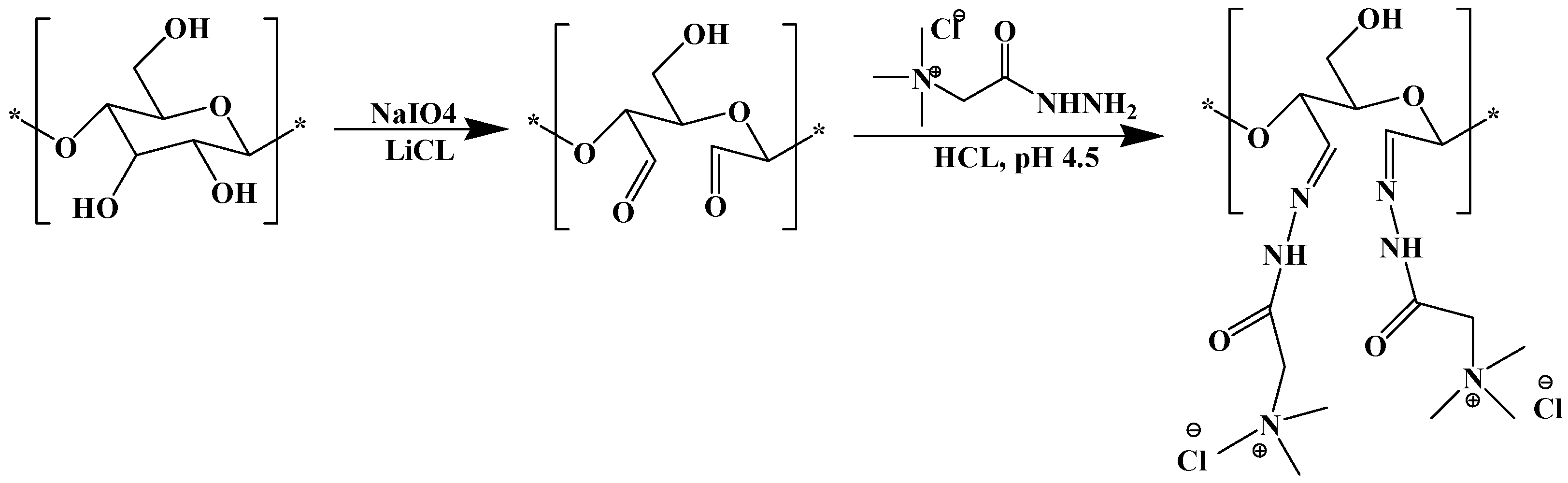
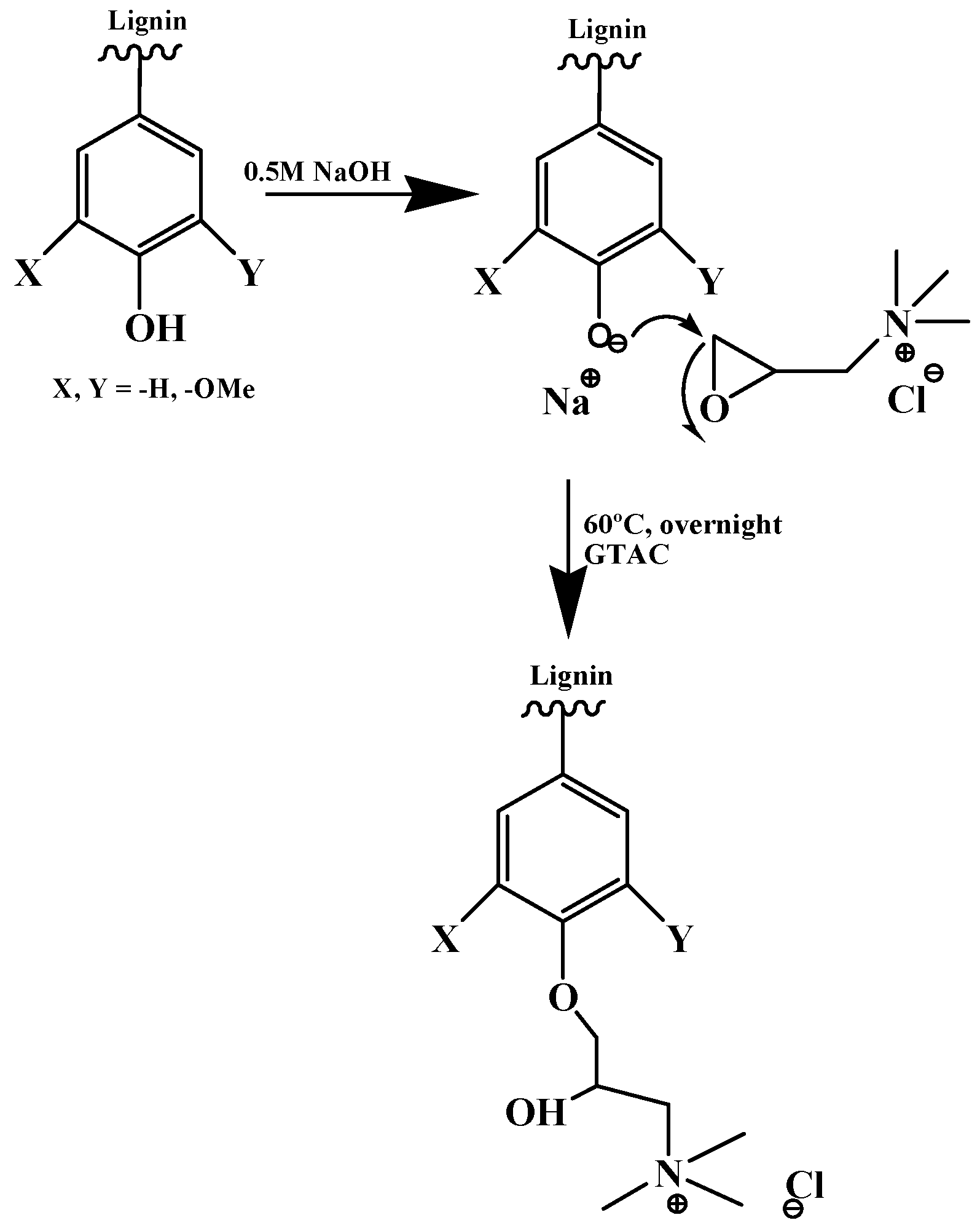
- Wahlström, R.; Kalliola, A.; Heikkinen, J.; Kyllönen, H.; Tamminen, T. Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications. Ind. Crop. Prod. 2017, 104, 188–194. [Google Scholar] [CrossRef]
- Grenda, K.; Gamelas, J.; Arnold, J.; Cayre, O.; Rasteiro, M. Evaluation of Anionic and Cationic Pulp-Based Flocculants With Diverse Lignin Contents for Application in Effluent Treatment From the Textile Industry: Flocculation Monitoring. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, I.; Ferreira, P.J.; Garcia, F.A.; Reis, M.S.; Pereira, A.C.; Wandrey, C.; Ahmadloo, H.; Amaral, J.L.; Hunkeler, D.; Rasteiro, M.G. An experimental design methodology to evaluate the importance of different parameters on flocculation by polyelectrolytes. Powder Technol. 2013, 238, 2–13. [Google Scholar] [CrossRef]
- Lourenço, A.; Reis, M.S.; Arnold, J.; Rasteiro, M.G. Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure. Processes 2020, 8, 349. [Google Scholar] [CrossRef]
- Laszlo, J.A. Solubility and Dye-Binding Properties of Quaternized and Peroxidase-Polymerized Kraft Lignin. Environ. Technol. 1999, 20, 607–615. [Google Scholar] [CrossRef]
- Kong, F.; Parhiala, K.; Wang, S.; Fatehi, P. Preparation of cationic softwood kraft lignin and its application in dye removal. Eur. Polym. J. 2015, 67, 335–345. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef] [PubMed]
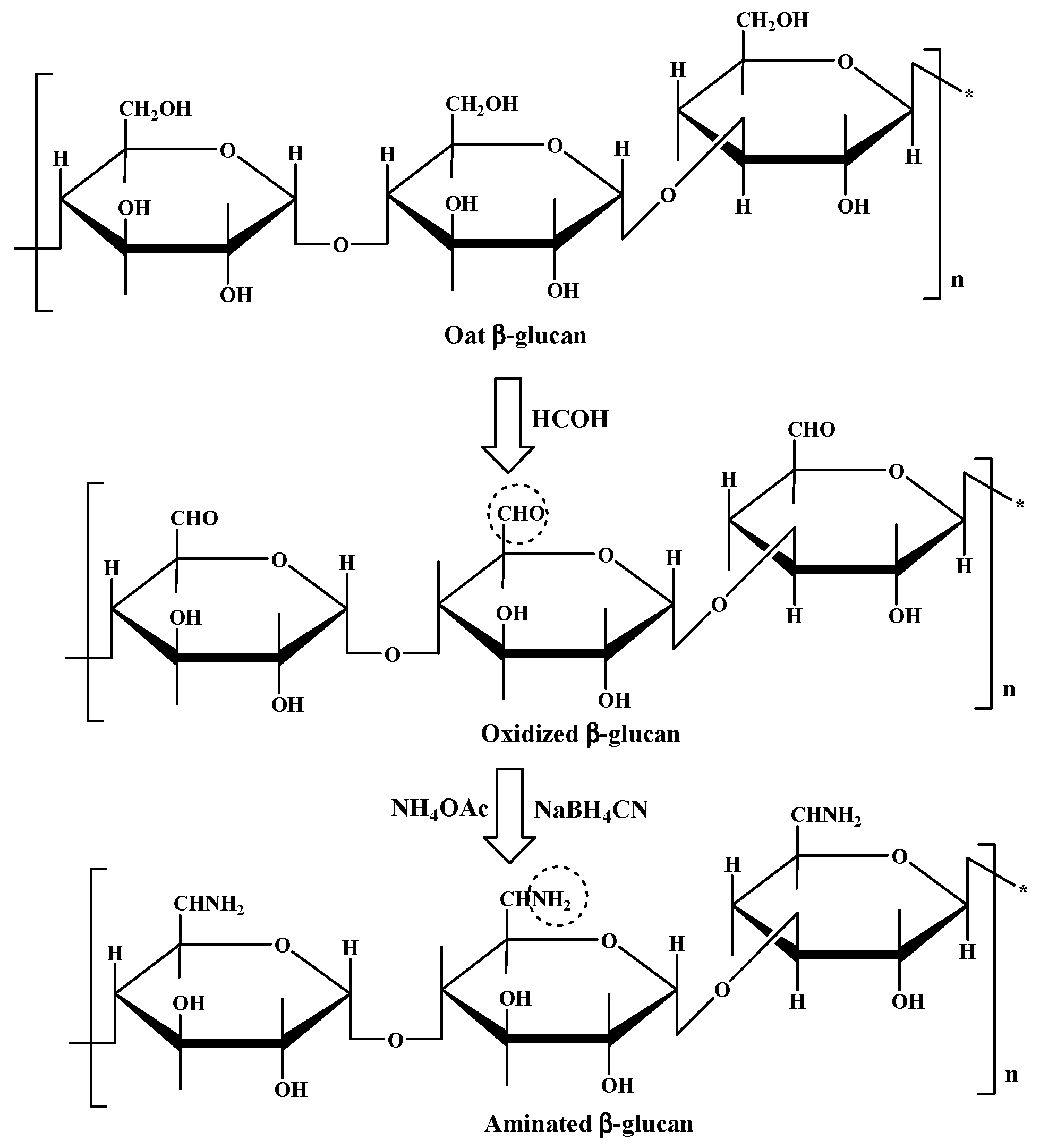
- Shin, M.S.; Lee, S.; Lee, K.Y.; Lee, H.G. Structural and Biological Characterization of Aminated-Derivatized Oat β-Glucan. J. Agric. Food Chem. 2005, 53, 5554–5558. [Google Scholar] [CrossRef] [PubMed]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Flocculation of silica nanoparticles by natural, wood-based polyelectrolytes. Sep. Purif. Technol. 2020, 231, 115888. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Rasteiro, M.G. Environmentally friendly cellulose-based polyelectrolytes in wastewater treatment. Water Sci. Technol. 2017, 76, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Grenda, K.; Gamelas, J.A.F.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Cationization of Eucalyptus wood waste pulps with diverse lignin contents for potential application in colored wastewater treatment. RSC Adv. 2019, 9, 34814–34826. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Hunkeler, D.; Gamelas, J.A.F.; Rasteiro, M.G. Tannin-based Coagulants from Laboratory to Pilot Plant Scales for Coloured Wastewater Treatment. BioResources 2018, 13, 21. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, Y.; Pu, Y.; Zheng, Z.; Shi, C.; Huang, X. Synthesis and characterization of quaternized β-chitin. Carbohydr. Res. 2010, 345, 1609–1612. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Hussein, M.Z.B.; Oksman, K. Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 2010, 17, 299–307. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, S.; Alves, L.; Medronho, B.; Romano, A.; Rasteiro, M.d.G. Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies. Molecules 2020, 25, 3954. https://doi.org/10.3390/molecules25173954
Magalhães S, Alves L, Medronho B, Romano A, Rasteiro MdG. Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies. Molecules. 2020; 25(17):3954. https://doi.org/10.3390/molecules25173954
Chicago/Turabian StyleMagalhães, Solange, Luís Alves, Bruno Medronho, Anabela Romano, and Maria da Graça Rasteiro. 2020. "Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies" Molecules 25, no. 17: 3954. https://doi.org/10.3390/molecules25173954
APA StyleMagalhães, S., Alves, L., Medronho, B., Romano, A., & Rasteiro, M. d. G. (2020). Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies. Molecules, 25(17), 3954. https://doi.org/10.3390/molecules25173954








