Bioinspired Heterocyclic Partnership in a Cyanine-Type Acidichromic Chromophore
Abstract
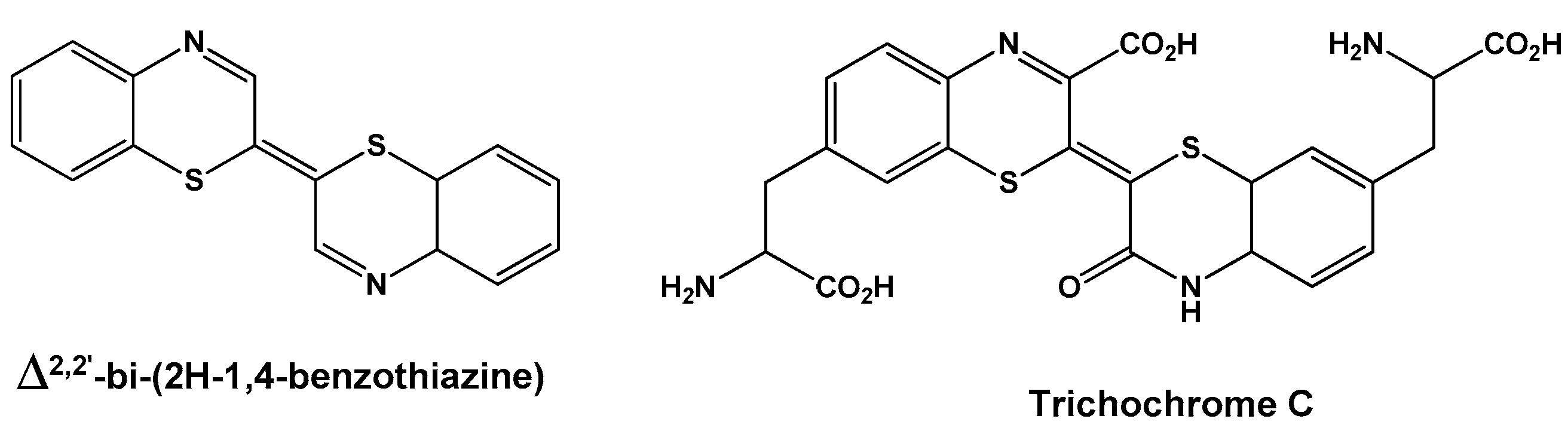
1. Introduction
2. Results and Discussion
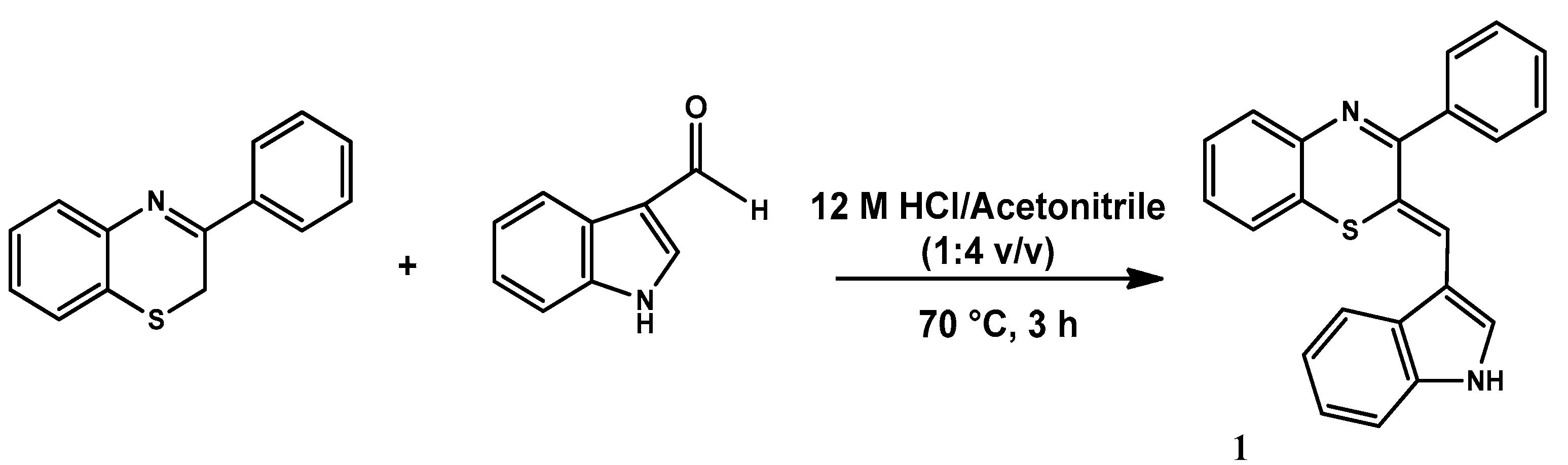
2.1. Synthesis and Structural Characterization of Cyanine 1
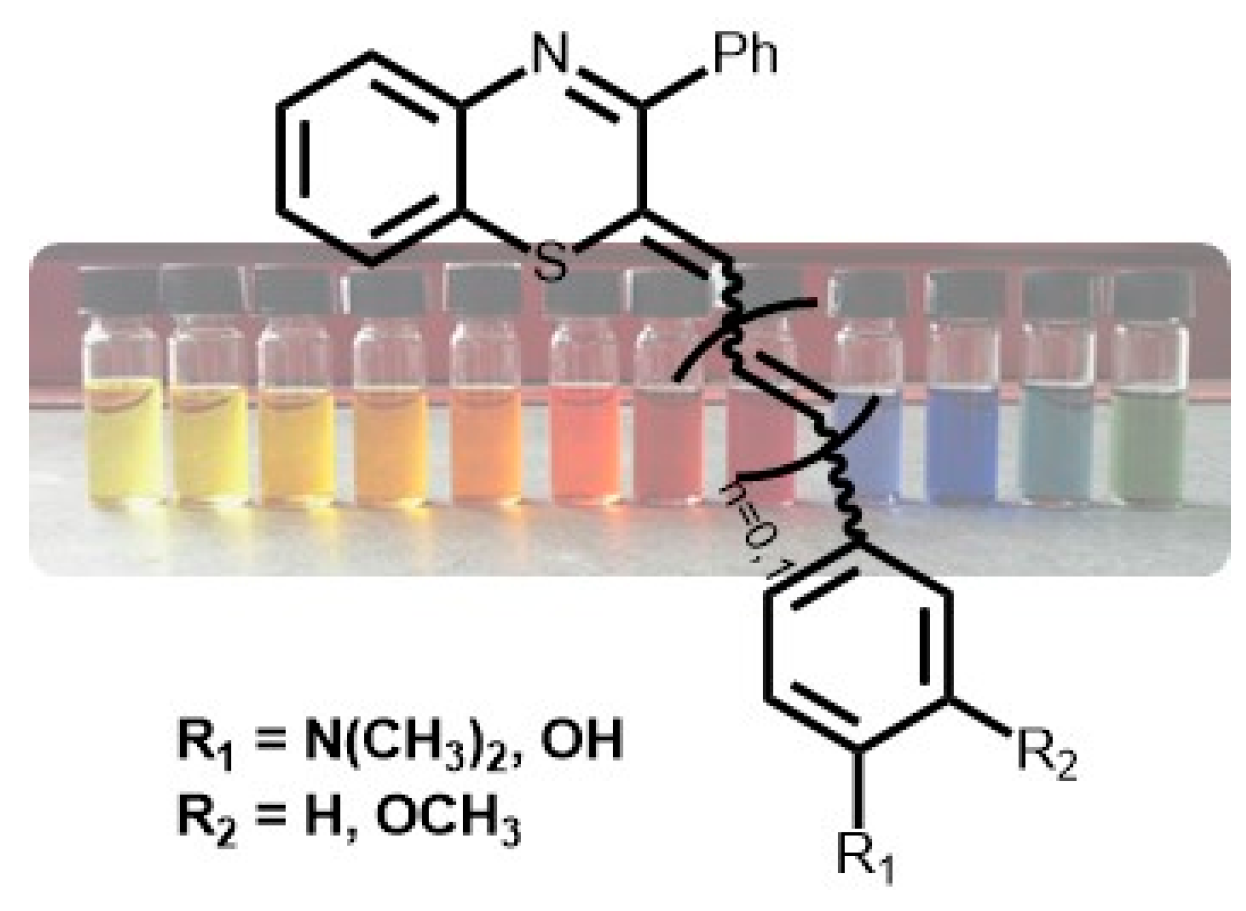
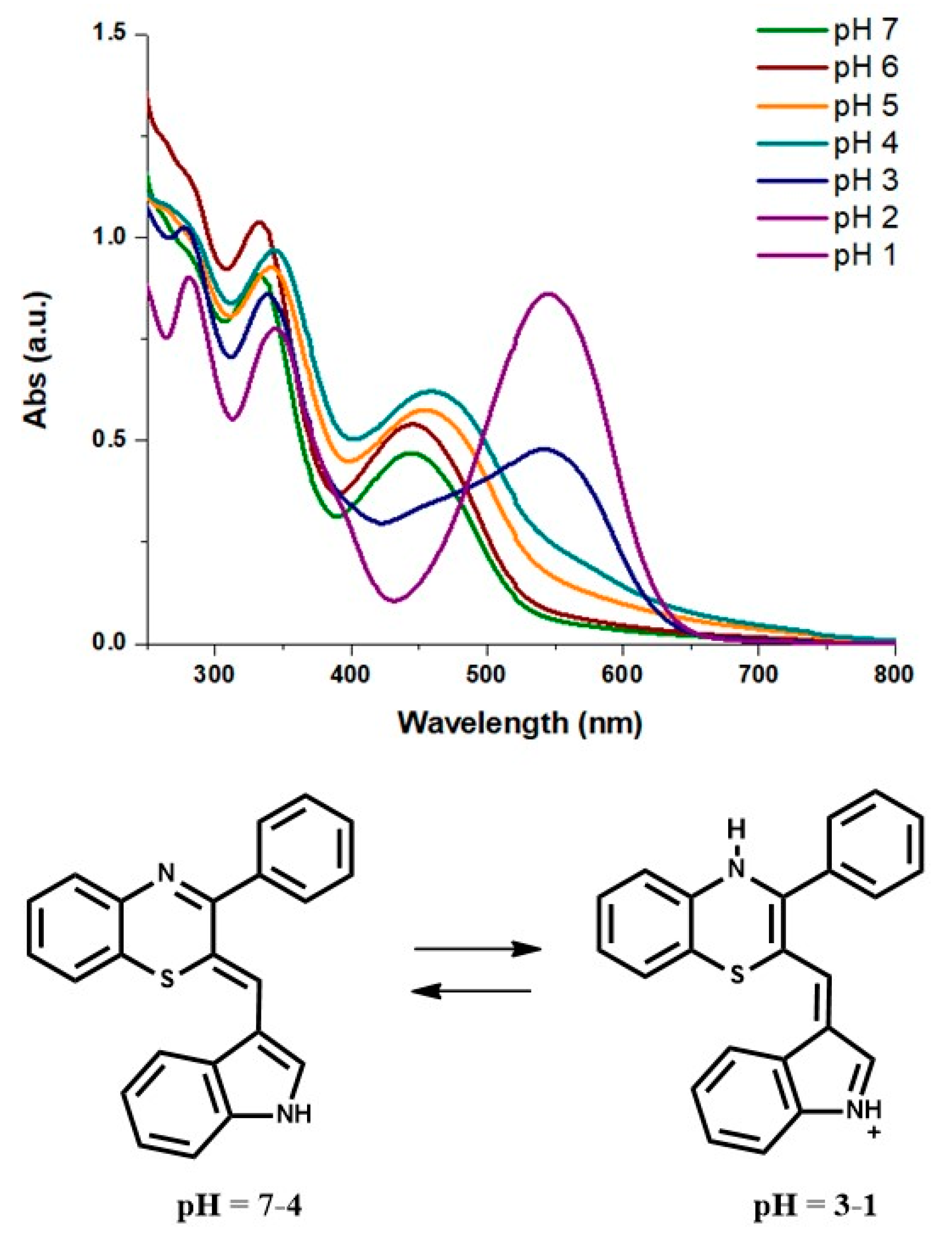
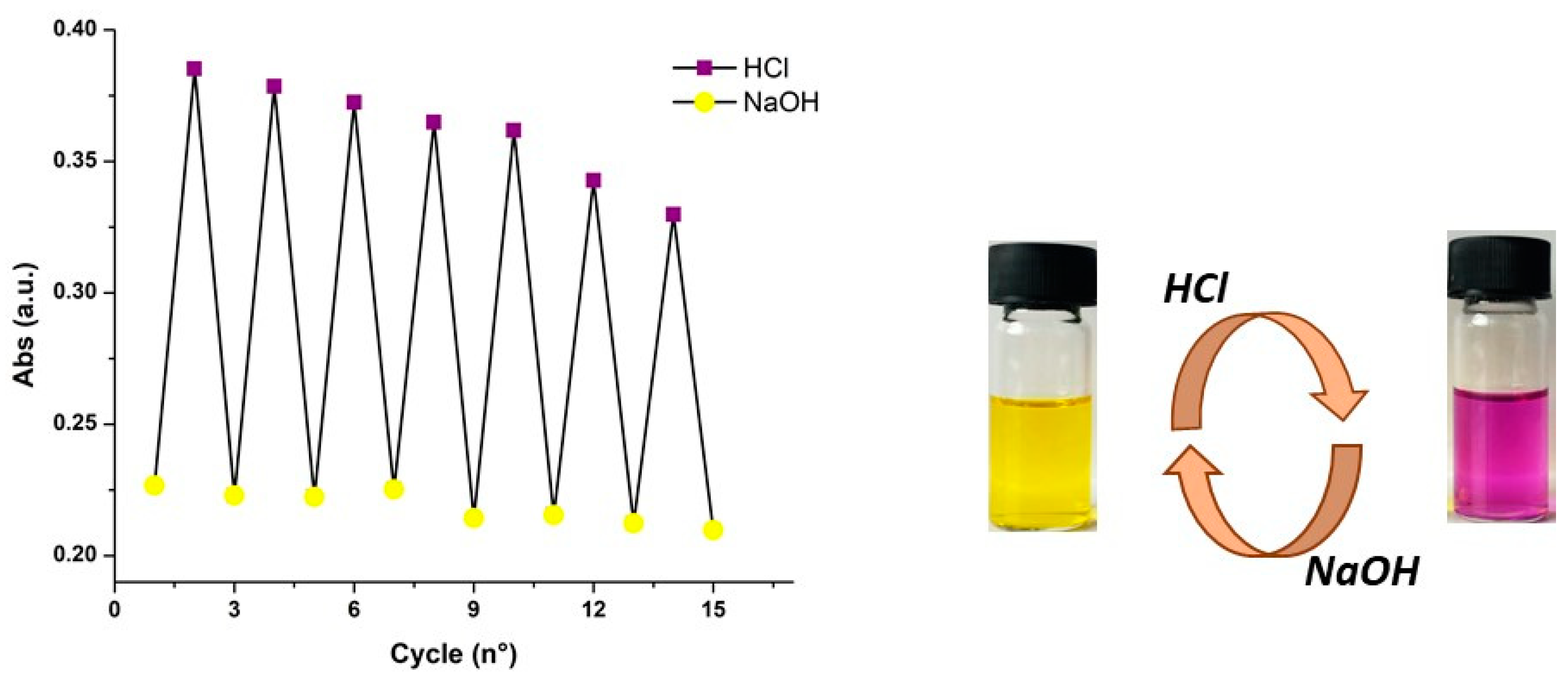
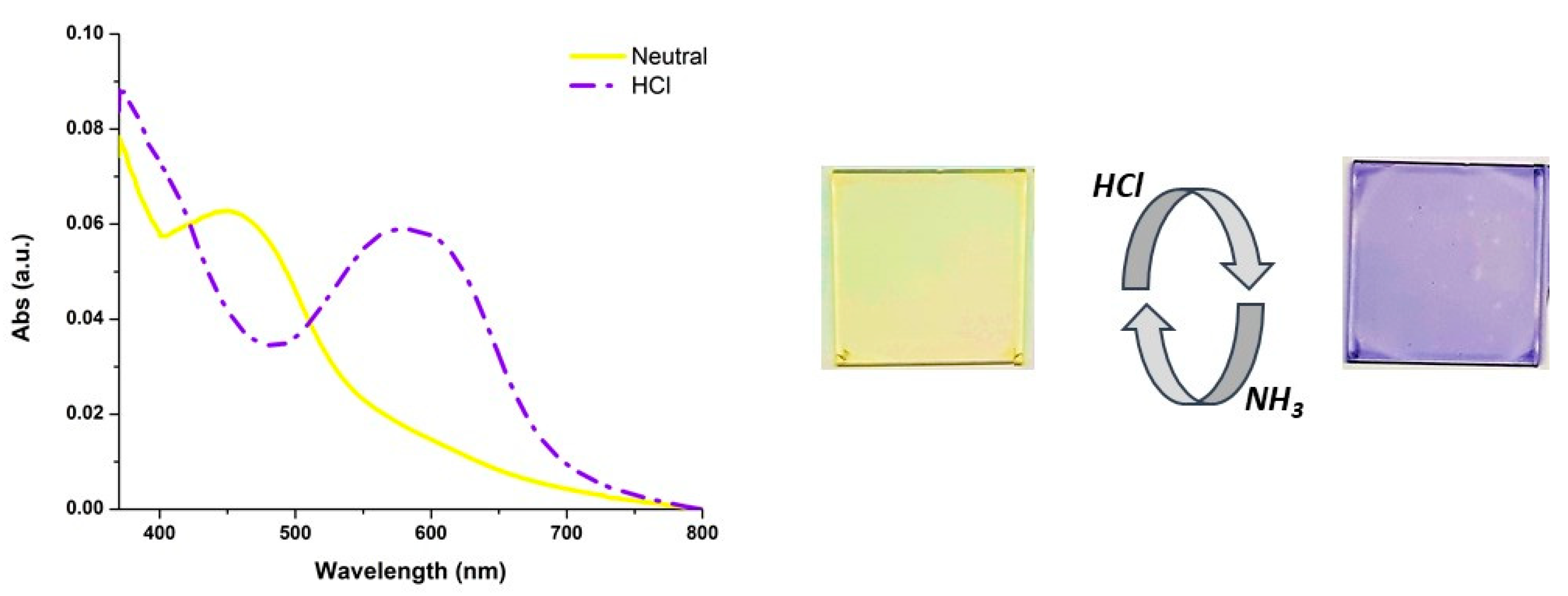
2.2. Characterization of the pH-Dependent Chromophore
3. Materials and Methods
3.1. Synthesis of 3-Phenyl-(2H)-1,4-benzothiazine
3.2. Synthesis of 2Z-((1H-Indol-3-yl)methylene)-3-phenyl-2H-1,4-benzothiazine)
3.3. Film Formation with 1 and Dyeing Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pepe, G.; Cole, J.M.; Waddell, P.G.; McKechnie, S. Molecular engineering of cyanine dyes to design a panchromatic response in co-sensitized dye-sensitized solar cells. Mol. Syst. Des. Eng. 2016, 1, 86–98. [Google Scholar] [CrossRef]
- Liu, H.; Liao, X.; Li, X.; Wu, D.; Guo, Q.; Wu, J.; Qian, S.; Lan, J.; Wang, R.; You, J. Molecular design of new organic sensitizers based on thieno[1,4]benzothiazine for dye-sensitized solar cells. RSC Adv. 2015, 5, 56865–56871. [Google Scholar] [CrossRef]
- Lerch, M.M.; Medved, M.; Lapini, A.; Laurent, A.D.; Iagatti, A.; Bussotti, L.; Szymański, W.; Jan Buma, W.; Foggi, P.; Di Donato, M.; et al. Tailoring photoisomerization pathways in donor-acceptor stenhouse adducts: The role of the hydroxy group. J. Phys. Chem. A 2018, 122, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Kuo, P.Y.; Yang, D.Y. Design and synthesis of a coumarin-based acidichromic colorant. Molecules 2007, 12, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ge, H.T.; Zhao, Y.; Zhao, D.; Fan, J.; Liao, L.S. Novel carbazole derivatives designed by an ortho-linkage strategy for efficient phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 4300–4307. [Google Scholar] [CrossRef]
- Gsänger, M.; Bialas, D.; Huang, L.; Stolte, M.; Würthner, F. Organic semiconductors based on dyes and color pigments. Adv. Mater. 2016, 28, 3615–3645. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Wu, J.; Xie, Z.; Zhang, Q. Synthesis and photovoltaic properties of new conjugated polymers based on red hair pigment skeleton. Dyes Pigm. 2019, 160, 823–829. [Google Scholar] [CrossRef]
- Luo, S.M.; Stellmach, K.A.; Ikuzwe, S.M.; Cao, D.D. Redox-active heteroacene chromophores derived from a nonlinear aromatic diimide. J. Org. Chem. 2019, 84, 10362–10370. [Google Scholar] [CrossRef]
- Cai, K.; Xie, J.; Yang, X.; Zhao, D. Heterohexacene diimides: Anti- and syn-isomers and quinonoid forms. Org. Lett. 2014, 16, 1852–1855. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, Y.; Xiao, L.; Chen, J.; Liao, Q.; Yao, J.; Fu, H. Organic phosphorescence nanowire lasers. J. Am. Chem. Soc. 2017, 139, 6376–6381. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Papalia, T.; Barattucci, A.; Salerno, T.M.G.; Rosano, C.; Castagnola, P.; Viale, M.; Monticone, M.; Campagna, S.; Puntoriero, F. Localization-controlled two-color luminescence imaging via environmental modulation of energy transfer in a multichromophoric species. Dalton Trans. 2018, 47, 4733–4738. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y.; Zou, J.; Huang, L.P.; Chordia, M.D.; Yue, W.; Wu, J.J.; Pan, D.F. Synthesis and biological evaluation of genistein-IR783 conjugate: Cancer cell targeted delivery in MCF-7 for superior anti-cancer therapy. Molecules 2019, 24, 4120. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Mou, Y.H.; Wang, Y.J.; Wang, J.; Li, Y.Y.; Kong, R.H.; Ding, M.; Wang, D.; Guo, C. Design, synthesis, and evaluation of monoamine oxidase a inhibitors–indocyanine dyes conjugates as targeted antitumor agents. Molecules 2019, 24, 1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Xia, S.; Mazi, W.; Wan, S.; Mikesell, L.; Rudy, L.L.; Liu, H. A near-infrared fluorescent probe based on a FRET rhodamine donor linked to a cyanine acceptor for sensitive detection of intracellular pH alternations. Molecules 2018, 23, 2679. [Google Scholar] [CrossRef] [PubMed]
- James, N.S.; Cheruku, R.R.; Missert, J.R.; Sunar, U.; Pandey, R.K. Measurement of cyanine dye photobleaching in photosensitizer cyanine dye conjugates could help in optimizing light dosimetry for improved photodynamic therapy of cancer. Molecules 2018, 23, 1842. [Google Scholar] [CrossRef]
- Ahoulou, E.O.; Drinkard, K.K.; Basnet, K.; St. Lorenz, A.; Taratula, O.; Henary, M.; Grant, K.B. DNA photocleavage in the near-infrared wavelength range by 2-quinolinium dicarbocyanine dyes. Molecules 2020, 25, 2926. [Google Scholar] [CrossRef]
- Lin, C.M.; Usama, S.M.; Burgess, K. Site-specific labeling of proteins with near-IR heptamethine cyanine dyes. Molecules 2018, 23, 2900. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Jia, S.; Liu, X.; Zhao, J.; Yin, G. Dicyanovinyl substituted push–pull chromophores: Effects of central C=C/phenyl spacers, crystal structures and application in hydrazine sensing. Phys. Chem. Chem. Phys. 2019, 21, 3218–3226. [Google Scholar] [CrossRef]
- Šarlah, D.; Juranovič, A.; Kožar, B.; Rejc, L.; Golobič, A.; Petrič, A. Synthesis of naphthalene-based push-pull molecules with a heteroaromatic electron acceptor. Molecules 2016, 21, 267. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, X.; Xiao, W. Theoretical studies of photophysical properties of D−π−A−π−D-type diketopyrrolopyrrole-based molecules for organic light-emitting diodes and organic solar cells. Molecules 2020, 25, 667. [Google Scholar] [CrossRef]
- Gayton, J.N.; Autry, S.; Fortenberry, R.C.; Hammer, N.I.; Delcamp, J.H. Counter anion effect on the photophysical properties of emissive indolizine-cyanine dyes in solution and solid state. Molecules 2018, 23, 3051. [Google Scholar] [CrossRef] [PubMed]
- El-Shishtawy, R.M.; Oliveira, A.S.; Almeida, P.; Ferreira, D.P.; Conceição, D.S.; Ferreira, L.F.V. Photophysical studies of a new water soluble indocarbocyanine dye adsorbed onto microcrystalline cellulose and beta-cyclodextrin. Molecules 2013, 18, 5648–5668. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Mandal, K.J.; Narasimha Moorthy, J. Anionic merocyanine dyes based on thiazol-2-hydrazides: Reverse solvatochromism, preferential solvation and multiparametric approaches to spectral shifts. Phys. Chem. Chem. Phys. 2018, 20, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Rönicke, F.; Schepersb, U.; Wagenknecht, H.A. A new structure-activity relationship for cyanine dyes to improve photostability and fluorescence properties for live cell imaging. Chem. Sci. 2018, 9, 6557–6563. [Google Scholar] [CrossRef]
- Soriano, E.; Holder, C.; Levitz, A.; Henary, M. Benz[c,d]indolium-containing monomethine cyanine dyes: Synthesis and photophysical properties. Molecules 2016, 21, 23. [Google Scholar] [CrossRef]
- Leone, L.; Pezzella, S.; Crescenzi, O.; Napolitano, A.; Barone, V.; d’Ischia, M. Trichocyanines: A red-hair-inspired modular platform for dye-Based one-time-pad molecular cryptography. ChemistryOpen 2015, 4, 370–377. [Google Scholar] [CrossRef]
- Napolitano, A.; Panzella, L.; Leone, L.; d’Ischia, M. Red hair benzothiazines and benzothiazoles: Mutation-inspired chemistry in the quest for functionality. Acc. Chem. Res. 2013, 46, 519–528. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef]
- Napolitano, A.; De Lucia, M.; Panzella, L.; d’Ischia, M. The “Benzothiazine” chromophore of pheomelanins: A reassessment. Photochem. Photobiol. 2008, 84, 593–599. [Google Scholar] [CrossRef]
- Napolitano, A.; Di Donato, P.; Prota, G. Zinc-catalyzed oxidation of 5-S-cysteinyldopa to 2,2’-bi(2H-1,4-benzothiazine): Tracking the biosynthetic pathway of trichochromes, the characteristic pigments of red hair. J. Org. Chem. 2001, 66, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Crescenzi, O.; Napolitano, A.; Barone, V.; d’Ischia, M. The Δ2,2′-bi(2H-1,4-benzothiazine) structural motif of red hair pigments revisited: Photochromism and acidichromism in a unique four-state system. Eur. J. Org. Chem. 2012, 27, 5136–5140. [Google Scholar] [CrossRef]
- Ye, T.; Lamb, L.E.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ultrafast absorption and photothermal studies of decarboxytrichochrome C in solution. Photochem. Photobiol. Sci. 2003, 2, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Crescenzi, O.; Amorati, R.; Valgimigli, L.; Napolitano, A.; Barone, V.; d’Ischia, M. Red-hair-inspired chromogenic system based on a proton-switched dehydrogenative free-radical coupling. Org. Lett. 2013, 15, 4944–4947. [Google Scholar] [CrossRef]
- Santacroce, C.; Sica, D.; Nicolaus, R.A. Sintesi di 1,4-benzotiazine. Gazz. Chim. Ital. 1968, 98, 85–96. [Google Scholar]
- MacKenzie, N.E.; Thomson, R.H.; Greenhalgh, C.W. New dyes based on 3-aryl-benzo- and -naphtho- l,4-thiazines. J. Chem. Soc. Perkin Trans. 1 1980, 1, 2923–2932. [Google Scholar] [CrossRef]
- Lin, Y.M.; Lu, G.P.; Wang, R.K.; Yi, W.B. Radical route to 1,4-benzothiazine derivatives from 2-aminobenzenethiols and ketones under transition-metal-free. Org. Lett. 2016, 18, 6424–6427. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3-phenyl-(2H)-1,4-benzothiazine and 2Z-((1H-indol-3-yl)methylene)-3-phenyl-2H-1,4-benzothiazine) are available from the authors. |
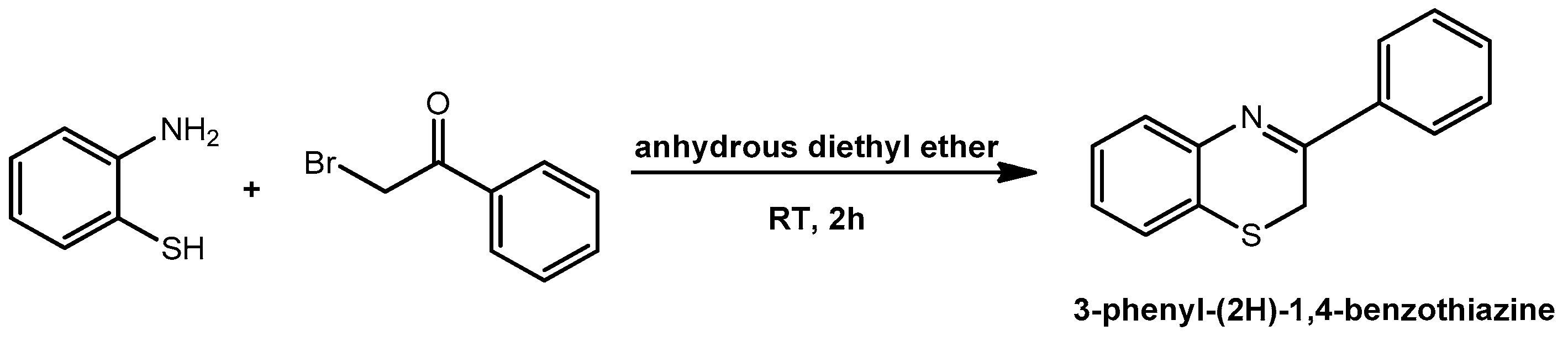

| Entry | Benzothiazine/Aldehyde (Molar Ratio) | Solvent | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| 1 | 1:1 | CH3CN/0.5 M HCl | r.t. | 24 |
| 2 | 1:1 | CH3CN/1.5 M HCl | r.t. | 24 |
| 3 | 1:1 | CH3CN/2.5 M HCl | r.t. | 24 |
| 4 | 1:1 | CH3CN/2.5 M HCl | 45 | 8 |
| 5 | 1:1 | CH3CN/2.5 M HCl | 70 | 3 |
| 6 | 0.5:1 | CH3CN/2.5 M HCl | 70 | 3 |
| 7 | 1:2 | CH3CN/2.5 M HCl | 70 | 3 |
| 8 | 1:1.5 | CH3CN/2.5 M HCl | 70 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.L.; Panzella, L.; d’Ischia, M.; Napolitano, A. Bioinspired Heterocyclic Partnership in a Cyanine-Type Acidichromic Chromophore. Molecules 2020, 25, 3817. https://doi.org/10.3390/molecules25173817
Alfieri ML, Panzella L, d’Ischia M, Napolitano A. Bioinspired Heterocyclic Partnership in a Cyanine-Type Acidichromic Chromophore. Molecules. 2020; 25(17):3817. https://doi.org/10.3390/molecules25173817
Chicago/Turabian StyleAlfieri, Maria Laura, Lucia Panzella, Marco d’Ischia, and Alessandra Napolitano. 2020. "Bioinspired Heterocyclic Partnership in a Cyanine-Type Acidichromic Chromophore" Molecules 25, no. 17: 3817. https://doi.org/10.3390/molecules25173817
APA StyleAlfieri, M. L., Panzella, L., d’Ischia, M., & Napolitano, A. (2020). Bioinspired Heterocyclic Partnership in a Cyanine-Type Acidichromic Chromophore. Molecules, 25(17), 3817. https://doi.org/10.3390/molecules25173817










