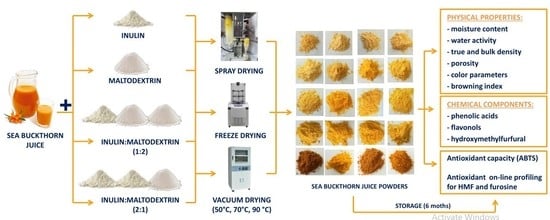
Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of Sea Buckthorn Juice Powders
2.1.1. Moisture Content
2.1.2. Water Activity
2.1.3. True and Bulk Density
2.1.4. Porosity
2.1.5. Color Parameters
2.1.6. Browning Index
2.2. Hydroxymethylfurfural in Sea Buckthorn Juice Powders
2.3. Phenolic Compounds in Sea Buckthorn Juice Powders
2.4. Antioxidant Capacity of Sea Buckthorn Juice Powders
3. Materials and Methods
3.1. Chemicals
3.2. Material and Sample Preparation
3.3. Drying Methods
3.4. Storage
3.5. Physical Properties
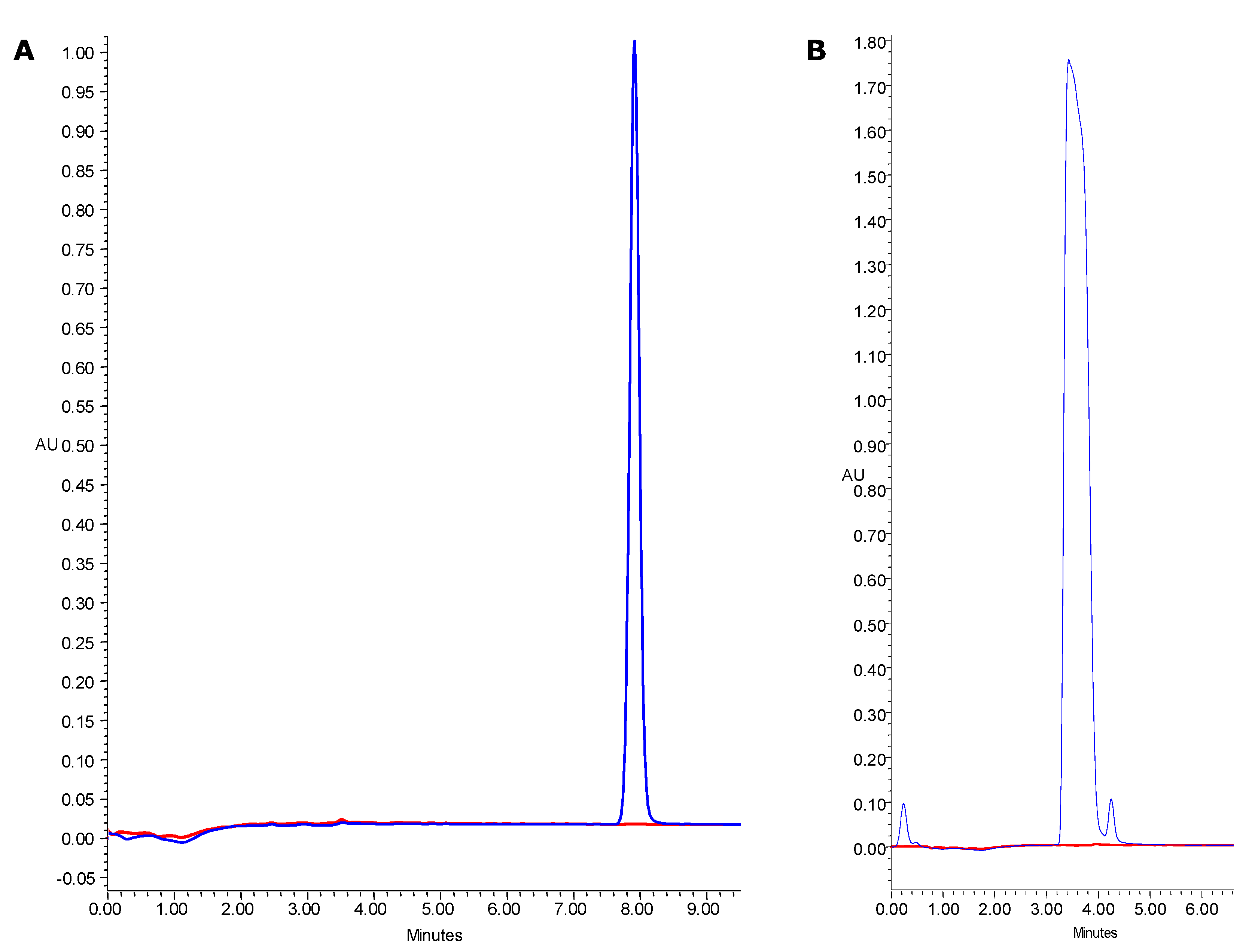
3.6. Determination of Phenolic Compounds and Hydroxymethylfurfural (HMF)
3.7. Determination of Antioxidant Capacity and Antioxidant On-Line Profiling by HPLC-PDA Coupled with Post-Column Derivatization with ABTS·+ Reagent
3.8. Statistical Analysis
4. Conclusions
- (1)
- Inulin caused stronger water retention of powders than maltodextrin. The drying method modulated the water activity more strongly than the type of carrier agents.
- (2)
- Powders with inulin had higher true density values than those with maltodextrin. Bulk density and porosity were significantly differentiated by drying methods, and vacuum drying seems to be a useful technique to obtain powders with high bulk density. The porosity of the spray-dried and freeze-dried powders was higher than after vacuum drying.
- (3)
- In view of the yellow color and its intensity, the use of maltodextrin was competitive compared to inulin. Moreover, spray-, freeze- and vacuum-drying at 50 °C and the addition of maltodextrin were not conducive to browning and HMF formation.
- (4)
- Powders spray- and vacuum-dried at 70 °C had the highest concentrations of phenolic acids and flavonols, respectively. However, in stored freeze-dried powders, phenolic compound losses were the lowest. More phenolic compounds were determined in powders with maltodextrin.
- (5)
- Storage for six months increased antioxidant capacity, but browning compounds, HMF and furosine did not affect this effect.
Author Contributions
Funding
Conflicts of Interest
References
- Teleszko, M.; Wojdyło, A.; Rudzinńska, M.; Oszmianński, J.; Golis, T. Analysis of lipophilic and hydrophilic bioactive compounds content in sea buckthorn (Hippophaë rhamnoides L.) berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS profiling of phenolic and carotenoid compounds and their influence on anticholinergic potential for AChE and BuChE inhibition and on-line antioxidant activity of selected Hippophaë rhamnoides L. cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef]
- Rafalska, A.; Abramowicz, K.; Krauze, M. Sea buckthorn (Hippophaë rhamnoides L.) as a plant for universal application. World Sci. News. 2017, 72, 123–140. [Google Scholar]
- Aziz, M.; Yusof, Y.; Blanchard, C.; Saifullah, M.; Farahnaky, A.; Scheiling, G. Material properties and tableting of fruit powders. Food Eng. Rev. 2018, 10, 66–80. [Google Scholar] [CrossRef]
- Boonyai, P.; Howes, T.; Bhandari, B. Applications of the cyclone stickiness test for characterization of stickiness in food powders. Dry. Technol. 2006, 24, 703–709. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT-Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Candido, R.C.; Oliveira, W.P. Spray drying microencapsulation of Lippia sidoides extracts in carbohydrate blends. Food Bioprod. Process. 2012, 90, 425–432. [Google Scholar] [CrossRef]
- Díaz-Bandera, D.; Villanueva-Carvajal, A.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A. Assessing release kinetics and dissolution of spray-dried Roselle (Hibiscus sabdariffa L.) extract encapsulated with different carrier agents. LWT-Food Sci. Technol. 2015, 64, 693–698. [Google Scholar] [CrossRef]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT-Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Caparino, O.; Tang, J.; Nindo, C.; Sablani, S.; Powers, J.; Fellman, J. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Michalska, A.; Lech, K. The effect of carrier quantity and drying method on the physical properties of apple juice powders. Beverages 2018, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.; Oliveira, A.; Pintado, M.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT-Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kołodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.; Calderas, F.; Laredo, R.G.; Sánchez-Olivares, G.; Gallegos-Infante, J.; Rocha-Guzmán, N.; Rodriguez-Ramirez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Khanum, F. Optimization of spray drying process for developing seabuckthorn fruit juice powder using response surface methodology. J. Food Sci. Technol. 2014, 51, 3731–3739. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.; Calderas, F.; González-Laredo, R.; Rocha-Guzmán, N.; Ochoa-Martínez, L.; Bernad-Bernad, M. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT-Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Lacerda, E.C.Q.; de Araújo Calado, V.M.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, inulin and maltodextrin as encapsulating agents affect the quality and stability of jussara pulp microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Villacrez, J.L.; Carriazo, J.G.; Osorio, C. Microencapsulation of Andes berry (Rubus glaucus Benth.) aqueous extract by spray drying. Food Bioprocess Tech. 2014, 7, 1445–1456. [Google Scholar] [CrossRef]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; Vissotto, F.Z.; de Aguirre, J.M. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. Int. J. Food Sci. Technol. 2012, 47, 1237–1245. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Solval, K.M.; Sundararajan, S.; Alfaro, L.; Sathivel, S. Development of cantaloupe (Cucumis melo) juice powders using spray drying technology. LWT-Food Sci. Technol. 2012, 46, 287–293. [Google Scholar] [CrossRef]
- Azizpour, M.; Mohebbi, M.; Khodaparast, M.H.H. Effects of foam-mat drying temperature on physico-chemical and microstructural properties of shrimp powder. Innov. Food Sci. Emerg. 2016, 34, 122–126. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-L.; Jin, S.-Y.; Chen, C.-S. Relative reactivities of glucose and galactose in browning and pyruvaldehyde formation in sugar/glycine model systems. Food Chem. 2005, 92, 597–605. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Evaluation of different methods for the production of juice concentrates and fruit powders from cactus pear. Innov. Food Sci. Emerg. 2006, 7, 275–287. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Lech, K.; Figiel, A. Functional relationships between phytochemicals and drying conditions during the processing of blackcurrant pomace into powders. Adv. Powder Technol. 2017, 28, 1340–1348. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.; Figiel, A. Chemical composition and antioxidant properties of powders obtained from different plum juice formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasrija, D.; Ezhilarasi, P.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT-Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Rocha-Parra, D.F.; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of storage conditions on phenolic compounds stability, antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT-Food Sci. Technol. 2016, 70, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Lech, K.; Michalska-Ciechanowska, A.; Nowicka, P. The influence of different carrier agents and drying techniques on physical and chemical characterization of Japanese quince (Chaenomeles japonica) microencapsulation powder. Food Chem. 2020, 323, 126830. [Google Scholar] [CrossRef]
- Šumić, Z.; Tepić, A.; Vidović, S.; Jokić, S.; Malbaša, R. Optimization of frozen sour cherries vacuum drying process. Food Chem. 2013, 136, 55–63. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Lech, K.; Tkacz, K.; Nowicka, P. Influence of different drying methods on the quality of Japanese quince fruit. LWT-Food Sci. Technol. 2019, 114, 108416. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkacz, K.; Wojdyło, A.; Nowicka, P.; Turkiewicz, I.; Golis, T. Characterization in vitro potency of biological active fractions of seeds, skins and flesh from selected Vitis vinifera L. cultivars and interspecific hybrids. J. Funct. Foods 2019, 56, 353–363. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |

| Drying Method | Carrier Agent | Moisture Content (%) | Water Activity (aw) | True Density (kg m−3) | Bulk Density (kg m−3) | Porosity (%) |
|---|---|---|---|---|---|---|
| SD | INU | 2.62 ± 0.11 d | 0.086 ± 0.001 d | 1408 ± 15 c | 488.2 ± 13 d | 65.34 ± 0.6 d |
| MALTO | 2.01 ± 0.16 de | 0.090 ± 0.002 cd | 1240 ± 9 e | 389.5 ± 5 f | 68.52 ± 0.1 c | |
| I:M 2:1 | 1.64 ± 0.10 e | 0.080 ± 0.001 d | 1375 ± 13 d | 459.6 ± 23 de | 66.59 ± 1.3 d | |
| I:M 1:2 | 2.19 ± 0.12 de | 0.089 ± 0.000 cd | 1471 ± 12 b | 466.5 ± 10 de | 68.29 ± 0.4 c | |
| FD | INU | 4.75 ± 0.18 b | 0.101 ± 0.001 c | 1543 ± 10 a | 448.1 ± 4 e | 70.95 ± 0.1 b |
| MALTO | 2.55 ± 0.12 d | 0.096 ± 0.000 c | 1529 ± 13 ab | 548.9 ± 18 bc | 64.10 ± 0.9 d | |
| I:M 2:1 | 3.28 ± 0.14 c | 0.097 ± 0.001 c | 1485 ± 15 b | 474.9 ± 11 d | 68.03 ± 0.4 c | |
| I:M 1:2 | 3.45 ± 0.16 c | 0.098 ± 0.001 c | 1519 ± 8 ab | 373.1 ± 3 f | 75.43 ± 0.1 a | |
| VD 50 °C | INU | 4.96 ± 0.20 ab | 0.099 ± 0.001 c | 1508 ± 11 ab | 541.9 ± 12 bc | 64.08 ± 0.5 d |
| MALTO | 2.42 ± 0.10 d | 0.096 ± 0.001 c | 1485 ± 13 b | 550.5 ± 18 bc | 62.94 ± 0.9 de | |
| I:M 2:1 | 3.68 ± 0.13 c | 0.097 ± 0.000 c | 1480 ± 15 b | 541.0 ± 19 bc | 63.45 ± 0.9 d | |
| I:M 1:2 | 4.04 ± 0.11 bc | 0.100 ± 0.001 c | 1462 ± 12 b | 519.1 ± 11 c | 64.49 ± 0.5 d | |
| VD 70 °C | INU | 4.31 ± 0.13 b | 0.098 ± 0.000 c | 1479 ± 10 b | 542.7 ± 12 bc | 63.30 ± 0.6 d |
| MALTO | 1.69 ± 0.15 e | 0.088 ± 0.002 d | 1467 ± 9 b | 549.4 ± 9 bc | 62.57 ± 0.4 de | |
| I:M 2:1 | 1.99 ± 0.18 de | 0.088 ± 0.001 d | 1473 ± 12 b | 539.9 ± 21 bc | 63.35 ± 1.1 d | |
| I:M 1:2 | 3.98 ± 0.22 bc | 0.096 ± 0.001 c | 1475 ± 13 b | 515.9 ± 6 c | 65.03 ± 0.1 d | |
| VD 90 °C | INU | 1.89 ± 0.11 e | 0.076 ± 0.000 e | 1421 ± 11 c | 551.7 ± 3 bc | 61.19 ± 0.1 e |
| MALTO | 1.36 ± 0.17 ef | 0.074 ± 0.000 e | 1393 ± 14 cd | 524.5 ± 5 c | 62.34 ± 0.1 de | |
| I:M 2:1 | 1.62 ± 0.12 e | 0.076± 0.002 e | 1412 ± 11 c | 597.1 ± 8 b | 57.71 ± 0.3 f | |
| I:M 1:2 | 1.29 ± 0.19 f | 0.075 ± 0.002 e | 1406 ± 12 c | 573.1 ± 15 b | 59.23 ± 0.7 e | |
| Pure carrier agents | INU | 2.08 ± 0.01 de | 0.129 ± 0.001 b | 1387 ± 11 d | 644.3 ± 9 a | 53.53 ± 0.3 h |
| MALTO | 5.70 ± 0.25 a | 0.397 ± 0.001 a | 1228 ± 12 ef | 472.3 ± 2 d | 61.65 ± 0.2 e | |
| I:M 2:1 | 5.07 ± 0.00 ab | 0.383 ± 0.001 a | 1273 ± 13 e | 547.5 ± 5 bc | 57.00 ± 0.1 f | |
| I:M 1:2 | 4.65 ± 0.08 b | 0.368 ± 0.001 a | 1308 ± 9 f | 575.4 ± 6 b | 56.01 ± 0.2 g | |
| Duncan’s Multiple Range Test | ||||||
| Drying method | SD | 2.12 C | 0.086 B | 1374 D | 451.2 D | 67.19 A |
| FD | 3.51 A | 0.098 A | 1519 A | 461.3 C | 69.63 A | |
| VD 50 °C | 3.78 A | 0.098 A | 1484 B | 538.1 B | 63.74 B | |
| VD 70 °C | 2.99 B | 0.093 AB | 1474 B | 537.0 B | 63.56 B | |
| VD 90 °C | 1.54 D | 0.075 C | 1408 C | 561.6 A | 60.12 C | |
| Carrier agent | INU | 3.71 A | 0.092 A | 1472 A | 514.5 B | 64.97 AB |
| MALTO | 2.01 D | 0.089 B | 1423 D | 512.7 B | 64.09 B | |
| I:M 2:1 | 2.44 C | 0.088 B | 1445 C | 522.5 A | 63.83 B | |
| I:M 1:2 | 2.99 B | 0.092 A | 1467 B | 489.6 C | 66.49 A | |
| Drying Method | Carrier Agent | Color Parameters | Browning Index (AU) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Chroma (C) | dE | Hue Angle (h°) | 0 Months | 6 Months | ||
| SD | INU | 86.57 ± 0.14 bc | 1.56 ± 0.03 g | 40.81 ± 0.08 d | 40.84 ± 0.38 d | 36.67 ± 0.13 c | 87.81 ± 0.04 d | 0.22 ± 0.00 de | 0.74 ± 0.02 e |
| MALTO | 89.26 ± 0.17 b | −1.21 ± 0.05 i | 36.77 ± 0.05 e | 36.79 ± 0.41 e | 41.64 ± 0.16 b | 91.88 ± 0.06 c | 0.24 ± 0.01 d | 0.50 ± 0.01 g | |
| I:M 2:1 | 88.53 ± 0.09 b | −0.63 ± 0.11 i | 38.76 ± 0.02 de | 38.77 ± 0.67 de | 40.08 ± 0.08 b | 90.93 ± 0.13 c | 0.43 ± 0.02 b | 0.58 ± 0.01 f | |
| I:M 1:2 | 87.12 ± 0.16 bc | 0.97 ± 0.05 h | 40.70 ± 0.08 d | 40.71 ± 0.65 d | 37.47 ± 0.15 c | 88.64 ± 0.06 d | 0.28 ± 0.01 cd | 0.59 ± 0.01 f | |
| FD | INU | 87.58 ± 0.17 bc | −0.36 ± 0.23 hi | 46.67 ± 0.05 c | 46.67 ± 0.44 c | 37.38 ± 0.15 c | 90.44 ± 0.24 c | 0.30 ± 0.02 c | 0.72 ± 0.01 e |
| MALTO | 81.99 ± 0.04 c | 6.32 ± 0.06 d | 50.16 ± 0.06 bc | 50.56 ± 0.37 b | 28.46 ± 0.03 e | 82.82 ± 0.07 g | 0.16 ± 0.00 e | 0.52 ± 0.01 fg | |
| I:M 2:1 | 78.87 ± 0.06 d | 9.66 ± 0.04 c | 57.43 ± 0.02 ab | 58.24 ± 0.20 a | 24.55 ± 0.05 f | 80.45 ± 0.05 g | 0.14 ± 0.01 e | 0.59 ± 0.01 f | |
| I:M 1:2 | 83.79 ± 0.19 c | 3.55 ± 0.03 ef | 55.24 ± 0.07 b | 55.35 ± 0.85 ab | 31.79 ± 0.16 d | 86.32 ± 0.04 de | 0.11 ± 0.01 ef | 0.47 ± 0.01 g | |
| VD 50 °C | INU | 84.59 ± 0.20 bc | 1.64 ± 0.10 g | 47.89 ± 0.06 c | 47.92 ± 0.34 c | 33.71 ± 0.18 d | 88.04 ± 0.12 d | 0.19 ± 0.01 e | 0.61 ± 0.01 f |
| MALTO | 77.00 ± 0.09 d | 11.98 ± 0.02 bc | 55.53 ± 0.03 b | 56.81 ± 0.40 ab | 21.31 ± 0.07 fg | 77.83 ± 0.04 h | 0.15 ± 0.02 e | 0.64 ± 0.01 f | |
| I:M 2:1 | 80.99 ± 0.03 c | 6.05 ± 0.05 d | 53.57 ± 0.02 b | 53.91 ± 0.12 b | 27.91 ± 0.01 e | 83.57 ± 0.06 f | 0.11 ± 0.01 ef | 0.66 ± 0.01 f | |
| I:M 1:2 | 82.88 ± 0.28 c | 2.96 ± 0.04 f | 53.70 ± 0.04 b | 53.78 ± 0.54 b | 31.40 ± 0.24 d | 86.85 ± 0.05 de | 0.18 ± 0.01 e | 0.55 ± 0.01 fg | |
| VD 70 °C | INU | 77.48 ± 0.03 d | 4.62 ± 0.04 e | 50.32 ± 0.02 bc | 50.53 ± 0.79 b | 26.48 ± 0.01 ef | 84.75 ± 0.05 f | 0.17 ± 0.01 e | 1.16 ± 0.01 d |
| MALTO | 77.59 ± 0.17 d | 9.12 ± 0.06 c | 59.19 ± 0.03 a | 59.89 ± 0.98 a | 24.43 ± 0.15 f | 81.24 ± 0.07 g | 0.09 ± 0.00 f | 0.70 ± 0.01 ef | |
| I:M 2:1 | 77.64 ± 0.04 d | 9.19 ± 0.06 c | 58.86 ± 0.10 a | 59.57 ± 0.11 a | 24.32 ± 0.03 f | 81.13 ± 0.07 g | 0.32 ± 0.02 c | 0.74 ± 0.01 e | |
| I:M 1:2 | 77.62 ± 0.14 d | 4.56 ± 0.05 e | 52.87 ± 0.05 b | 53.07 ± 0.31 b | 26.61 ± 0.13 ef | 85.57 ± 0.06 e | 0.25 ± 0.01 d | 1.00 ± 0.01 d | |
| VD 90 °C | INU | 54.85 ± 0.09 f | 12.03 ± 0.07 b | 33.06 ± 0.05 f | 35.80 ± 0.69 e | 23.34 ± 0.08 f | 70.00 ± 0.08 i | 0.71 ± 0.02 a | 2.98 ± 0.03 a |
| MALTO | 71.52 ± 0.03 e | 10.57 ± 0.08 bc | 58.60 ± 0.01 a | 59.55 ± 0.87 a | 19.33 ± 0.03 g | 79.78 ± 0.08 g | 0.28 ± 0.01 cd | 1.57 ± 0.01 c | |
| I:M 2:1 | 54.55 ± 0.04 f | 35.78 ± 0.04 a | 33.70 ± 0.09 f | 49.15 ± 0.83 b | 21.91 ± 0.03 fg | 43.29 ± 0.09 k | 0.30 ± 0.01 c | 2.84 ± 0.03 ab | |
| I:M 1:2 | 56.21 ± 0.12 f | 13.26 ± 0.07 b | 39.62 ± 0.07 d | 41.78 ± 0.69 d | 17.30 ± 0.10 h | 71.50 ± 0.08 i | 0.42 ± 0.01 b | 2.72 ± 0.01 b | |
| Pure carrier agents | INU | 97.58 ± 0.01 a | −1.51 ± 0.01 i | 4.53 ± 0.01 g | 4.78 ± 0.01 f | 65.60 ± 0.01 a | 108.44 ± 0.01 b | - | - |
| MALTO | 98.03 ± 0.00 a | −1.24 ± 0.01 i | 2.74 ± 0.01 h | 3.01 ± 0.01 f | 67.05 ± 0.01 a | 114.35 ± 0.01 a | - | - | |
| I:M 2:1 | 97.90 ± 0.01 a | 1.47 ± 0.01 i | 3.75 ± 0.01 gh | 4.03 ± 0.01 f | 65.22 ± 0.01 a | 68.60 ± 0.02 ij | - | - | |
| I:M 1:2 | 98.02 ± 0.00 a | 1.37 ± 0.01 i | 3.33 ± 0.01 gh | 3.60 ± 0.01 f | 65.63 ± 0.01 a | 67.64 ± 0.01 j | - | - | |
| Duncan’s Multiple Range Test | |||||||||
| Drying method | SD | 87.87 A | 0.17 C | 39.26 D | 39.28 C | 38.96 A | 89.82 A | 0.29 B | 0.60 C |
| FD | 83.06 AB | 4.79 B | 52.38 B | 52.70 A | 30.55 B | 85.01 B | 0.18 CD | 0.58 C | |
| VD 50 °C | 81.37 B | 5.66 B | 52.67 B | 53.10 A | 28.58 B | 84.07 BC | 0.16 D | 0.62 C | |
| VD 70 °C | 77.58 C | 6.87 B | 55.31 A | 55.76 A | 25.46 C | 83.17 C | 0.21 C | 0.90 B | |
| VD 90 °C | 59.28 D | 17.91 A | 41.25 C | 46.41 B | 20.47 D | 66.14 D | 0.43 A | 2.53 A | |
| Carrier agents | INU | 78.21 AB | 3.90 D | 43.75 C | 44.23 D | 31.52 A | 70.17 A | 0.32 A | 1.24 A |
| MALTO | 79.47 A | 7.36 B | 52.05 A | 55.72 A | 27.03 C | 68.93 A | 0.18 C | 0.79 C | |
| I:M 2:1 | 76.12 C | 12.01 A | 48.46 B | 51.93 B | 27.75 BC | 62.23 B | 0.26 B | 1.08 B | |
| I:M 1:2 | 77.52 BC | 5.06 C | 48.43 B | 48.94 C | 28.91 B | 69.81 A | 0.25 B | 1.07 B | |
| Drying Method | Carrier Agent | HMF | Phenolic Acids | Flavonols | Antioxidant Capacity | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Months | 6 Months | 0 Months | 6 Months | 0 Months | 6 Months | 0 Months | 6 Months | ||
| SD | INU | 1.50 ± 0.13 e | 1.79 ± 0.19 f | 2.86 ± 0.11 ab | 2.71 ± 0.15 a | 210.83 ± 4.05 f | 203.92 ± 2.23 e | 1.62 ± 0.08 ab | 2.25 ± 0.23 c |
| MALTO | 0.39 ± 0.09 g | 0.72 ± 0.11 g | 3.00 ± 0.29 a | 2.96 ± 0.19 a | 222.21 ± 2.45 e | 209.51 ± 2.46 e | 1.56 ± 0.08 b | 1.43 ± 0.19 ef | |
| I:M 2:1 | 0.82 ± 0.10 f | 1.89 ± 0.20 f | 2.49 ± 0.09 b | 2.26 ± 0.13 b | 242.05 ± 5.17 d | 174.96 ± 2.01 f | 1.60 ± 0.02 ab | 2.20 ± 0.03 c | |
| I:M 1:2 | 1.05 ± 0.02 ef | 1.88 ± 0.18 f | 1.70 ± 0.14 c | 1.26 ± 0.10 de | 266.43 ± 3.55 b | 144.05 ± 2.12 g | 1.64 ± 0.10 ab | 2.27 ± 0.22 c | |
| FD | INU | 0.04 ± 0.01 i | 0.67 ± 0.14 gh | 1.71 ± 0.10 c | 1.67 ± 0.21 d | 213.28 ± 4.44 ef | 200.40 ± 2.32 ef | 1.73 ± 0.19 a | 2.10 ± 0.34 cd |
| MALTO | 0.41 ± 0.10 g | 0.57 ± 0.11 h | 1.85 ± 0.24 bc | 1.80 ± 0.14 cd | 251.48 ± 4.64 c | 249.02 ± 3.54 ab | 1.45 ± 0.20 c | 2.09 ± 0.12 cd | |
| I:M 2:1 | 0.07 ± 0.01 i | 0.15 ± 0.08 i | 1.44 ± 0.18 d | 1.37 ± 0.24 d | 255.78 ± 2.47 c | 233.09 ± 3.53 c | 1.56 ± 0.07 b | 1.85 ± 0.22 d | |
| I:M 1:2 | 0.09 ± 0.01 i | 0.17 ± 0.10 i | 2.12 ± 0.22 b | 2.07 ± 0.07 c | 245.40 ± 3.89 d | 236.53 ± 2.79 c | 1.40 ± 0.07 c | 1.72 ± 0.29de | |
| VD 50 °C | INU | 0.45 ± 0.06 g | 0.51 ± 0.10 h | 1.39 ± 0.16 d | 1.00 ± 0.25 e | 217.69 ± 2.56 e | 150.42 ± 3.72 g | 1.56 ± 0.08 b | 1.80 ± 0.34 d |
| MALTO | 0.44 ± 0.03 g | 0.50 ± 0.07 h | 1.57 ± 0.20 cd | 1.49 ± 0.24 d | 274.25 ± 3.52 b | 256.46 ± 2.64 a | 1.46 ± 0.11 c | 1.96 ± 0.17 d | |
| I:M 2:1 | 0.13 ± 0.01 i | 0.89 ± 0.22 g | 1.60 ± 0.15 cd | 1.23 ± 0.17 de | 248.55 ± 3.12 cd | 222.51 ± 2.70 d | 1.55 ± 0.14 b | 2.61 ± 0.17 b | |
| I:M 1:2 | 0.32 ± 0.09 h | 0.90 ± 0.06 g | 1.02 ± 0.09 e | 0.92 ± 0.15 e | 273.93 ± 2.25 b | 223.91 ± 3.75 d | 1.46 ± 0.05 c | 2.35 ± 0.08 bc | |
| VD 70 °C | INU | 14.09 ± 1.53 c | 21.12 ± 2.14 c | 1.21 ± 0.13 de | 0.94 ± 0.13 e | 243.86 ± 2.51 d | 212.50 ± 2.34 e | 1.40 ± 0.18 c | 1.65 ± 0.20 e |
| MALTO | 0.46 ± 0.08 g | 2.15 ± 0.67 ef | 1.59 ± 0.08 cd | 1.12 ± 0.13 e | 290.34 ± 4.02 a | 244.73 ± 2.27 b | 1.29 ± 0.05 d | 2.92 ± 0.11 a | |
| I:M 2:1 | 0.05 ± 0.01 i | 2.94 ± 0.23 e | 0.24 ± 0.01 g | 0.18 ± 0.09 g | 267.62 ± 1.87 b | 234.59 ± 2.75 c | 1.64 ± 0.33 ab | 1.99 ± 0.18 d | |
| I:M 1:2 | 9.22 ± 0.45 d | 15.35 ± 2.53 d | 1.06 ± 0.11 e | 0.79 ± 0.17 f | 285.03 ± 2.16 a | 232.87 ± 3.64 c | 1.32 ± 0.11 cd | 1.42 ± 0.17 ef | |
| VD 90 °C | INU | 75.21 ± 1.74 a | 94.20 ± 4.35 a | 0.71 ± 0.12 ef | 0.14 ± 0.03 g | 191.24 ± 1.54 g | 195.87 ± 1.58 ef | 1.12 ± 0.06 d | 1.82 ± 0.25 d |
| MALTO | 0.39 ± 0.02 gh | 11.53 ± 1.04 c | 1.16 ± 0.17 de | 0.89 ± 0.12 ef | 298.68 ± 5.34 a | 200.13 ± 2.28 ef | 1.29 ± 0.03 c | 1.35 ± 0.19 f | |
| I:M 2:1 | 75.07 ± 1.19 a | 101.40 ± 4.93 a | 0.71 ± 0.09 ef | 0.25 ± 0.08 g | 219.68 ± 3.74 e | 188.92 ± 2.22 f | 1.42 ± 0.05 c | 1.76 ± 0.22 de | |
| I:M 1:2 | 47.12 ± 0.80 b | 70.53 ± 3.66 b | 0.24 ± 0.03 g | 0.10 ± 0.02 g | 181.80 ± 2.81 h | 175.41 ± 3.64 f | 0.85 ± 0.07 e | 2.19 ± 0.11 c | |
| Duncan’s Multiple Range Test | |||||||||
| Drying method | SD | 0.94 C | 1.57 C ↑67.0% | 2.51 A | 2.30 A ↓8.4% | 235.38 BC | 183.11 C ↓22.2% | 1.61 A | 2.04 A ↑26.7% |
| FD | 0.15 C | 0.33 C ↑120.0% | 1.78 B | 1.73 B ↓2.8% | 241.49 BC | 229.76 A ↓4.8% | 1.54 A | 1.94 AB ↑26.0% | |
| VD 50 °C | 0.33 C | 0.90 C ↑173% | 1.39 B | 1.16 B ↓16.5% | 253.61 B | 213.33 B ↓15.9% | 1.51 A | 2.18 A ↑44.4% | |
| VD 70 °C | 5.95 B | 10.39 B ↑74,6% | 1.03 BC | 0.76 C ↓26.2% | 271.71 A | 231.17 A ↓14.9% | 1.41 AB | 2.00 A ↑41.8% | |
| VD 90 °C | 49.45 A | 69.42 A ↑40.4% | 0.71 C | 0.35 D ↓50.7% | 222.85 C | 190.08 C ↓14.7% | 1.17 B | 1.75 B ↑49.6% | |
| Carrier agents | INU | 18.26 A | 29.45 A ↑61.3% | 1.58 B | 1.29 B ↓18.4% | 215.38 C | 192.62 C ↓10.6% | 1.49 A | 1.92 A ↑28.9% |
| MALTO | 0.41 D | 4.80 C ↑1070.7% | 1.83 A | 1.65 A ↓9.8% | 267.39 A | 231.97 A ↓13.2% | 1.41 AB | 1.93 A ↑36.9% | |
| I:M 2:1 | 15.23 B | 26.59 A ↑74.6% | 1.30 C | 1.06 C ↓18.5% | 246.74 B | 210.81 B ↓14.6% | 1.55 A | 2.08 A ↑34.2% | |
| I:M 1:2 | 11.56 C | 17.77 B ↑53.7% | 1.23 C | 1.03 C ↓16.3% | 250.52 B | 202.55 BC ↓19.1% | 1.33 B | 1.99 A ↑49.6% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkacz, K.; Wojdyło, A.; Michalska-Ciechanowska, A.; Turkiewicz, I.P.; Lech, K.; Nowicka, P. Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders. Molecules 2020, 25, 3801. https://doi.org/10.3390/molecules25173801
Tkacz K, Wojdyło A, Michalska-Ciechanowska A, Turkiewicz IP, Lech K, Nowicka P. Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders. Molecules. 2020; 25(17):3801. https://doi.org/10.3390/molecules25173801
Chicago/Turabian StyleTkacz, Karolina, Aneta Wojdyło, Anna Michalska-Ciechanowska, Igor Piotr Turkiewicz, Krzysztof Lech, and Paulina Nowicka. 2020. "Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders" Molecules 25, no. 17: 3801. https://doi.org/10.3390/molecules25173801
APA StyleTkacz, K., Wojdyło, A., Michalska-Ciechanowska, A., Turkiewicz, I. P., Lech, K., & Nowicka, P. (2020). Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders. Molecules, 25(17), 3801. https://doi.org/10.3390/molecules25173801