Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin
Abstract
1. Introduction
2. Results and Discussion
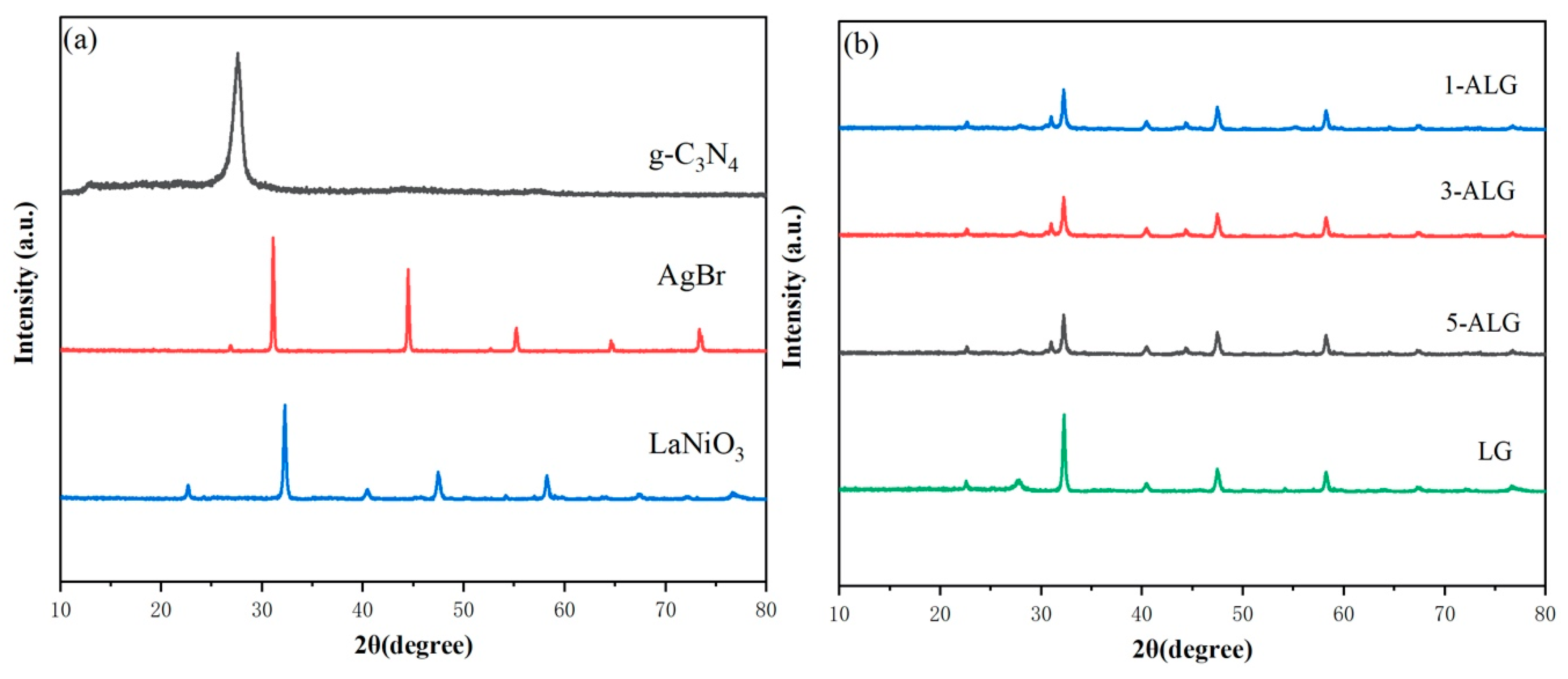
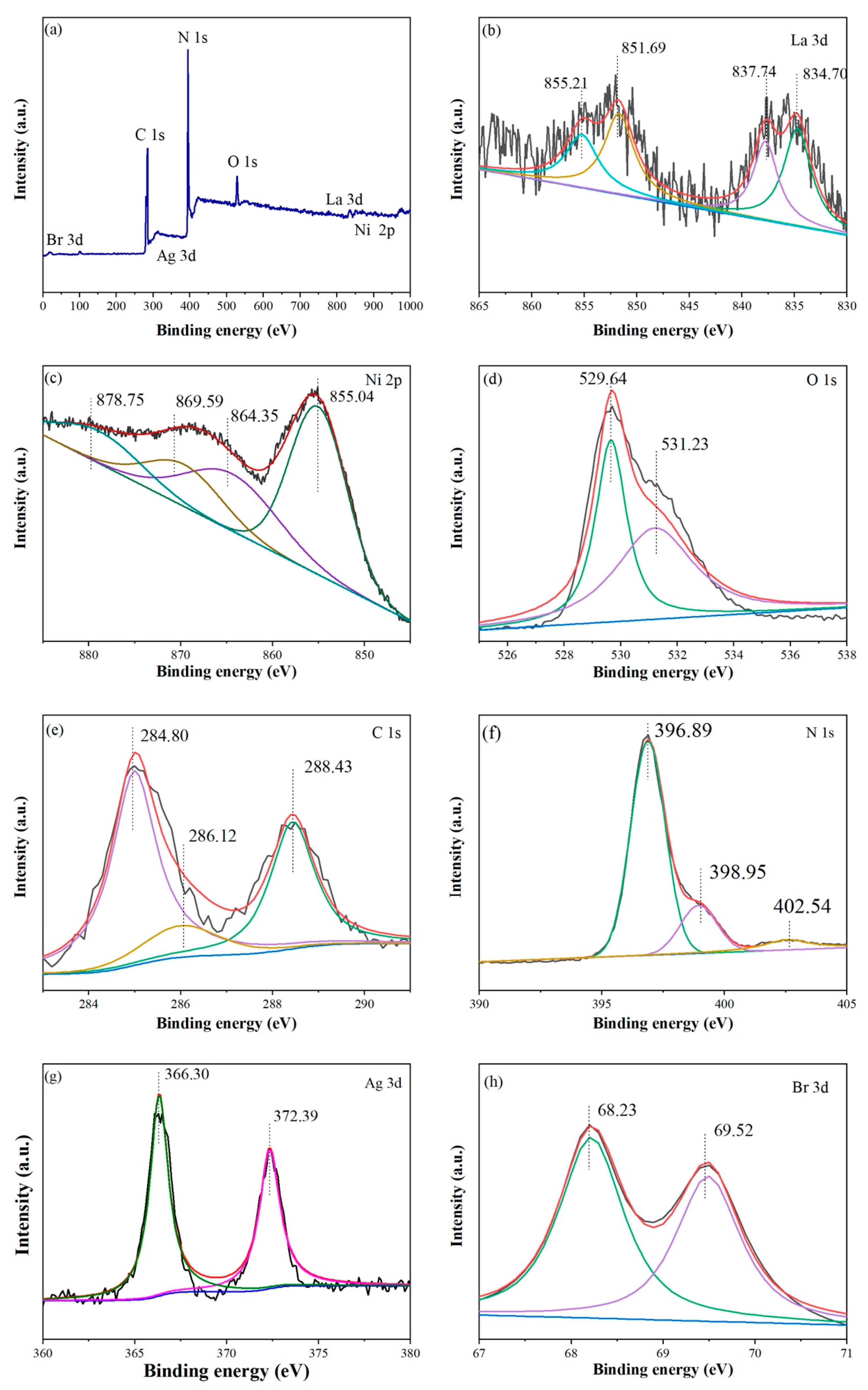
2.1. Microstructure and Surface Morphology
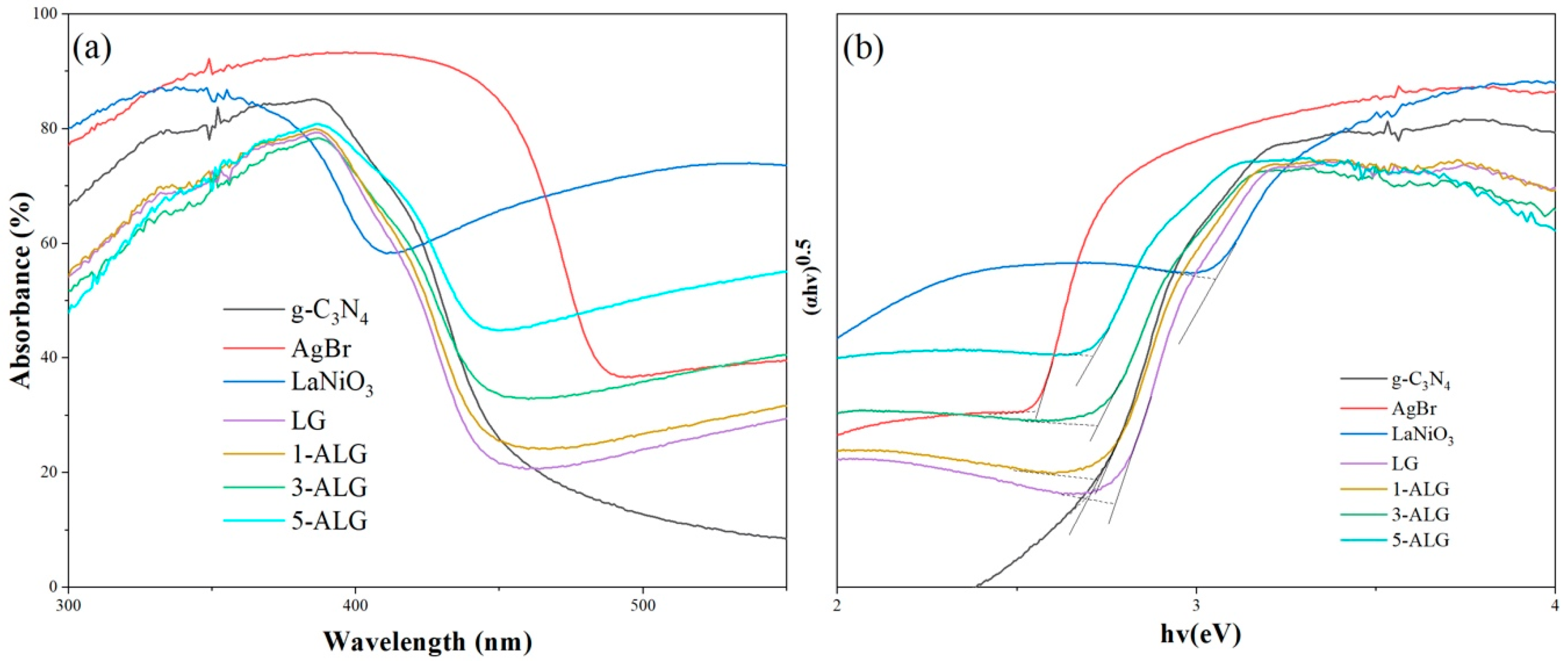
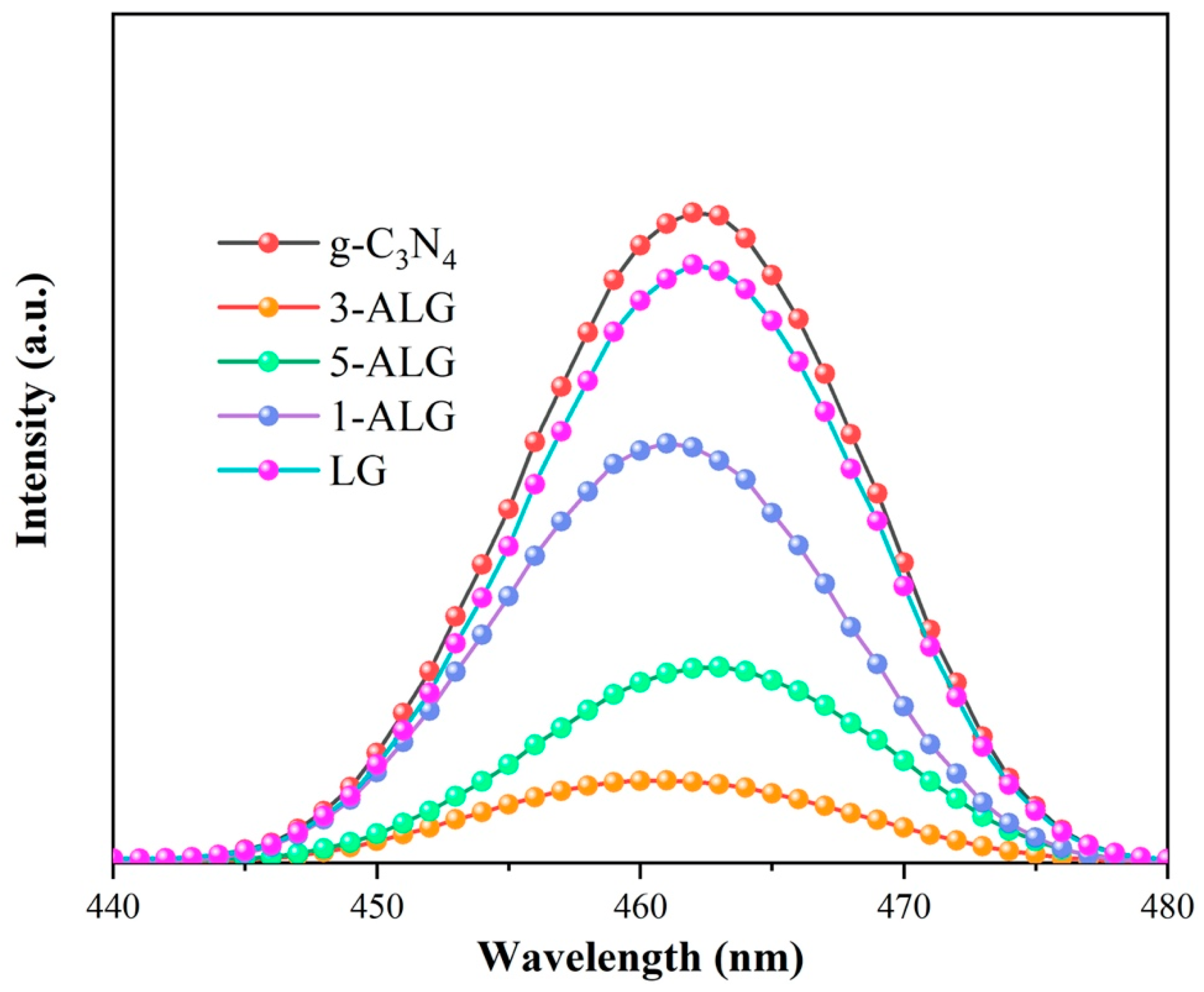
2.2. Optical Properties
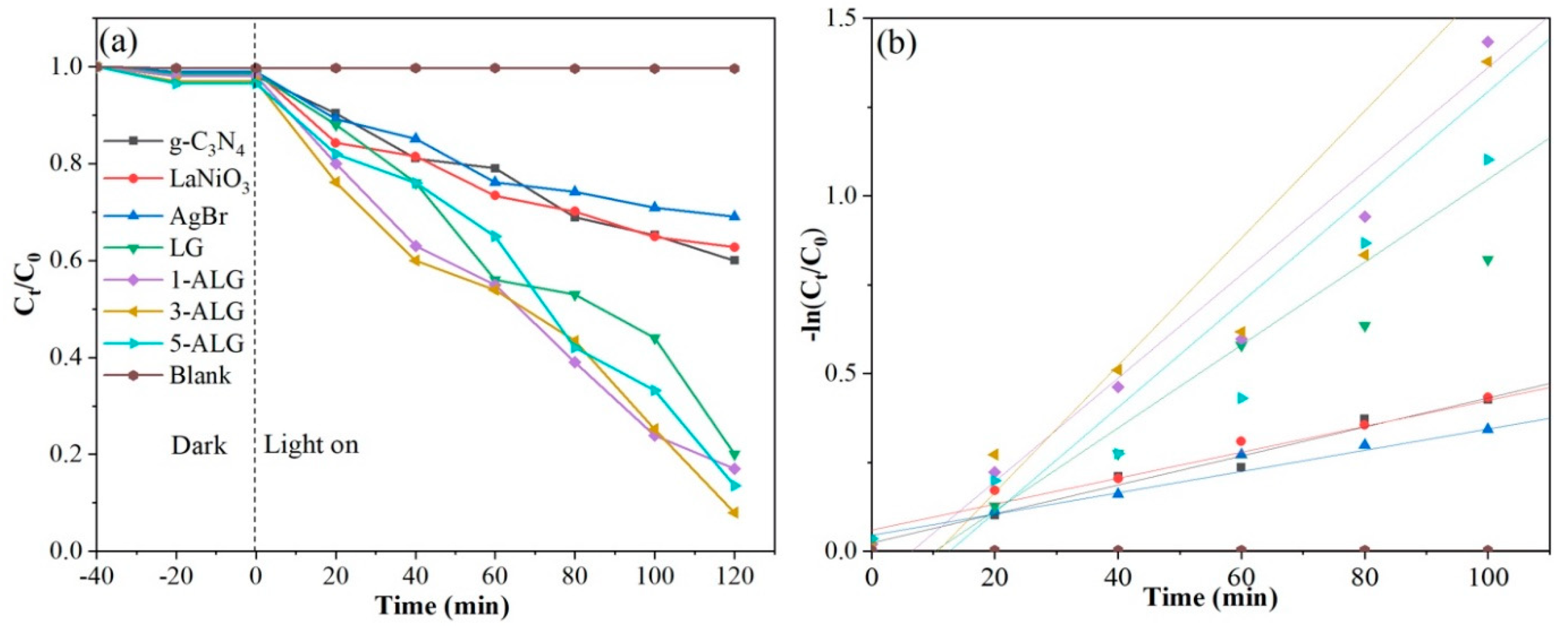
2.3. Photocatalytic Activity
2.4. Photocatalytic Degradation of NOF Comparing with Other Materials
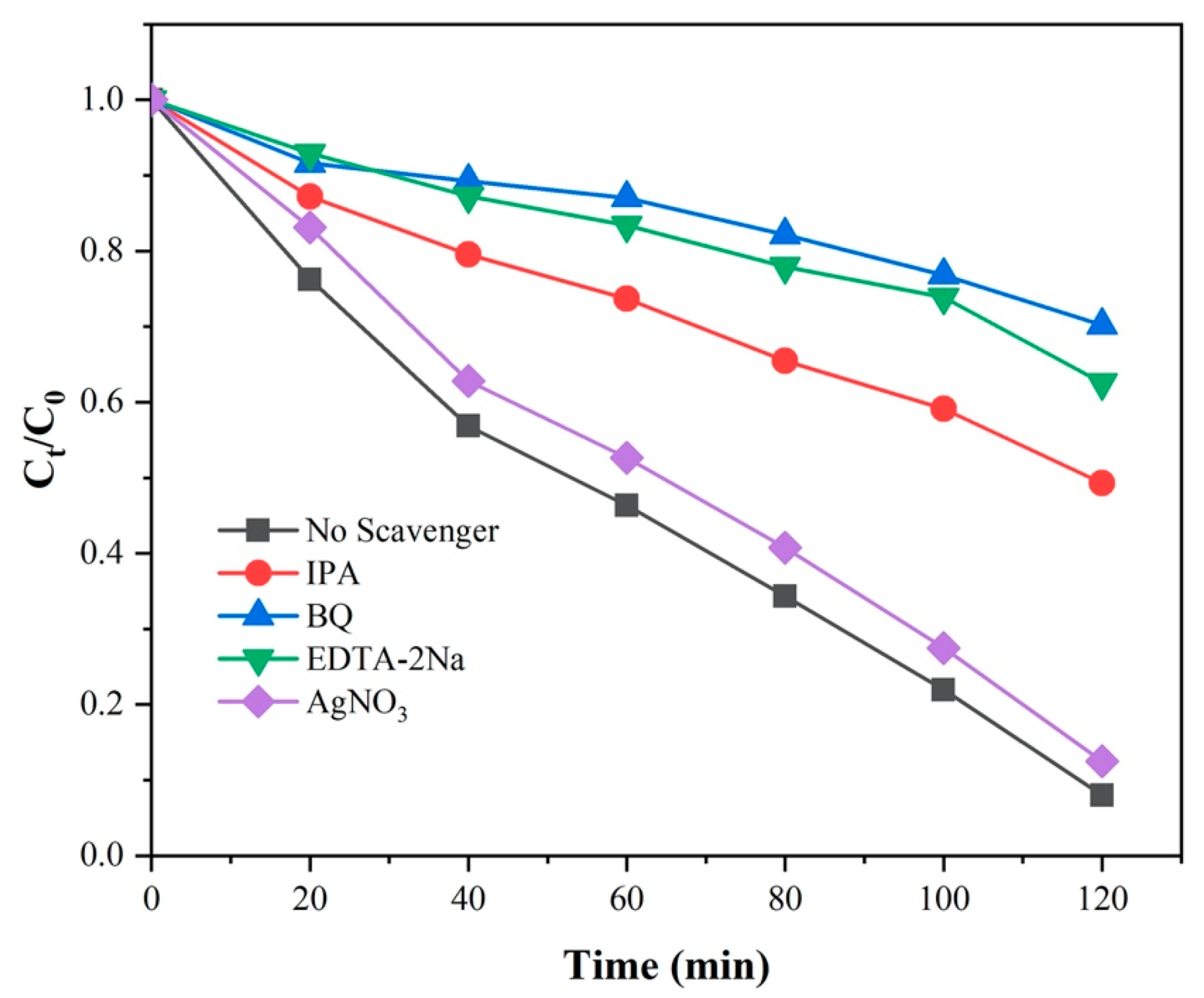
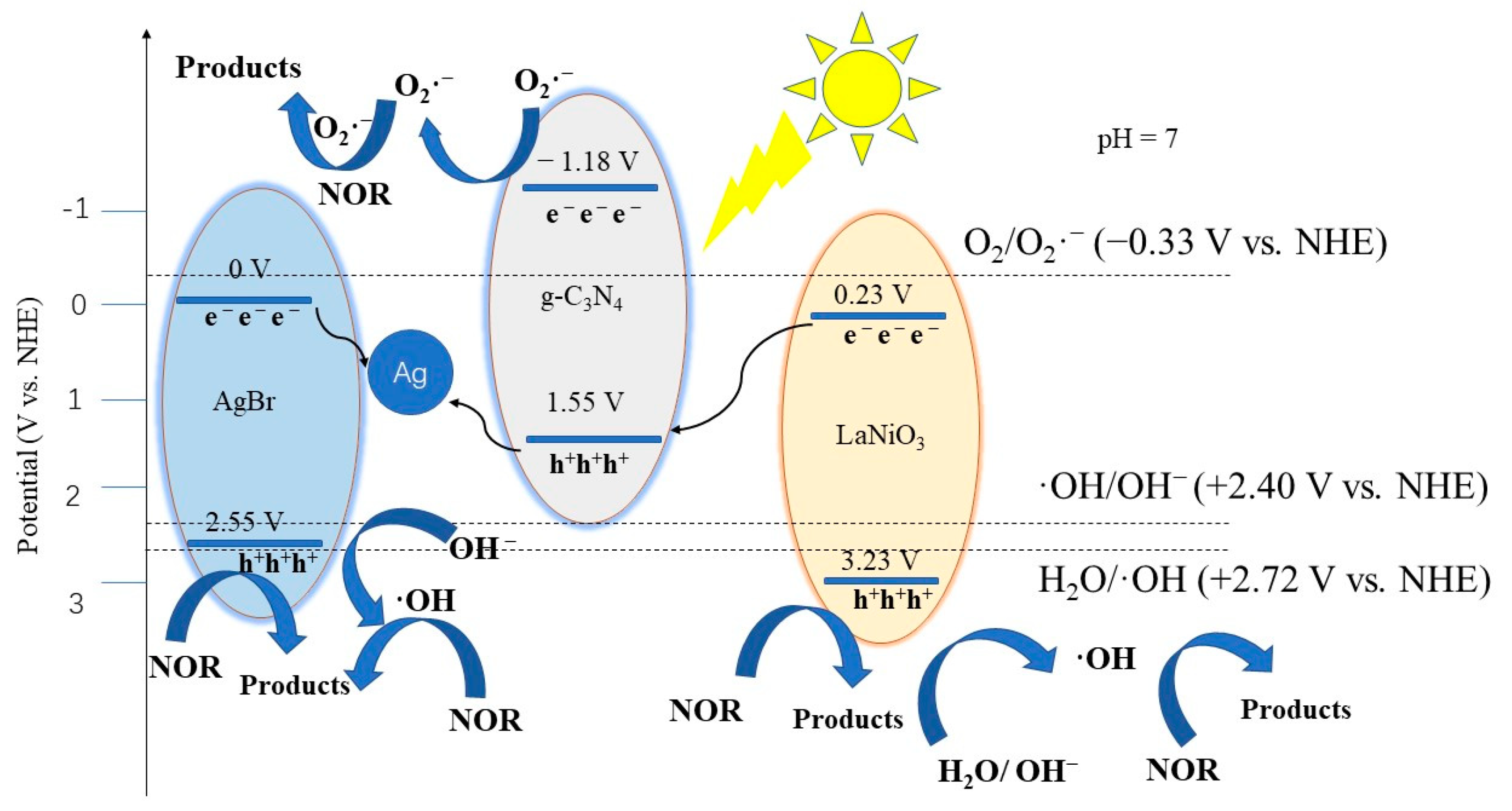
2.5. Photocatalytic Mechanism
3. Materials and Methods
3.1. Synthesis of g-C3N4 Nanosheets
3.2. Synthesis of LaNiO3 Nanospheres
3.3. Synthesis of AgBr Nanoparticles
3.4. Synthesis of LG Hybrid
3.5. Synthesis of ALG Photocatalysts
3.6. Characterization of Samples
3.7. Photodegradation Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Chu, W. Photocatalytic degradation and decomposition mechanism of fluoroquinolones norfloxacin over bismuth tungstate: Experiment and mathematic model. Appl. Catal. B Environ. 2015, 168, 175–182. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Zeng, G.; Liu, Y.; Deng, Y.; Zhou, Y.; Tang, J.; Wang, J.; Guo, Z. Enhanced photocatalytic degradation of norfloxacin in aqueous Bi2WO6 dispersions containing nonionic surfactant under visible light irradiation. J. Hazard. Mater. 2016, 306, 295–304. [Google Scholar] [CrossRef]
- Xu, M.; Ye, M.; Zhou, X.; Cheng, J.; Huang, C.; Wong, W.; Wang, Z.; Wang, Y.; Li, C. One-pot controllable synthesis of BiOBr/β-Bi2O3 nanocomposites with enhanced photocatalytic degradation of norfloxacin under simulated solar irradiation. J. Alloys Compd. 2020, 816, 152664. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Jin, P.; Wang, J.; Liu, L. Facile preparation 3D ZnS nanospheres-reduced graphene oxide composites for enhanced photodegradation of norfloxacin. J. Alloys Compd. 2017, 729, 809–815. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Yang, S.; Cai, T.; Ding, D. Rational design and synthesis of hollow Co3O4@Fe2O3 core-shell nanostructure for the catalytic degradation of norfloxacin by coupling with peroxymonosulfate. Chem. Eng. J. 2019, 359, 373–384. [Google Scholar] [CrossRef]
- Behera, A.; Kandi, D.; Sahoo, S.; Parida, K. Construction of Isoenergetic Band Alignment between CdS QDs and CaFe2O4@ZnFe2O4 Heterojunction: A Promising Ternary Hybrid toward Norfloxacin Degradation and H2 Energy Production. J. Phys. Chem. C. 2019, 123, 17112–17126. [Google Scholar] [CrossRef]
- Baoum, A.A.; Amin, M.S.; Mohamed, R.M. Decoration of SnO2 nanosheets by AgI nanoparticles driven visible light for norfloxacin degradation. Appl. Nanosci. 2018, 8, 2093–2102. [Google Scholar] [CrossRef]
- Wang, W.C.; Li, S.; Wen, Y.Y.; Gong, M.C.; Zhang, L.; Yao, Y.L.; Chen, Y.Q. Synthesis and characterization of TiO2/YFeO3 and its photocatalytic oxidation of gaseous benzene. Acta Phys.-Chim. Sin. 2008, 24, 1761–1766. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, C. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Jourshabani, M.; Lee, B.K.; Shariatinia, Z. From Traditional Strategies to Z-scheme Configuration in Graphitic Carbon Nitride Photocatalysts: Recent Progress and Future Challenges. Appl. Catal. B Environ. 2020, 276, 119157. [Google Scholar] [CrossRef]
- Ali, S.; Humayun, M.; Pi, W.; Yuan, Y.; Wang, M.; Khan, A.; Yue, P.; Shu, L.; Zheng, Z.; Fu, Q.; et al. Fabrication of BiFeO3-g-C3N4-WO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for water reduction and 2,4-dichlorophenol degradation: Insight mechanism. J. Hazard. Mater. 2020, 397, 122708. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Huang, D.; Li, J.; Zeng, G.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.; Gong, X.; Li, B. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3 /g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. Ternary magnetic g-C3N4/Fe3O4/AgI nanocomposites: Novel recyclable photocatalysts with enhanced activity in degradation of different pollutants under visible light. Mater. Chem. Phys. 2016, 174, 59–69. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Xu, Y.; Song, Y.; Li, H.; Xia, J.; Huang, C.; Wan, H. Novel visible-light-driven AgX/graphite-like C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B Environ. 2013, 129, 182–193. [Google Scholar] [CrossRef]
- Jung, W.Y.; Hong, S.S. Synthesis of LaCoO3 nanoparticles by microwave process and their photocatalytic activity under visible light irradiation. J. Ind. Eng. Chem. 2013, 19, 157–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Peng, S.; Zhang, Y.; Shen, Z.; Cao, J.J.; Ho, W.; Lee, S.C.; Pui, D.Y.H. Perovskite LaFeO3-SrTiO3 composite for synergistically enhanced NO removal under visible light excitation. Appl. Catal. B Environ. 2017, 204, 346–357. [Google Scholar] [CrossRef]
- Wang, Z.; Su, B.; Xu, J.; Hou, Y.; Ding, Z. Direct Z-scheme ZnIn2S4/LaNiO3 nanohybrid with enhanced photocatalytic performance for H2 evolution. Int. J. Hydrog. Energy 2020, 45, 4113–4121. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, R.; Li, R.; Chen, Y.; Zhou, X.; Luo, J. Construction of Z-scheme LaNiO3/SnS2 composite for boosting visible light photodegradation of tetracycline. J. Taiwan Inst. Chem. Eng. 2019, 100, 186–193. [Google Scholar] [CrossRef]
- Xu, J.; Sun, C.; Wang, Z.; Hou, Y.; Ding, Z.; Wang, S. Perovskite Oxide LaNiO3 Nanoparticles for Boosting H2 Evolution over Commercial CdS with Visible Light. Chem.-A Eur. J. 2018, 24, 18512–18517. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Yu, P.; Zhou, X.; Yan, Y.; Li, Z.; Zheng, T. Enhanced visible-light-driven photocatalytic disinfection using AgBr-modified g-C3N4 composite and its mechanism. Colloids Surf. B Biointerfaces 2019, 179, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, F.; Huo, P.; Zulfiqarc, S.; Xu, J.; Yan, Y.; Tang, H. Constructing novel visible-light-driven ternary photocatalyst of AgBr nanoparticles decorated 2D/2D heterojunction of g-C3N4/BiOBr nanosheets with remarkably enhanced photocatalytic activity for water-treatment. Ceram. Int. 2019, 45, 19197–19205. [Google Scholar] [CrossRef]
- Yu, H.; Wang, D.; Zhao, B.; Lu, Y.; Wang, X.; Zhu, S.; Qin, W.; Huo, M. Enhanced photocatalytic degradation of tetracycline under visible light by using a ternary photocatalyst of Ag3PO4/AgBr/g-C3N4 with dual Z-scheme heterojunction. Sep. Purif. Technol. 2020, 237, 116365. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yu, Y.; Huang, J.; Song, X.; Zhang, W. Z-scheme and multipathway photoelectron migration properties of a bayberry-like structure of BiOBr/AgBr/LaPO4 nanocomposites: Improvement of photocatalytic performance using simulated sunlight. J. Alloys Compd. 2020, 821, 153472. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Lv, J.; Zhang, Y.; Zhang, H.; Xu, J. A novel Z-scheme AgBr/P-g-C3N4 heterojunction photocatalyst: Excellent photocatalytic performance and photocatalytic mechanism for ephedrine degradation. Appl. Catal. B Environ. 2020, 266, 118614. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, D.; Ma, J.; Wu, D.; Yang, M.; Komarneni, S. Fabrication of AgBr/Ag2CrO4 composites for enhanced visible-light photocatalytic activity. Ceram. Int. 2015, 41, 12509–12513. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Rida, K.; Peña, M.A.; Sastre, E.; Martínez-Arias, A. Effect of calcination temperature on structural properties and catalytic activity in oxidation reactions of LaNiO3 perovskite prepared by Pechini method. J. Rare Earths 2012, 30, 210–216. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. A new application of Z-Schene Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2014, 49, 649–656. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; He, T.; Liu, X.; Deng, Q.; Mao, Y.; Wang, S. Enhanced visible-light-driven photocatalytic activities of Bi2Fe4O9/g-C3N4 composite photocatalysts. Mater. Res. Bull. 2018, 104, 104–111. [Google Scholar] [CrossRef]
- Spectroscopy, O. Water content and water profiles in skin measured by FTIR and Raman spectroscopy. Proc. SPIE 2000, 4162, 39–45. [Google Scholar]
- Zhou, X.; Chen, Y.; Li, C.; Zhang, L.; Zhang, X.; Ning, X.; Zhan, L.; Luo, J. Construction of LaNiO3 nanoparticles modified g-C3N4 nanosheets for enhancing visible light photocatalytic activity towards tetracycline degradation. Sep. Purif. Technol. 2019, 211, 179–188. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Azizi-Toupkanloo, H.; Karimi-Nazarabad, M.; Shakeri, M.; Eftekhari, M. Photocatalytic mineralization of hard-degradable morphine by visible light-driven Ag@g-C3N4 nanostructures. Environ. Sci. Pollut. Res. 2019, 26, 30941–30953. [Google Scholar] [CrossRef]
- Du, H.; Pu, W.; Wang, Y.; Yan, K.; Feng, J.; Zhang, J.; Yang, C.; Gong, J. Synthesis of BiVO4/WO3 composite film for highly efficient visible light induced photoelectrocatalytic oxidation of norfloxacin. J. Alloys Compd. 2019, 787, 284–294. [Google Scholar] [CrossRef]
- Li, J.; Han, M.; Guo, Y.; Wang, F.; Sun, C. Fabrication of FeVO4/Fe2TiO5 composite catalyst and photocatalytic removal of norfloxacin. Chem. Eng. J. 2016, 298, 300–308. [Google Scholar] [CrossRef]
- Prabavathi, S.L.; Govindan, K.; Saravanakumar, K.; Jang, A.; Muthuraj, V. Construction of heterostructure CoWO4/g-C3N4 nanocomposite as an efficient visible-light photocatalyst for norfloxacin degradation. J. Ind. Eng. Chem. 2019, 80, 558–567. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Yao, J. Shuttle-like CeO2/g-C3N4 composite combined with persulfate for the enhanced photocatalytic degradation of norfloxacin under visible light. Ecotoxicol. Environ. Saf. 2020, 190, 110062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Liu, Y.; Wang, L.; Zhou, Y.; Guo, Z.; Wang, J.; Zhang, C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 285–294. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Dong, H.; Liu, Y.; Wang, L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D interface engineering of carbon quantum dots modified Bi2WO6 ultrathin nanosheets with enhanced photoactivity for full spectrum light utilization and mechanism insight. Appl. Catal. B Environ. 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Hu, L.; Hu, H.; Lu, W.; Lu, Y.; Wang, S. Novel composite BiFeO3/ZrO2 and its high photocatalytic performance under white LED visible-light irradiation. Mater. Res. Bull. 2019, 120, 110605. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


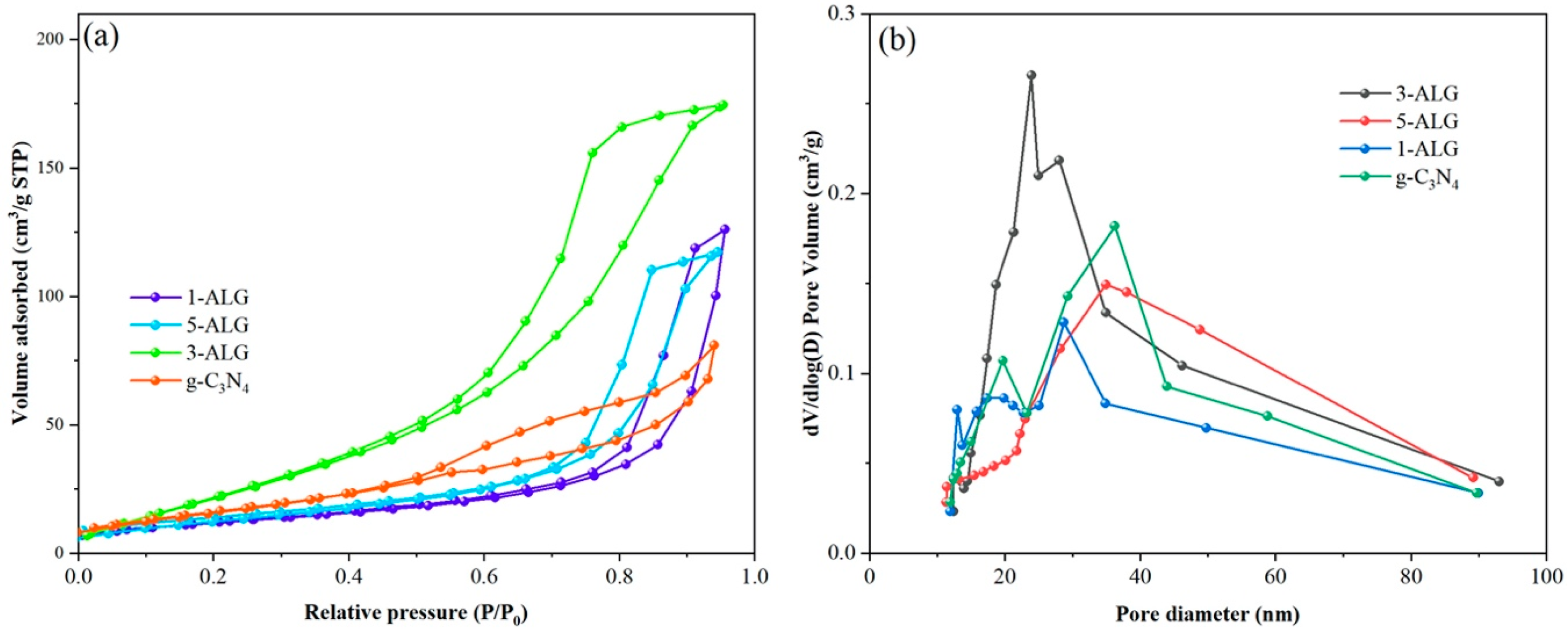


| Sample Name | Average Pore Sizes (nm) | Specific Surface Areas (m2/g) | Pore Volume (cm3/g) | Crystalline Size (nm) |
|---|---|---|---|---|
| g-C3N4 | 27.99 | 27.32 | 0.78 | 2.57 |
| 1-ALG | 27.50 | 45.81 | 0.72 | 20.56 |
| 3-ALG | 26.19 | 65.39 | 0.64 | 25.68 |
| 5-ALG | 30.54 | 30.72 | 0.89 | 26.47 |
| Sample Name | CB (V vs. NHE) | VB (V vs. NHE) |
|---|---|---|
| g-C3N4 | −1.18 | 1.55 |
| AgBr | 0 | 2.55 |
| LaNiO3 | 0.23 | 3.23 |
| Sample Name | Degradation (%) | k (min−1) | Standard Deviation | Band Gap (eV) |
|---|---|---|---|---|
| g-C3N4 | 40 | 0.00408 | 0.01425 | 2.73 |
| AgBr | 31 | 0.00365 | 0.02134 | 2.55 |
| LaNiO3 | 38 | 0.00299 | 0.02348 | 3.00 |
| LG | 80 | 0.01166 | 0.14129 | 2.77 |
| 1-ALG | 83 | 0.01456 | 0.08799 | 2.73 |
| 3-ALG | 92 | 0.01790 | 0.24918 | 2.72 |
| 5-ALG | 87 | 0.01481 | 0.18515 | 2.70 |
| Photocatalyst Name | Degradation Rate (%) | Photocatalyst Name | Degradation Rate (%) |
|---|---|---|---|
| 3-ALG | 92 | FeVO4/Fe2TiO5 | 92 |
| CeO2/g-C3N4 | 88.6 | CoWO4/g-C3N4 | 97 |
| ZnS | 75 | BiWO4/WO3 | 67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, Z.; Jiang, J.; Li, H. Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin. Molecules 2020, 25, 3706. https://doi.org/10.3390/molecules25163706
Zhang J, Zhu Z, Jiang J, Li H. Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin. Molecules. 2020; 25(16):3706. https://doi.org/10.3390/molecules25163706
Chicago/Turabian StyleZhang, Junjiao, Zhengru Zhu, Junchao Jiang, and Hong Li. 2020. "Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin" Molecules 25, no. 16: 3706. https://doi.org/10.3390/molecules25163706
APA StyleZhang, J., Zhu, Z., Jiang, J., & Li, H. (2020). Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin. Molecules, 25(16), 3706. https://doi.org/10.3390/molecules25163706




