Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical and Morphological Properties of Studied Cabbage Cultivars
2.2. Chemical Properties of Studied Cabbage Cultivars
2.3. Phenolic Acid Content of Studied Cabbage Cultivars
2.4. Volatile Profile of Studied Cabbage Cultivars
3. Materials and Methods
3.1. Chemicals
3.2. Cabbages Used for the Experiment
3.3. Physical Analysis
3.4. Texture Analysis
3.5. Colour Measurement
3.6. Morphological Analysis
3.7. Chemical Analysis
3.8. Total Polyphenol Determination
3.9. Antioxidant Activity Measured with DPPH Method
3.10. Vitamin C Method
3.11. Volatile Extraction and Analysis
3.12. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gong, Z.; Zhang, M.; Sun, J. Physico-chemical properties of cabbage powder as affected by drying methods. Dry. Technol. 2007, 25, 913–916. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Fernando Reyes, L.; Emilio Villarreal, J.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Changes in antioxidant compounds in white cabbage during winter storage. Postharvest Biol. Technol. 2009, 52, 173–179. [Google Scholar] [CrossRef]
- Silambarasan, T.; Manivannan, J.; Priya, M.K.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid protects heart against ischemia/reperfusion injury and H9c2 cardiomyoblast cells against oxidative stress. Biochem. Biophys. Res. Commun. 2015, 456, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, T.; Manivannan, J.; Raja, B.; Chatterjee, S. Prevention of cardiac dysfunction, kidney fibrosis and lipid metabolic alterations in l-NAME hypertensive rats by sinapic acid - Role of HMG-CoA reductase. Eur. J. Pharmacol. 2016, 777, 113–123. [Google Scholar] [CrossRef]
- Kaulmann, A.; André, C.M.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chem. 2016, 197, 325–332. [Google Scholar] [CrossRef]
- Jakobek, L.; Tomac, I.; Matic, P.; Sabo, M.; Dugum, J.; Subaric, D. Bioactive polyphenolic compounds from white cabbage cultivars. Croat. J. Food Sci. Technol. 2018, 10, 164–172. [Google Scholar] [CrossRef]
- Lonchamp, J.; Barry-Ryan, C.; Devereux, M. Identification of volatile quality markers of ready-to-use lettuce and cabbage. Food Res. Int. 2009, 42, 1077–1086. [Google Scholar] [CrossRef]
- Radovich, T.J.K. Cabbage Flavor. In Handbook of Fruit and Vegetable Flavors; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 741–750. ISBN 9780470227213. [Google Scholar]
- Akpolat, H.; Barringer, S.A. The Effect of pH and Temperature on Cabbage Volatiles During Storage. J. Food Sci. 2015, 80, S1878–S1884. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Jiang, N.; Choi, S.H.; Lim, Y.P.; Park, J.T.; Al-Dhabi, N.A.; Kim, S.J. Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (Brassica oleracea var. capitata). Ind. Crops Prod. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar]
- Balkaya, A.; Yanmaz, R.; Apaydin, A.; Kar, H. Morphological characterisation of white head cabbage (Brassica oleracea var. Capitata subvar. alba) genotypes in Turkey. New Zeal. J. Crop Hortic. Sci. 2005. [Google Scholar] [CrossRef]
- Cvetković, B.R.; Pezo, L.L.; Pestorić, M.; Filipčev, B.; Kevrešan, Ž.; Mastilović, J. Comparative study of white cabbage, traditional variety and hybrid intended for biological fermentation. J. Appl. Bot. Food Qual. 2014, 87, 286–290. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; de Sousa Galvâo, M.; Dutra Sandes, R.D.; Leite Neta, M.T.S.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT Food Sci. Technol. 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Yan-Hwa, C.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Rubatzky, V.R. Handbook of Vegetable Science and Technogogy: Production, Composition, Storage and Processing. Horttechnology 2018, 8, 622. [Google Scholar] [CrossRef]
- Podsȩdek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Hanif, R.; Iqbal, Z.; Iqbal, M.; Hanif, S.; Rasheed, M. Use of vegetables as nutritional food: Role in human health. J. Agric. Biol. Sci. 2006, 1, 18–22. [Google Scholar]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayirlioglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Martínez, S.; Olmos, I.; Carballo, J.; Franco, I. Quality parameters of Brassica spp. Grown in northwest Spain. Int. J. Food Sci. Technol. 2010, 45, 776–783. [Google Scholar] [CrossRef]
- Wennberg, M.; Ekvall, J.; Olsson, K.; Nyman, M. Changes in carbohydrate and glucosinolate composition in white cabbage (Brassica oleracea var. capitata) during blanching and treatment with acetic acid. Food Chem. 2006. [Google Scholar] [CrossRef]
- Del Carmen Mondragón-Portocarrero, A.; Pena-Martínez, B.; Fernández-Fernández, E.; Romero-Rodríguez, A.; Vázquez-Odériz, L. Effects of different pre-freezing blanching procedures on the physicochemical properties of Brassica rapa leaves (Turnip Greens, Grelos). Int. J. Food Sci. Technol. 2006, 41, 1067–1072. [Google Scholar] [CrossRef]
- Artés, F.; Vallejo, F.; Martínez, J.A. Quality of broccoli as influenced by film wrapping during shipment. Eur. Food Res. Technol. 2001, 213, 480–483. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Kim, J.K.; Kim, D.H.; Yook, H.S.; Byun, M.W. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.). Food Chem. 2005, 89, 589–597. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant properties and lipidic profile as quality indexes of cauliflower (Brassica oleracea L. var. botrytis) in relation to harvest time. Food Chem. 2007, 100, 1019–1025. [Google Scholar] [CrossRef]
- Sreeramulu, D.; Raghunath, M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Phenolic phytochemicals in cabbage inhibit amyloid β protein-induced neurotoxicity. LWT Food Sci. Technol. 2006, 39, 331–337. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Jacob, J.A.; Mahal, H.S.; Mukherjee, T.; Kapoor, S. Free radical reactions with the extract of brassica family. Food Chem. 2011, 129, 1132–1138. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Šamec, D.; Piljac-Žegarac, J.; Bogović, M.; Habjanič, K.; Grúz, J. Antioxidant potency of white (Brassica oleracea L. var. capitata) and Chinese (Brassica rapa L. var. pekinensis (Lour.)) cabbage: The influence of development stage, cultivar choice and seed selection. Sci. Hortic. (Amsterdam) 2011, 128, 78–83. [Google Scholar] [CrossRef]
- Šamec, D.; Bogović, M.; Vincek, D.; Martinčić, J.; Salopek-Sondi, B. Assessing the authenticity of the white cabbage (Brassica oleracea var. capitata f. alba) cv. “Varaždinski” by molecular and phytochemical markers. Food Res. Int. 2014, 60, 266–272. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.). LWT Food Sci. Technol. 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Hui, Y.H. Handbook of Fruits and Fruit Processing; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780813819815. [Google Scholar]
- Hong, E.; Kim, G.H. GC-MS analysis of the extracts from korean cabbage (Brassica campestris L. ssp. pekinensis) and Its seed. Prev. Nutr. Food Sci. 2013, 18, 218–221. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Schijvens, E.P.H.M.; Biekman, E.S.A. Modelling the change in colour of broccoli and green beans during blanching. Innov. Food Sci. Emerg. Technol. 2001, 2, 303–313. [Google Scholar] [CrossRef]
- Baumann, G.; Gierschner, K. Determination of Sugars in Fruit Juices. Comparison of Results Obtained With Enzymic and Luff-Schoorl Methods; International Fruchtsaft-Union, Wissenschaftlich-Technische Kommission, [Berichte]: Paris, France, 1970; Volume 10, pp. 115–140. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
Sample Availability: Samples of the four cabbage cultivars are available from the authors |
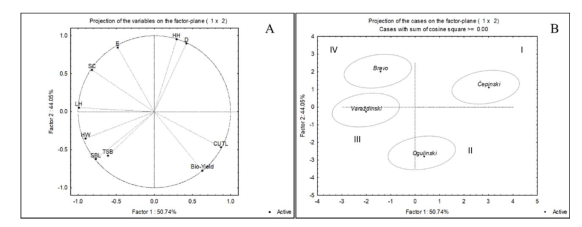
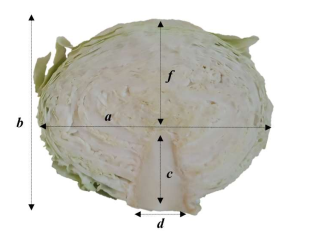
| ‘Čepinski’ | ‘Varaždinski’ | ‘Bravo’ | ‘Ogulinski’ | |
|---|---|---|---|---|
| Head mass (g) | 1549.28 ± 267.47bc | 1487.62 ± 348.77bc | 1677.73 ± 191.17ab | 1384.12 ± 316.44cd |
| Head width (cm) | 14.70 ± 0.20c | 18.40 ± 0.36a | 16.63 ± 1.46b | 16.90 ± 0.69ab |
| Head height (cm) | 14.70 ± 1.59a | 12.37 ± 1.00bc | 14.17 ± 0.61ab | 10.47 ± 0.65c |
| Height/width ratio | 1 | 0.67 | 0.85 | 0.62 |
| Stem base length (cm) | 5.30 ± 0.98c | 8.30 ± 0.61a | 6.70 ± 0.70b | 7.87 ± 0.32ab |
| Thickness of stem base (cm) | 3.50 ± 0.10a | 3.83 ± 0.21a | 3.93 ± 0.51a | 4.10 ± 0.62a |
| Distance from the top of stem base to the top of the head (cm) | 8.20 ± 0.30a | 4.70 ± 1.21b | 7.60 ± 0.87a | 3.80 ± 0.61b |
| Texture | ||||
| Bio-yield point (g) | 1105.23 ± 8.99b | 833.62 ± 8.99c | 557.24 ± 9.07d | 1308.65 ± 10.75a |
| Leaves hardness (g) | 369.71 ± 22.72b | 430.57 ± 11.74a | 432.85 ± 16.63a | 404.83 ± 14.26ab |
| Elasticity (mm) | 69.71 ± 2.71b | 73.57 ± 1.75b | 88.57 ± 1.19a | 60.84 ± 1.89c |
| Surface colour | ||||
| L | 68.66 ± 5.23a | 54.10 ± 7.23c | 62.77 ± 8.25ab | 72.69 ± 3.03b |
| a* | −18.78 ± 1.43b | −18.75 ± 2.80ab | −18.65 ± 1.35b | −21.37 ± 0.84a |
| b* | 34.98 ± 0.83b | 31.31 ± 7.36bc | 31.12 ± 4.31bc | 40.03 ± 1.20a |
| greenness level (-a*/b*) | 0.54 | 0.60 | 0.60 | 0.53 |
| Colour underneath two leaves | ||||
| L | 75.77 ± 3.50b | 77.12 ± 1.81b | 81.23 ±1.09a | 81.33 ± 1.38a |
| a* | −15.37 ± 2.65ab | −14.54 ± 1.11b | −12.40 ± 0.43c | −16.36 ± 0.97a |
| b* | 29.71 ± 2.96b | 30.83 ± 2.76ab | 24.74 ± 0.76c | 31.69 ± 1.72a |
| greenness level (-a*/b*) | 0.52 | 0.50 | 0.50 | 0.52 |
| ‘Čepinski’ | ‘Varaždinski’ | ‘Bravo’ | ‘Ogulinski’ | |
|---|---|---|---|---|
| Water content (%) | 93.10 ± 0.025b | 90.41 ± 0.080a | 93.61 ± 0.065cd | 93.73 ± 0.055d |
| Crude protein (g/100g) | 1.89 ± 0.025b | 2.42 ± 0.015a | 1.52 ± 0.025d | 1.59 ± 0.005c |
| Crude ash (g/100g) | 0.63 ± 0.010b | 0.70 ± 0.005a | 0.61 ± 0.005c | 0.53 ± 0.010d |
| Insoluble fibres % | 1.62 ± 0.002d | 2.12 ± 0.005a | 2.06 ± 0.003b | 1.87 ± 0.001c |
| Soluble fibres % | 0.74 ± 0.001b | 0.43 ± 0.000d | 0.83 ±0.001a | 0.67 ± 0.002c |
| Total fibres % | 2.35 ± 0.007d | 2.55 ± 0.001b | 2.89 ± 0.003a | 2.54 ±0.000c |
| Invert sugars (g/100 g) | 3.64 ± 0.002b | 3.65 ± 0.002a | 3.48 ± 0.004c | 2.97 ± 0.001d |
| Total sugars (g/100 g) | 3.96 ± 0.001b | 4.23 ± 0.002a | 3.62 ± 0.000c | 3.53 ± 0.001d |
| Soluble solids content (°Brix) | 6.90 ± 0.02b | 8.50 ± 0.01a | 6.70 ± 0.01c | 5.70 ± 0.01d |
| pH | 6.11 ± 0.13a | 5.87 ± 0.07b | 6.25 ± 0.06a | 6.16 ± 0.09a |
| TPC (mg (GAE)/kg FW) | 496.02 ± 5.88b | 594.80 ± 7.43a | 401.62 ± 11.10d | 480.95 ± 1.40c |
| DPPH (µmoL DPPH/g sample) | 1.18 ± 0.008a | 1.16 ± 0.057ab | 0.71 ± 0.003d | 1.02 ± 0.001c |
| Ascorbic acid (mg/100 g) | 18.52 ± 0.64a | 15.19 ± 0.97b | 13.45 ± 0.49d | 14.35 ± 0.66c |
| ‘Čepinski’ | ‘Varaždinski’ | ‘Bravo’ | ‘Ogulinski’ | |
|---|---|---|---|---|
| Caffeic acid | 1.76 ± 0.10b | 2.06 ± 0.16a | 1.49 ± 0.04c | 2.16 ± 0.08a |
| p-coumaric acid | 0.64 ± 0.06c | 1.27 ± 0.04a | 0.41 ± 0.03d | 0.66 ± 0.02c |
| Ferulic acid | 6.52 ± 0.15d | 8.18 ± 0.09a | 4.96 ± 0.17e | 7.77 ± 0.013b |
| Sinapic acid | 78.15 ± 0.28b | 96.45 ± 0.18a | 68.56 ± 0.16c | 65.9 ± 0.22d |
| Chlorogenic acid | 0.24 ± 0.05c | 0.43 ± 0.06a | 0.33 ± 0.01b | 0.25 ± 0.04c |
| 3,5-Dihydroxy-benzoic acid | 4.11 ± 0.13c | 5.74 ± 0.08a | 3.64 ± 0.06d | 4.61 ± 0.08b |
| Compound | RT | RI | ‘Čepinski’ | ‘Varaždinski’ | ‘Bravo’ | ‘Ogulinski’ |
|---|---|---|---|---|---|---|
| Aldehydes and Ketones | 2953.99 ± 56.23 | 4478.59 ± 181.72 | 1883.3 ± 19.68 | 1839.62 ± 136.91 | ||
| Hexanal | 13.0730 | 1078 | 98.99 ± 0.1 | 71.41 ± 1.16 | 100.62 ± 1.05 | 136.41 ± 2.53 |
| Cis-3-hexenal | 15.4762 | 1151 | n.d. | n.d. | 8.11 ± 0.07 | n.d. |
| 2-hexenal | 17.6132 | 1215 | 2207.64 ± 34.96 | 3287.63 ± 50.67 | 948.58 ± 3.75 | 1035.35 ± 98.32 |
| Octanal | 19.1960 | 1268 | 20.15 ± 13.68 | 18.41 ± 1.07 | 7.5 ± 0.04 | 7.05 ± 0.43 |
| 2-heptenal | 20.8480 | 1322 | 44.93 ± 0.46 | 19.45 ± 0.2 | 30.88 ± 0.17 | 36.74 ± 1.99 |
| 6-methyl-5-heptene-2-one | 21.1484 | 1333 | 13.69 ± 0.32 | n.d. | 11.76 ± 0.01 | 4.05 ± 0.1 |
| Nonanal | 22.7485 | 1388 | 112.82 ± 2.45 | 208.46 ± 4 | 176.75 ± 6.83 | 124.24 ± 12.33 |
| 2,4-heksadienal | 23.1586 | 1402 | 27.72 ± 0.04 | 167.51 ± 9.43 | 61.24 ± 0.45 | 75.46 ± 5.63 |
| 2-octanal | 23.8287 | 1428 | 73.86 ± 0.58 | 310.61 ± 11.87 | 229.82 ± 3.2 | 201.14 ± 11.62 |
| 2,4-heptadienal | 24.7991 | 1464 | 43.01 ± 0.12 | 32.23 ± 0.63 | 30.78 ± 0.19 | 35.66 ± 0.1 |
| Benzaldehyde | 26.4743 | 1528 | 17.67 ± 0.34 | 22.75 ± 0.85 | 23.39 ± 0.13 | 27.85 ± 0.56 |
| 2-nonenal | 26.5956 | 1533 | 24.55 ± 0.25 | 43.21 ± 0.39 | 30.55 ± 0.23 | 34.13 ± 0.89 |
| 3,5-octadiene-2-one | 27.5025 | 1569 | 31.08 ± 0.02 | 59.58 ± 1.1 | 33.56 ± 1.31 | 33.25 ± 0.62 |
| 2,6-nonadienal | 27.8895 | 1584 | 22.85 ± 0.2 | 14.57 ± 0.49 | 12.61 ± 0.52 | 16.46 ± 0.14 |
| Undecanal | 28.2188 | 1597 | 34.86 ± 0.02 | n.d. | 23.27 ± 0.13 | n.d. |
| 2-decenal | 29.2470 | 1641 | 11.76 ± 0.18 | 23.3 ± 0 | 9.1 ± 0.02 | 18.14 ± 0.03 |
| 4-ethyl benzaldehyde | 30.9684 | 1714 | 29.9 ± 0.82 | 61.07 ± 50.69 | 22.82 ± 0.35 | 13.01 ± 0.78 |
| 2 4-decadienal | 32.0774 | 1763 | 28.99 ± 0.3 | 58.24 ± 47.46 | 14.61 ± 0.35 | 33.06 ± 0.81 |
| Geranyl acetone | 33.9201 | 1847 | 102.23 ± 1.15 | 80.17 ± 1.71 | 90.18 ± 0.59 | n.d. |
| Tetradecanal | 35.3527 | 1914 | 7.29 ± 0.24 | n.d. | 17.17 ± 0.29 | 7.62 ± 0.04 |
| Terpens | 243.34 ± 1.88 | 217.19 ± 7.05 | 241.36 ± 1.19 | 165.87 ± 7.37 | ||
| Verbenen | 14.3033 | 1113 | 23.76 ± 0.07 | 12.46 ± 0.36 | 6.66 ± 0.07 | 11.18 ± 0.51 |
| D-limonene | 16.7987 | 1189 | 40.75 ± 0.22 | 26.11 ± 1.24 | 14.24 ± 0.02 | 21.77 ± 0.6 |
| β-cyclocitral | 28.8637 | 1625 | 13.74 ± 0.1 | 8.72 ± 0.18 | 8.48 ± 0.09 | 8.59 ± 0.46 |
| Estragole | 29.9343 | 1670 | 14.48 ± 0.31 | 8.7 ± 0.04 | n.d. | n.d. |
| Felandral | 31.3149 | 1729 | 28.57 ± 0.12 | 21.23 ± 0.02 | 22.68 ± 0.54 | 24.93 ± 0.74 |
| Camphene | 31.8233 | 1752 | 19.64 ± 0.41 | 12.93 ± 1.21 | 15.75 ± 0.13 | 9.19 ± 0.78 |
| Carveol | 33.5389 | 1829 | 59.7 ± 0.07 | 54.17 ± 0.54 | 72.99 ± 0.04 | 30.48 ± 1.49 |
| Elemicin | 41.2796 | 2234 | n.d. | 9.19 ± 0.06 | n.d. | 6.12 ± 0.13 |
| Myristicin | 42.1746 | 2276 | 42.7 ± 0.56 | 56.77 ± 3.25 | 75.79 ± 0.14 | 36.6 ± 1.7 |
| Farnesol | 42.2844 | 2281 | n.d. | 6.92 ± 0.16 | 24.78 ± 0.15 | 17.01 ± 0.96 |
| Alcohols | 382.85 ± 2.34 | 195.63 ± 5.1 | 255.18 ± 1.18 | 311.48 ± 15.6 | ||
| 2-Penten-1-ol | 20.5708 | 1312 | 6.91 ± 0.08 | 12.97 ± 0.12 | 4.84 ± 0.09 | 8.81 ± 0.17 |
| 2-ethyl hexanol | 25.1630 | 1478 | 89.83 ± 0.33 | 77.24 ± 1.72 | 47.32 ± 0.68 | 45.91 ± 1.81 |
| Octanol | 26.9017 | 1545 | 48.53 ± 0.29 | 21.98 ± 0.32 | 21.9 ± 0.05 | 30.84 ± 2.04 |
| 2-octenol | 28.3690 | 1603 | 30.96 ± 0.01 | 8.48 ± 0.03 | 8.76 ± 0.02 | 9.3 ± 0.85 |
| 1-nonanol | 29.3856 | 1647 | 55.68 ± 0.34 | 27.68 ± 0.79 | 33.59 ± 0.1 | 38.86 ± 1.04 |
| Decanol | 31.7540 | 1749 | 35.23 ± 0.36 | 16.67 ± 1.03 | 32.1 ± 0.02 | 40.87 ± 2.82 |
| Tetradecanol | 36.1152 | 1952 | 88.45 ± 0.56 | 30.63 ± 1.1 | 56.11 ± 0.16 | 18.83 ± 0.44 |
| Perillyl alcohol | 37.1667 | 2003 | 27.28 ± 0.36 | n.d. | 50.56 ± 0.07 | 118.06 ± 6.41 |
| Acids | 23.61 ± 0.32 | 57.1 ± 2.75 | 30.01 ± 0.34 | 18.53 ± 0.69 | ||
| Nonanoic acid | 40.1586 | 2173 | 9.24 ± 0.12 | 25.42 ± 0.36 | n.d. | n.d. |
| Decanoic acid | 42.0649 | 2271 | 8.61 ± 0.17 | 23.7 ± 2.37 | 15.5 ± 0.02 | 6.06 ± 0.14 |
| Lauric acid | 46.0102 | 2479 | 5.75 ± 0.04 | 7.98 ± 0.01 | 14.5 ± 0.31 | 12.47 ± 0.55 |
| Esters | 2758.96 ± 17.7 | 2860.92 ± 85.24 | 2191.54 ± 34.13 | 2247.88 ± 131.69 | ||
| Isobutyl isothiocyanate | 20.6978 | 1316 | 23.26 ± 0.1 | 27.88 ± 2.25 | 36.38 ± 0.37 | 27.52 ± 1.28 |
| Allyl isothiocyanate | 22.4019 | 1377 | 1090.26 ± 9.37 | 311.14 ± 14.9 | 66.55 ± 0.71 | 93.92 ± 5.27 |
| Pentyl isothiocyanate | 25.3768 | 1485 | 12.88 ± 0.35 | 26.32 ± 1.16 | 16.7 ± 0.13 | 5.97 ± 0.09 |
| Isopropyl myristate | 37.4784 | 2019 | 12.54 ± 0.21 | 59.14 ± 0.07 | 46.55 ± 0.37 | 7.73 ± 0.06 |
| Benzyl isothiocyanate | 39.2922 | 2116 | 163.21 ± 1.72 | 868.1 ± 1.89 | 403.96 ± 5.82 | 1507.64 ± 93.14 |
| Phenyl ethyl isothiocyanate | 41.6086 | 2250 | 1056.69 ± 2.2 | 1050.93 ± 19.66 | 1382.36 ± 22.7 | 408.61 ± 23.48 |
| Methyl dihydrojasmonate | 42.4461 | 2289 | 51.05 ± 0.92 | 24.6 ± 0.51 | 16.3 ± 0.08 | 48.68 ± 2.14 |
| Diethyl phthalate | 44.0173 | 2376 | 10.88 ± 0.46 | 52.63 ± 43.06 | 12.21 ± 0.33 | 5.83 ± 0.3 |
| Diisobutyl phthalate | 47.4659 | 2546 | 293.28 ± 2.21 | 393.85 ± 0.42 | 166.9 ± 3.42 | 126.49 ± 5.32 |
| Dibutyl phthalate | 51.7347 | 2708 | 44.92 ± 0.17 | 46.32 ± 1.34 | 43.64 ± 0.2 | 15.5 ± 0.63 |
| Miscellaneous compounds | 954.32 ± 13.7 | 741.33 ± 64.88 | 615.02 ± 6.4 | 859.77 ± 28.65 | ||
| 3-butene nitrile | 16.3829 | 1177 | 30.32 ± 0.02 | 26.51 ± 1 | 23.53 ± 0.06 | 31.36 ± 1.62 |
| 2-pentilfuran | 17.8732 | 1224 | 11.74 ± 0.05 | 61.37 ± 49.94 | 96.09 ± 0.46 | 83.77 ± 13.71 |
| Phenylacetonitrile | 35.8263 | 1938 | 157.98 ± 7.44 | 56.26 ± 0.37 | 32.07 ± 0.41 | 339.59 ± 2.82 |
| 2,4-di-t-butylphenol | 42.6772 | 2299 | 754.28 ± 6.19 | 597.19 ± 13.58 | 463.33 ± 5.47 | 405.05 ± 10.51 |
| * internal standard: mirtenol | 32.7417 | 1792 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, A.; Marček, T.; Šubarić, D.; Jozinović, A.; Babić, J.; Miličević, B.; Sinković, K.; Šubarić, D.; Ačkar, Đ. Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages. Molecules 2020, 25, 3696. https://doi.org/10.3390/molecules25163696
Lončarić A, Marček T, Šubarić D, Jozinović A, Babić J, Miličević B, Sinković K, Šubarić D, Ačkar Đ. Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages. Molecules. 2020; 25(16):3696. https://doi.org/10.3390/molecules25163696
Chicago/Turabian StyleLončarić, Ante, Tihana Marček, Domagoj Šubarić, Antun Jozinović, Jurislav Babić, Borislav Miličević, Karmen Sinković, Drago Šubarić, and Đurđica Ačkar. 2020. "Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages" Molecules 25, no. 16: 3696. https://doi.org/10.3390/molecules25163696
APA StyleLončarić, A., Marček, T., Šubarić, D., Jozinović, A., Babić, J., Miličević, B., Sinković, K., Šubarić, D., & Ačkar, Đ. (2020). Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages. Molecules, 25(16), 3696. https://doi.org/10.3390/molecules25163696









