Platinum (IV) Recovery from Waste Solutions by Adsorption onto Dibenzo-30-crown-10 Ether Immobilized on Amberlite XAD7 Resin–Factorial Design Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
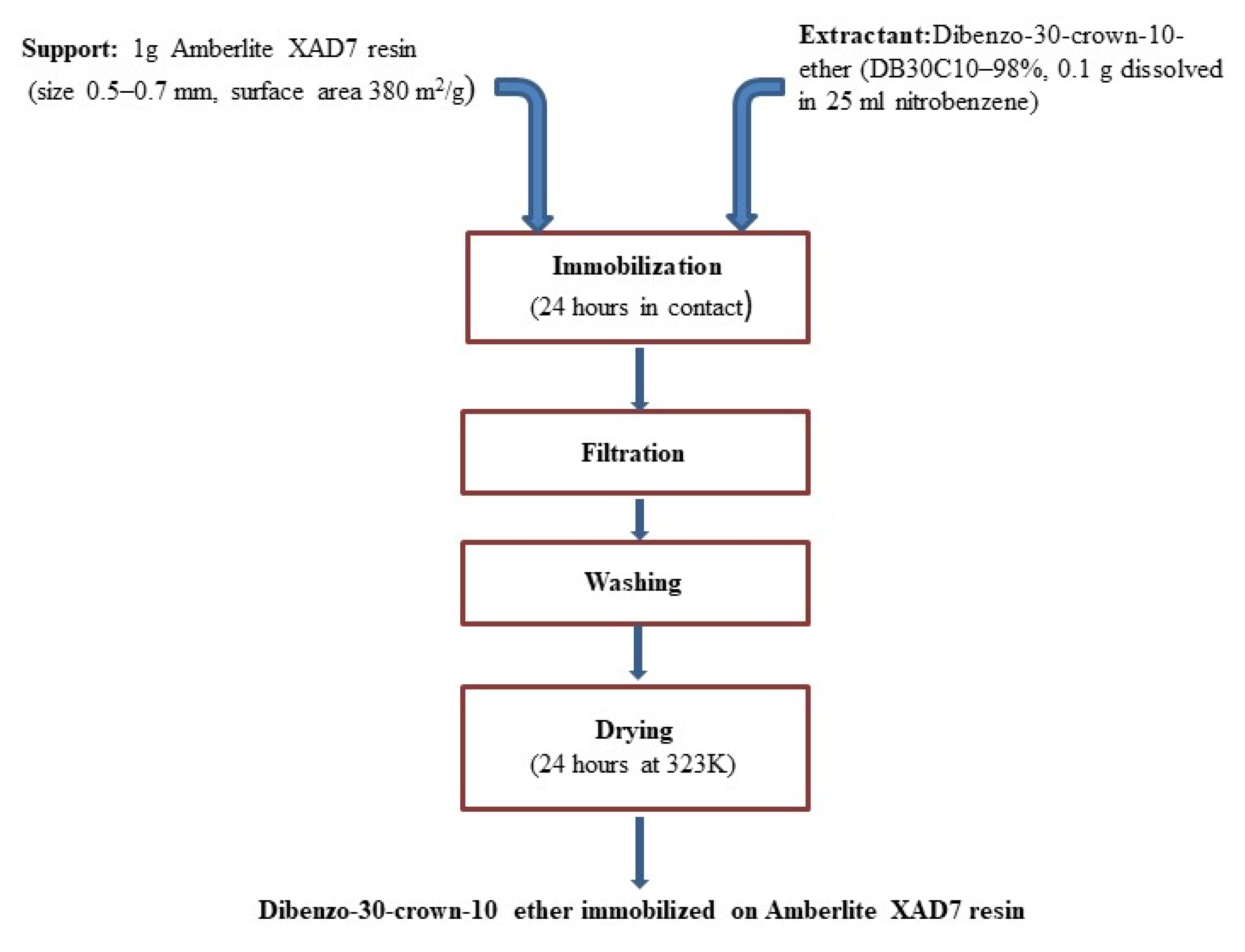
2.2. Immobilization of the DB30C10 onto XAD7 Resin
2.3. Influence of Physicochemical Parameters on Pt (IV) Adsorption
2.4. Factorial Design
3. Results and Discussions
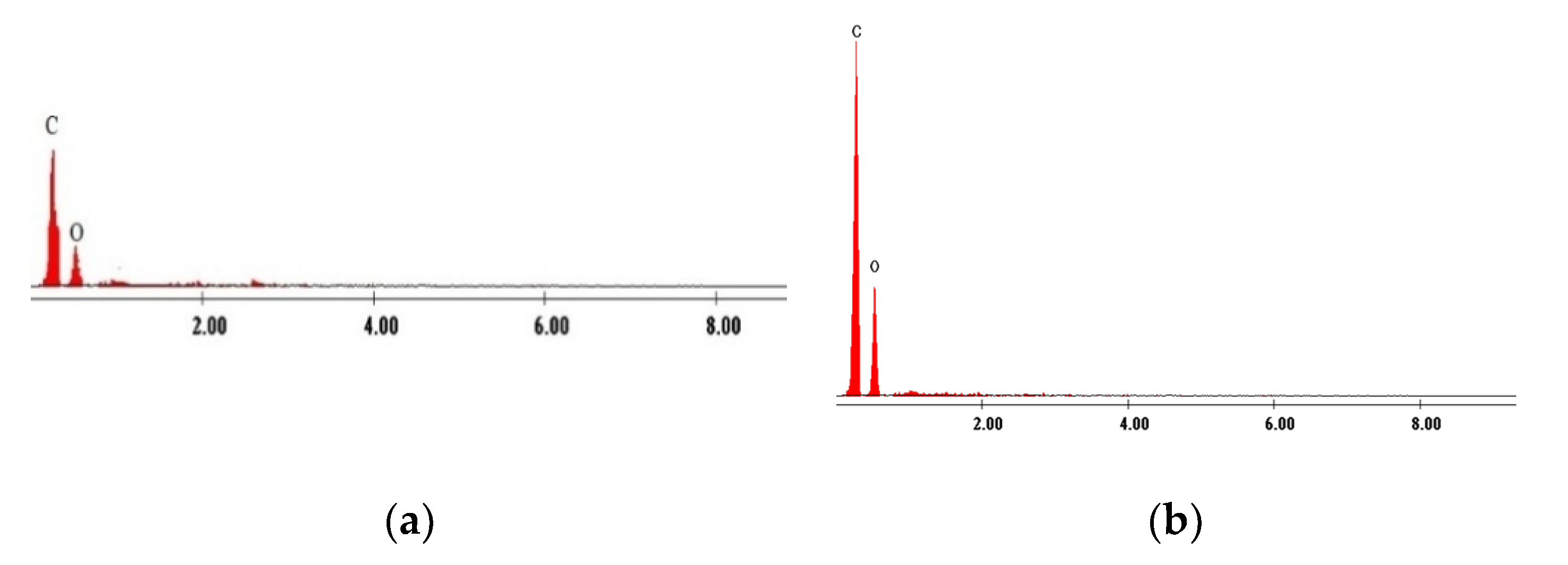
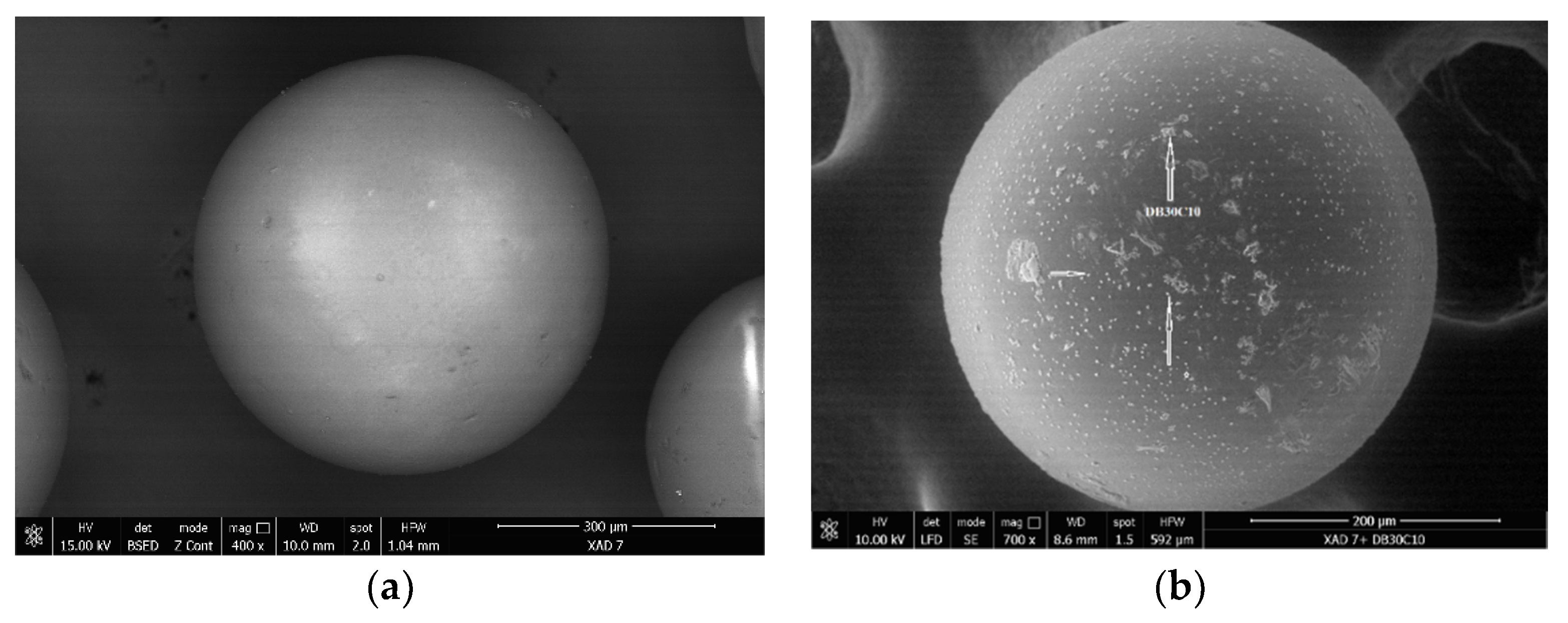
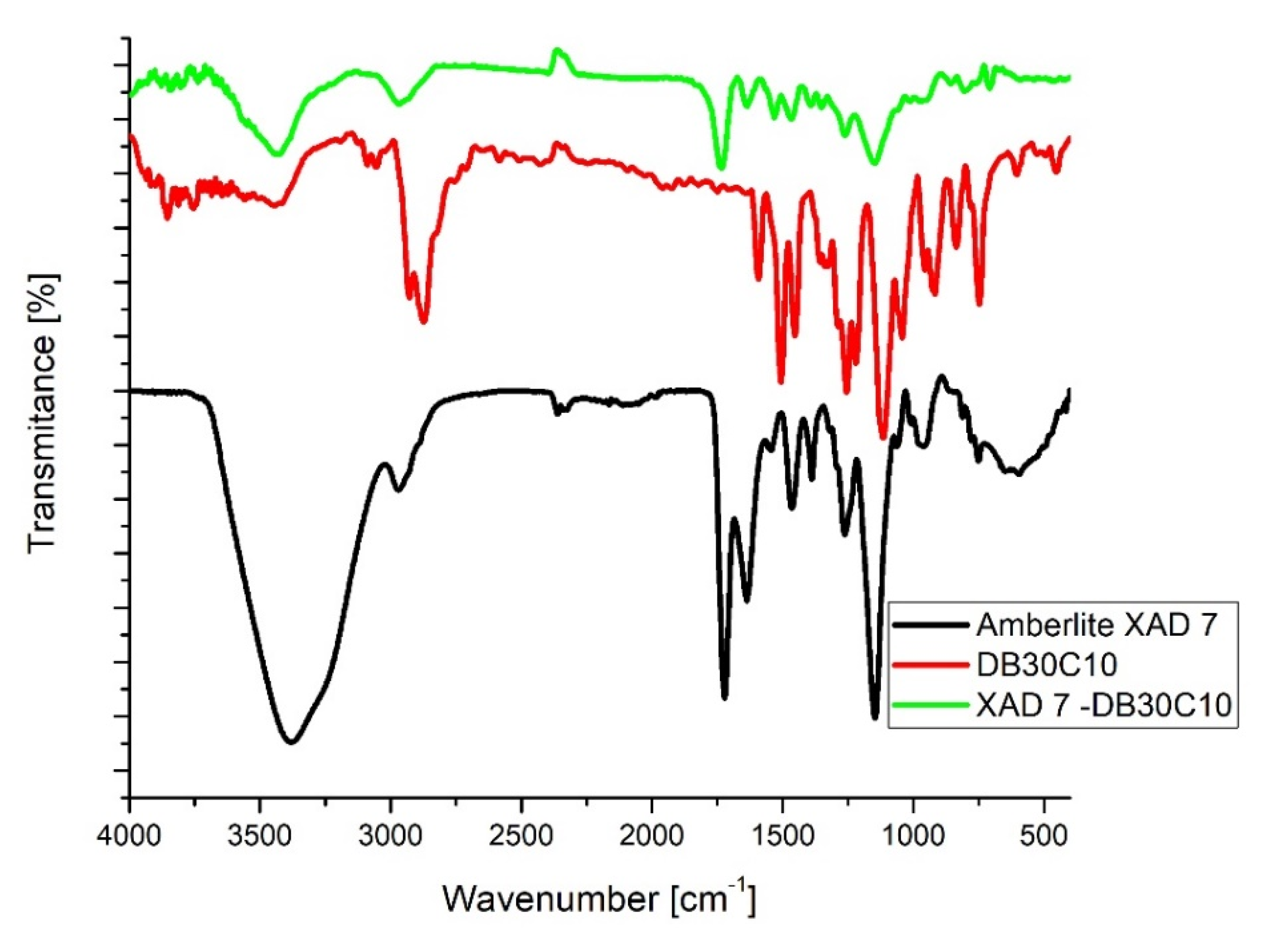
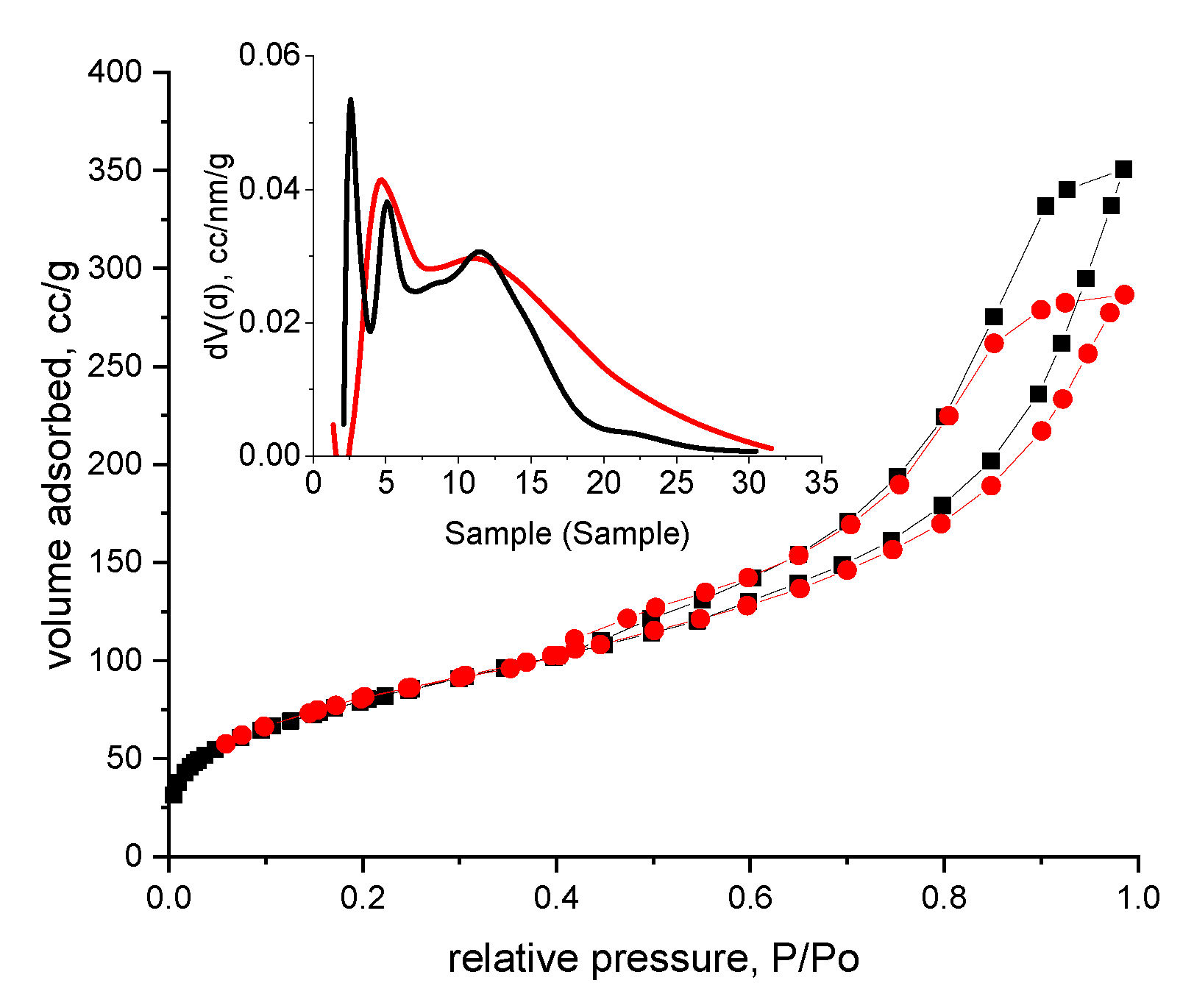
3.1. Evaluation of the Interaction between XAD7 Resin and Dibenzo-30-crown-10 Ether
3.2. Adsorption of Pt (IV) on XAD7-DB30C10
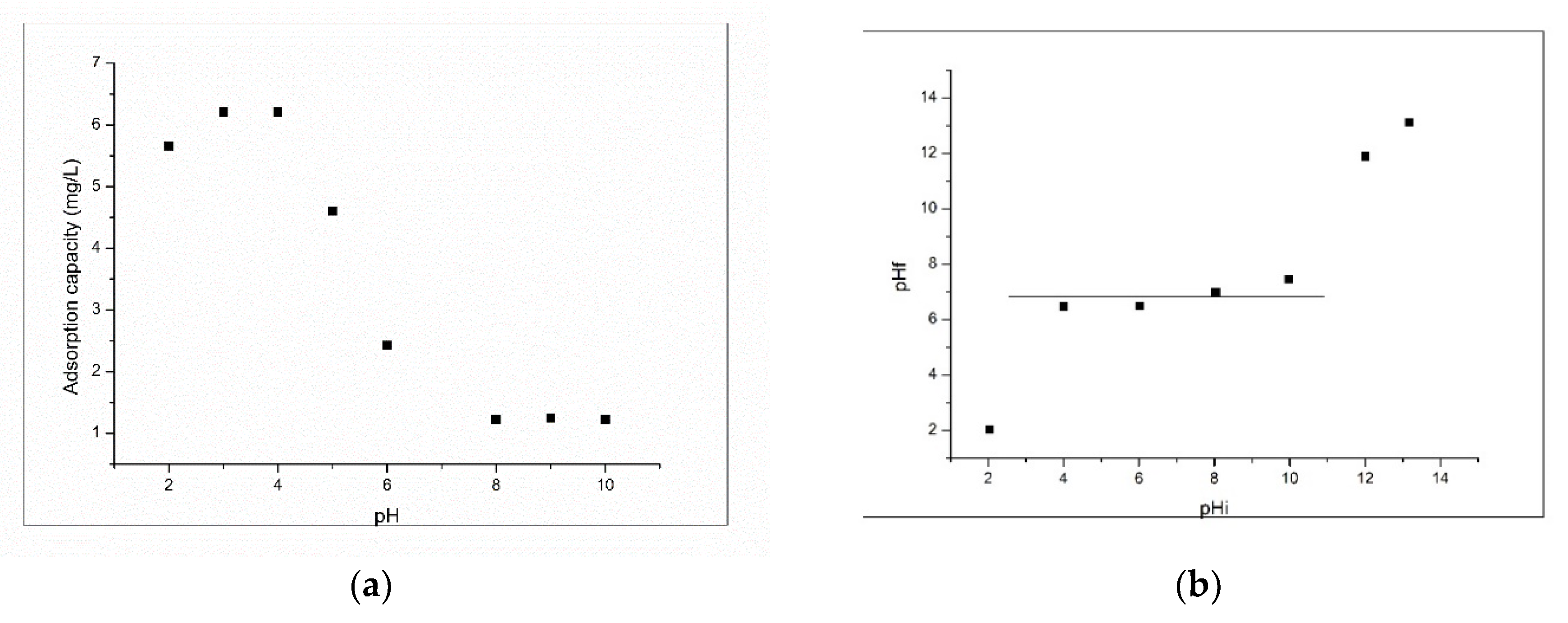
3.2.1. Influence of pH
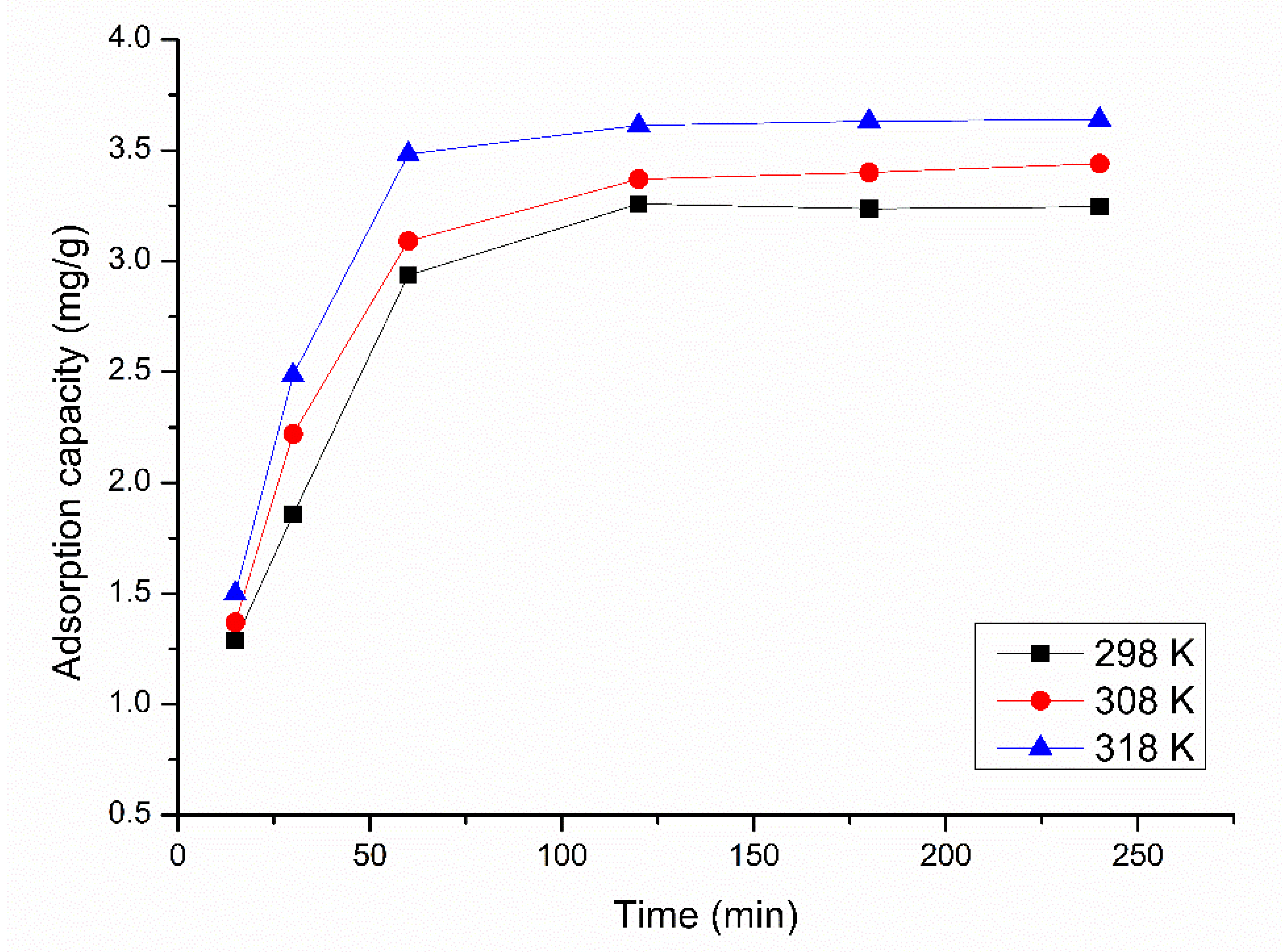
3.2.2. Influence of Contact Time and Temperature
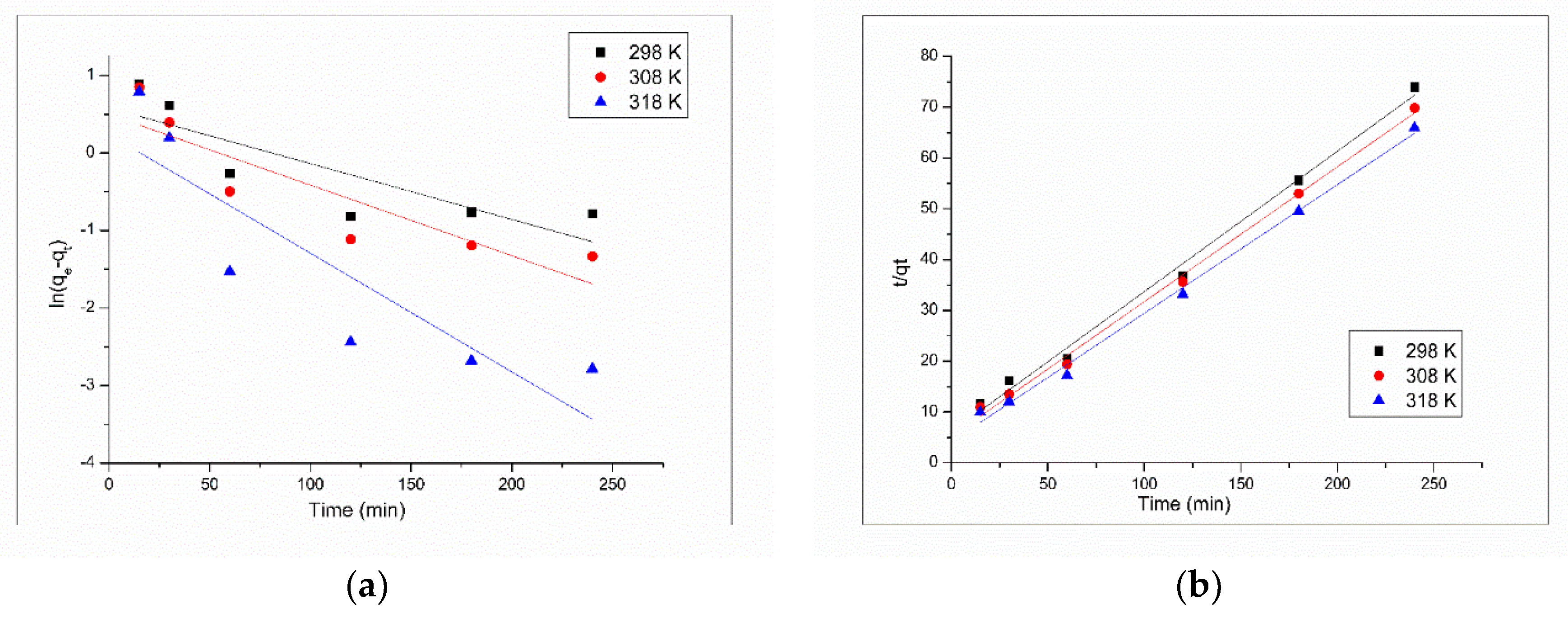
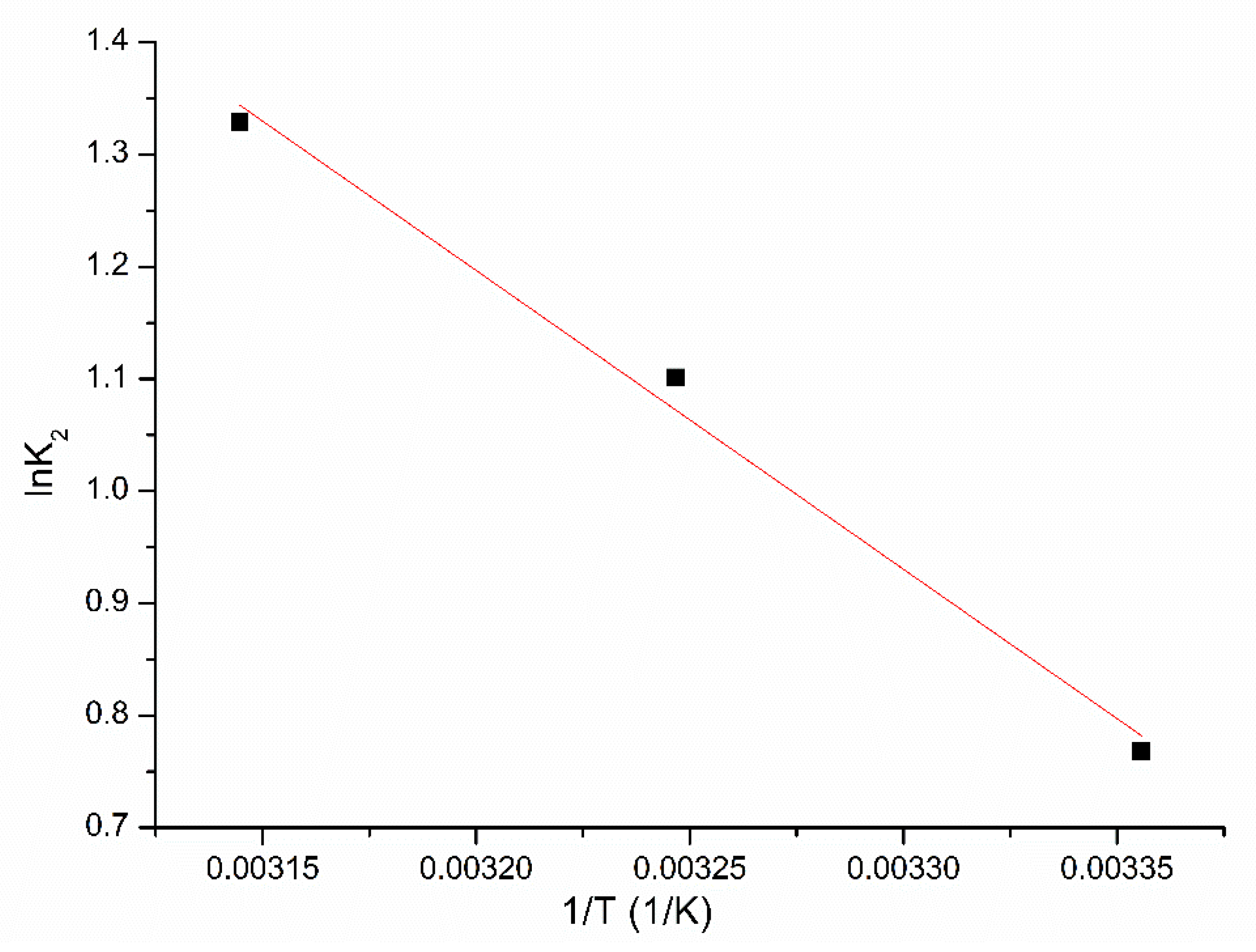
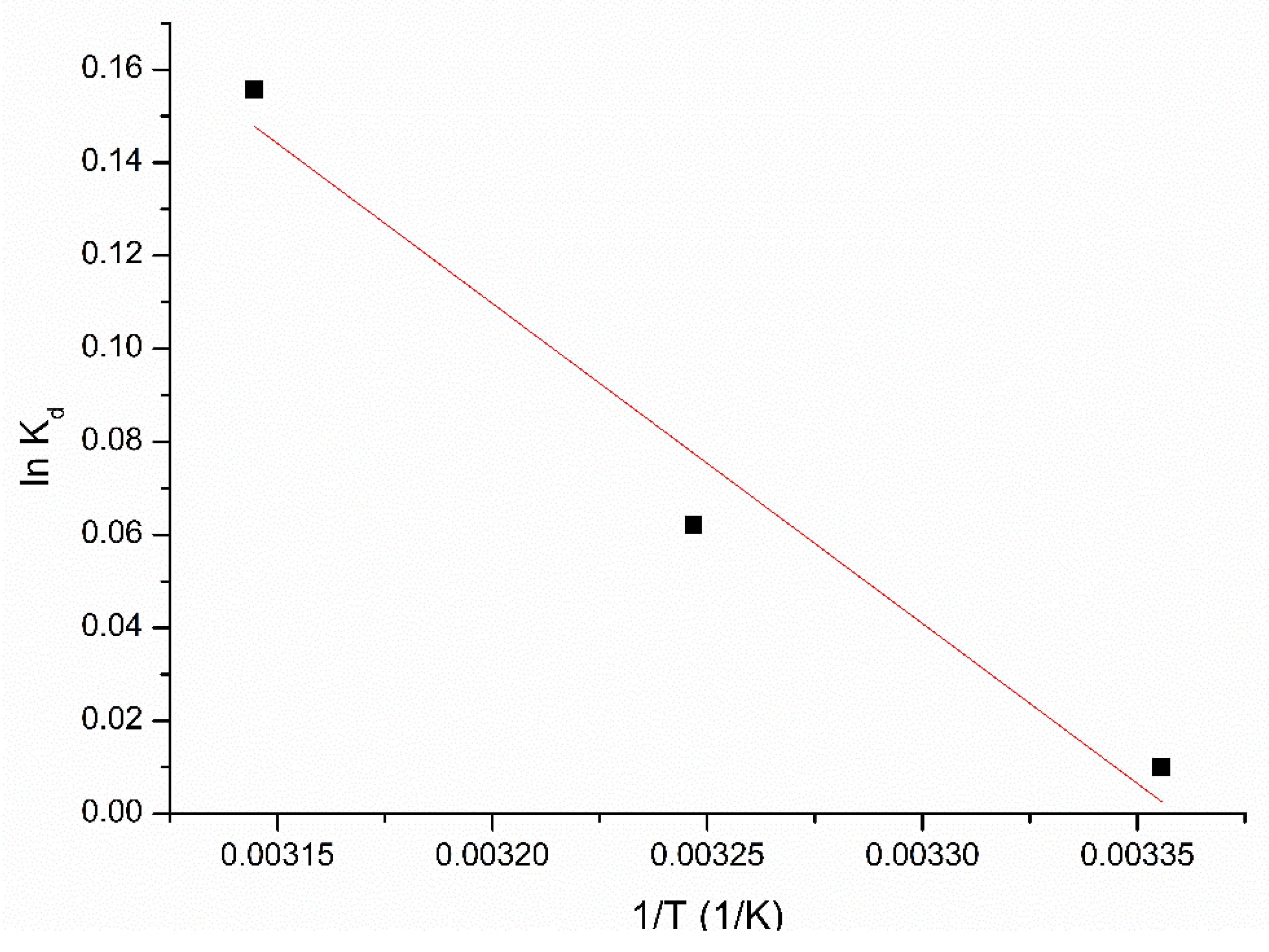
3.2.3. Kinetics and Thermodynamics Studies
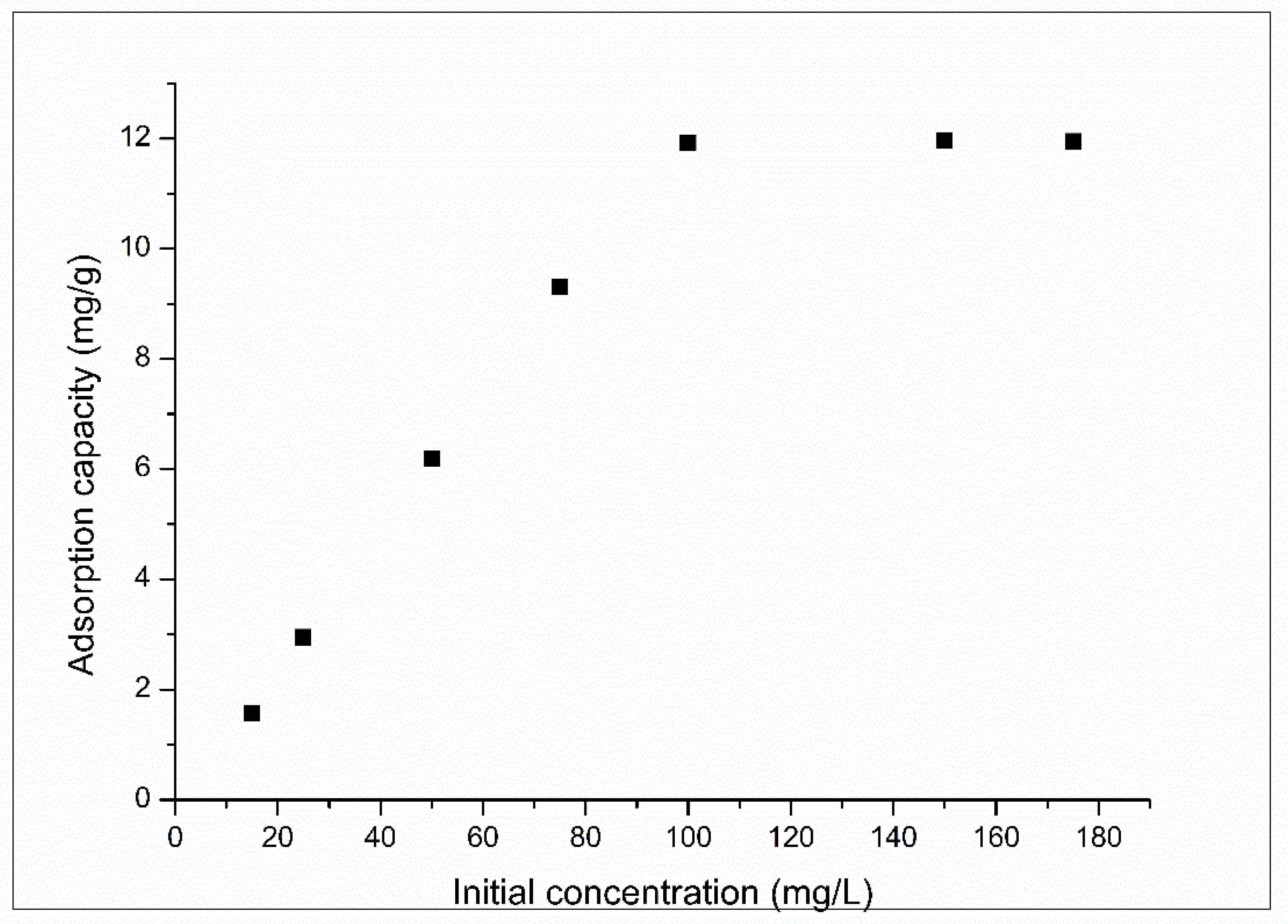
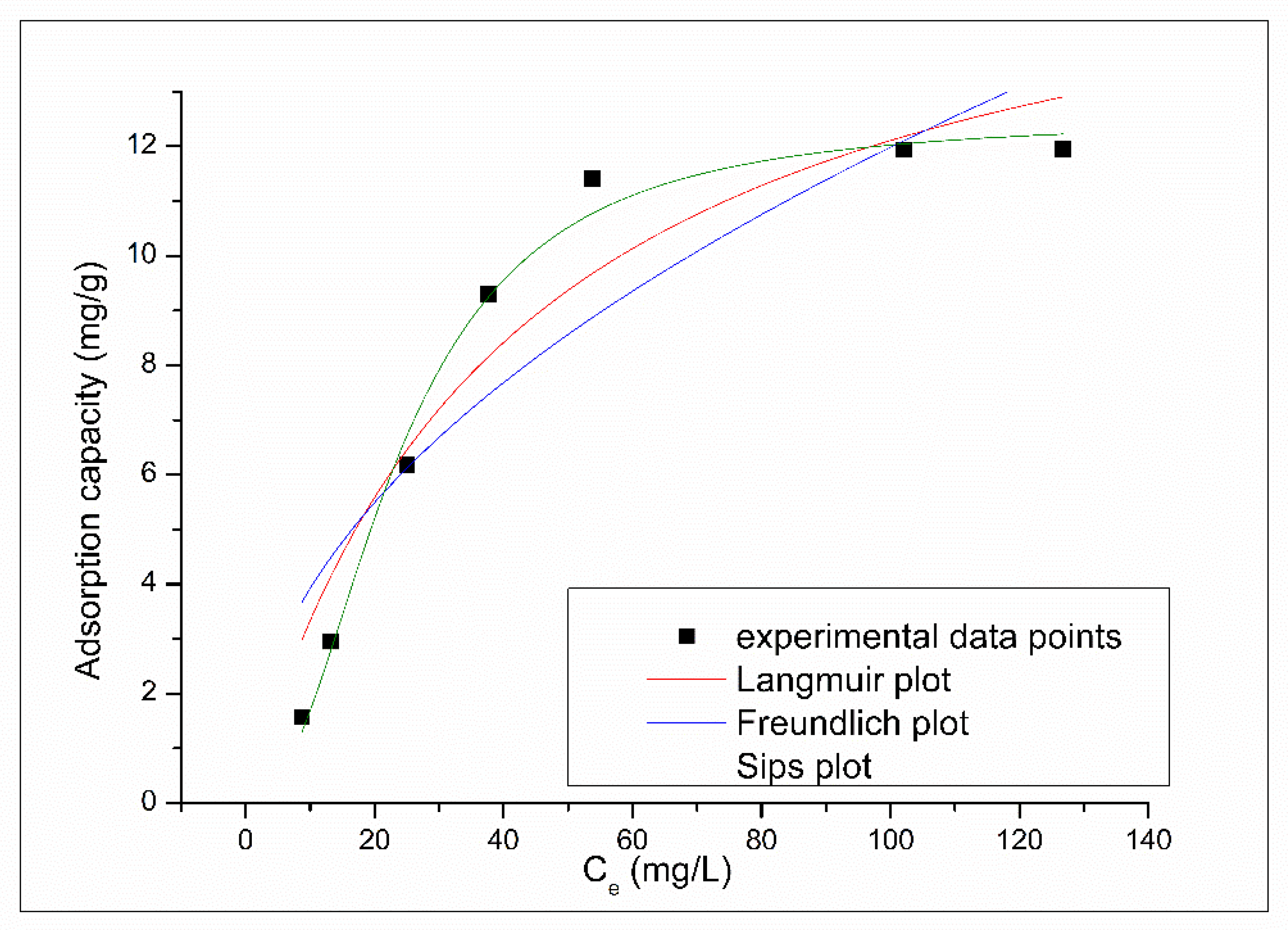
3.2.4. Effect of Initial Concentration and Equilibrium Study
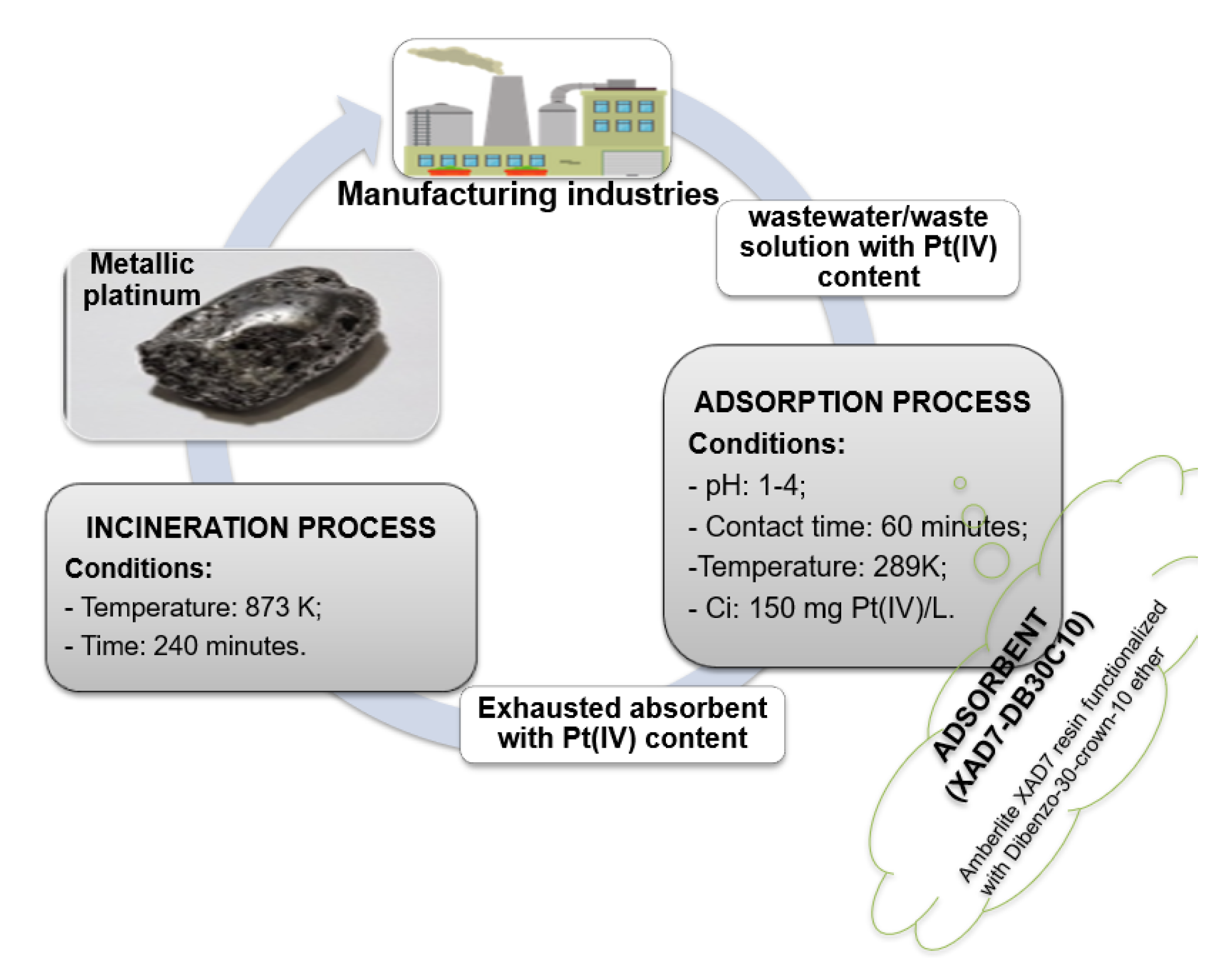
3.2.5. Platinum Recovery from Exhausted Material
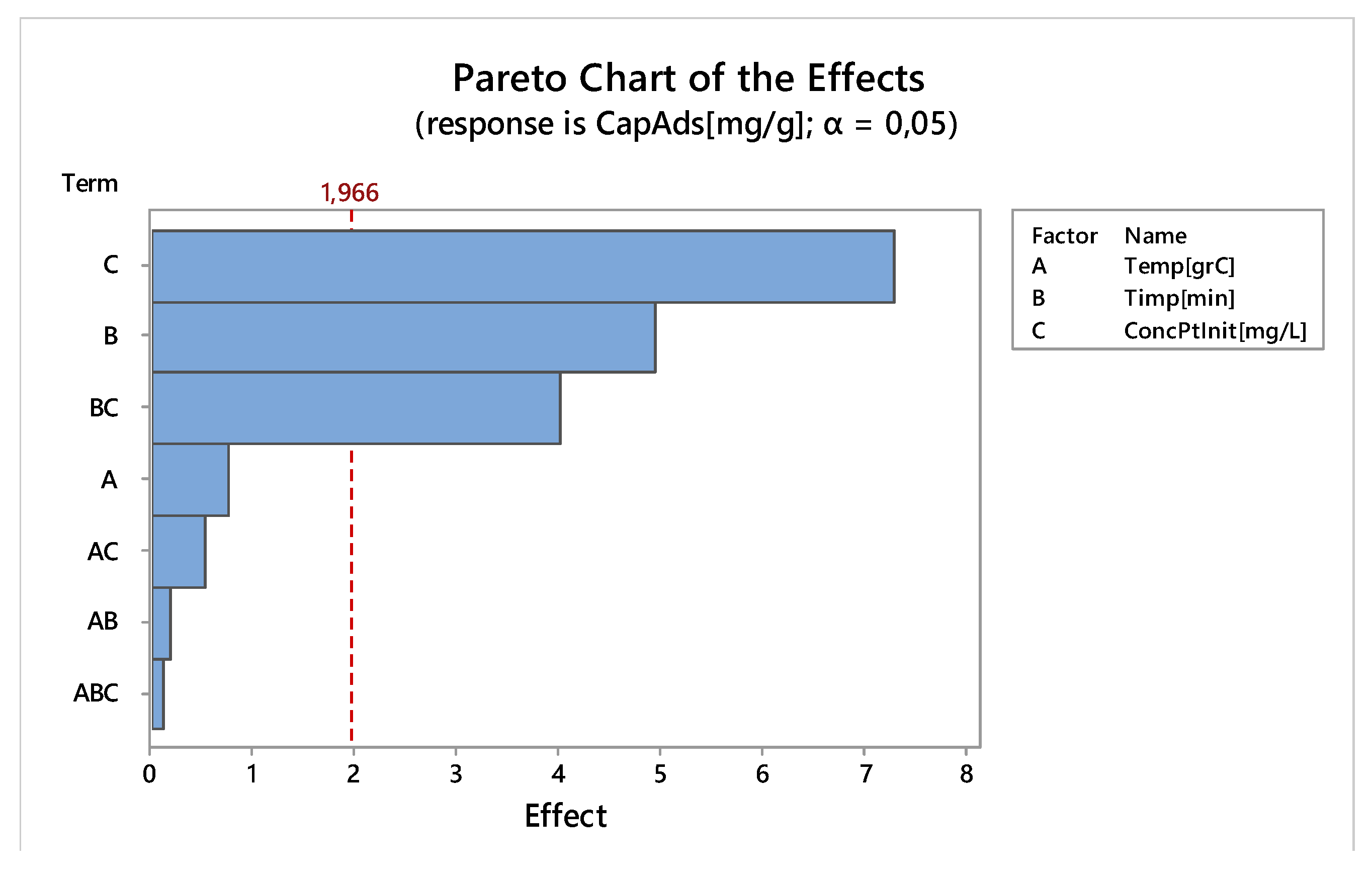
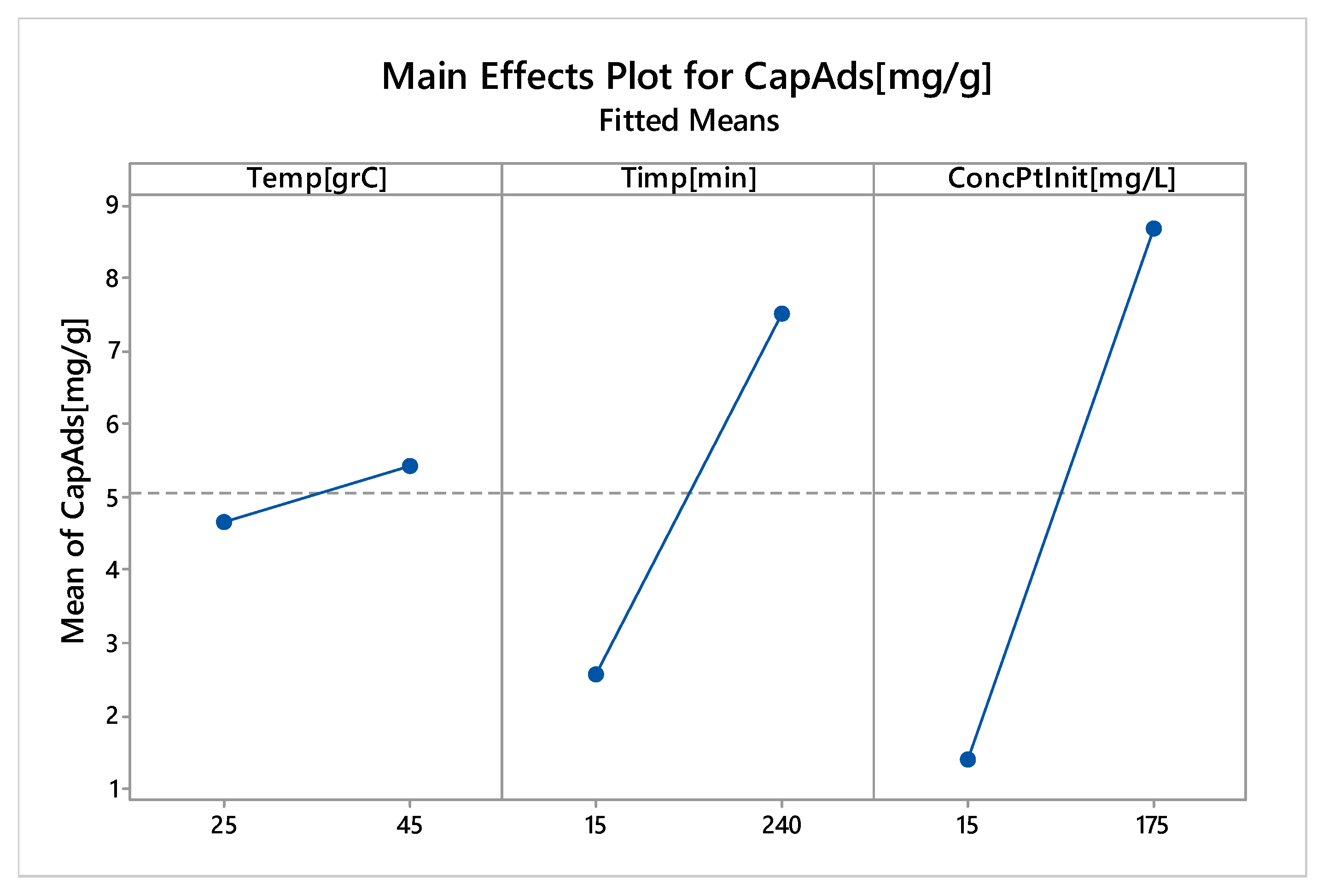
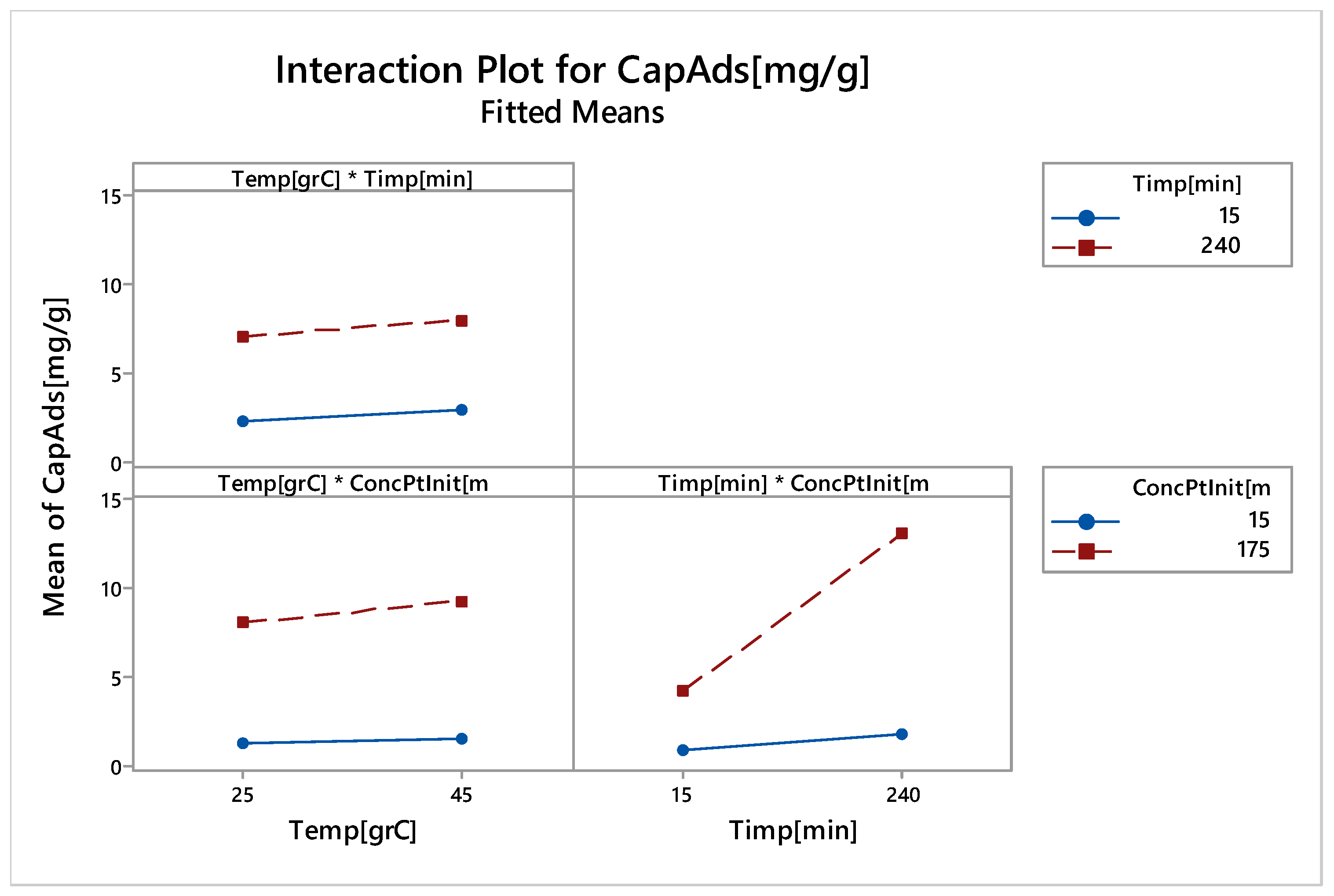
3.2.6. Factorial Design
Linear Experiments
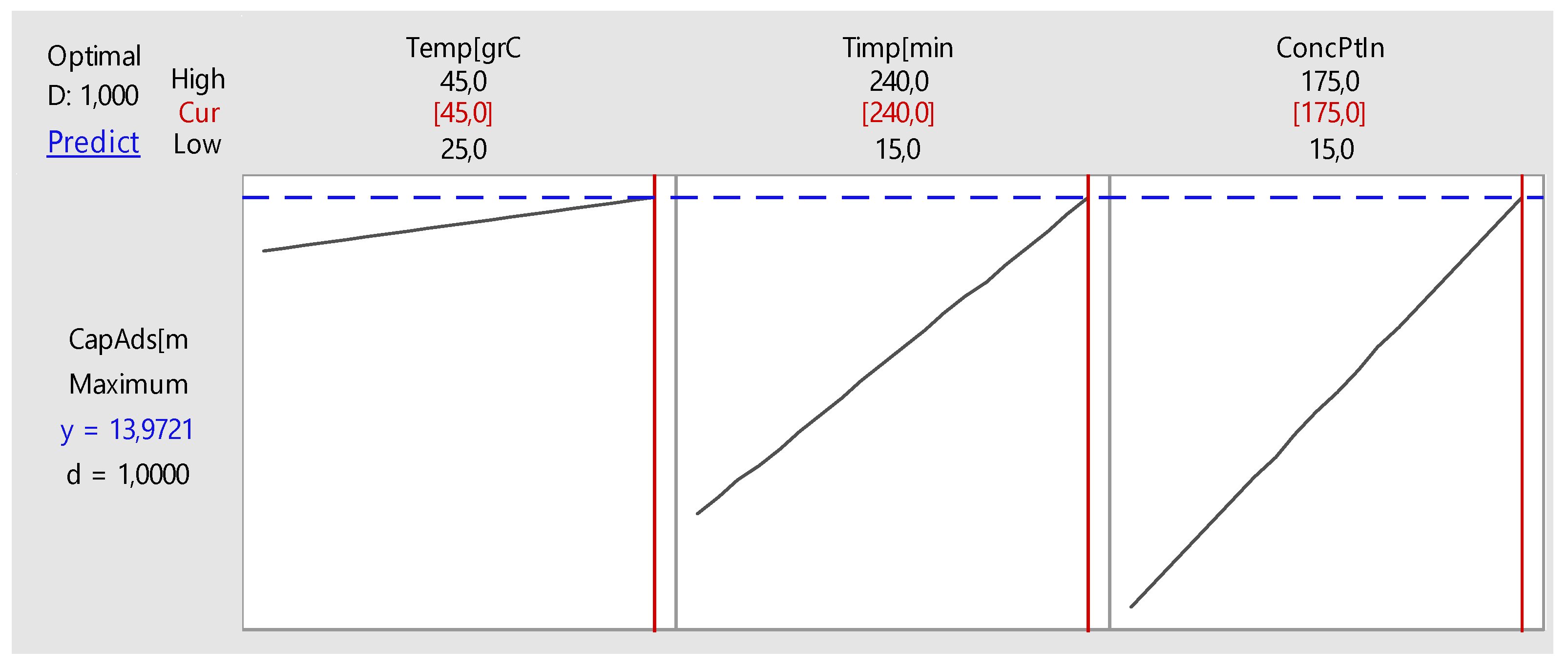
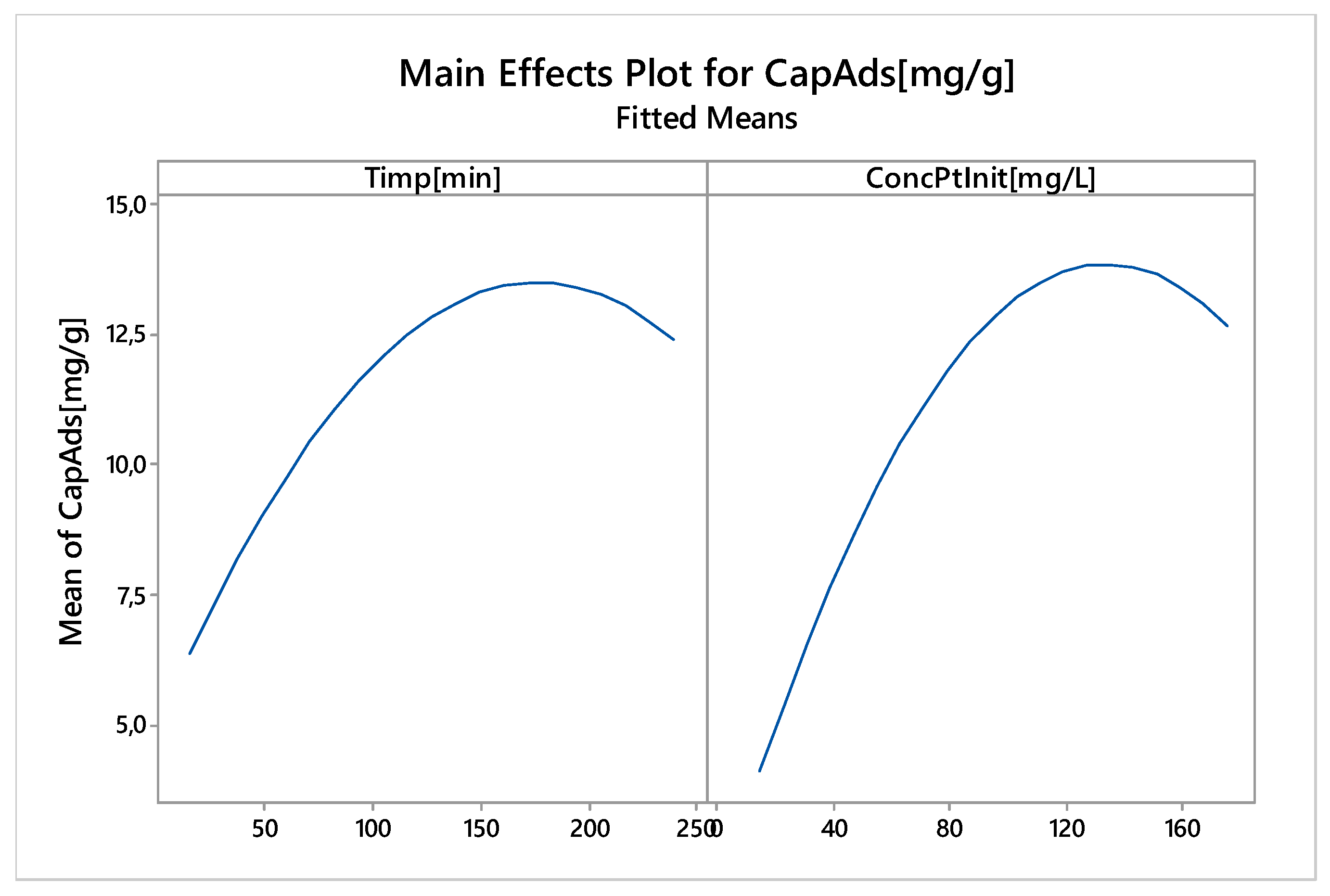
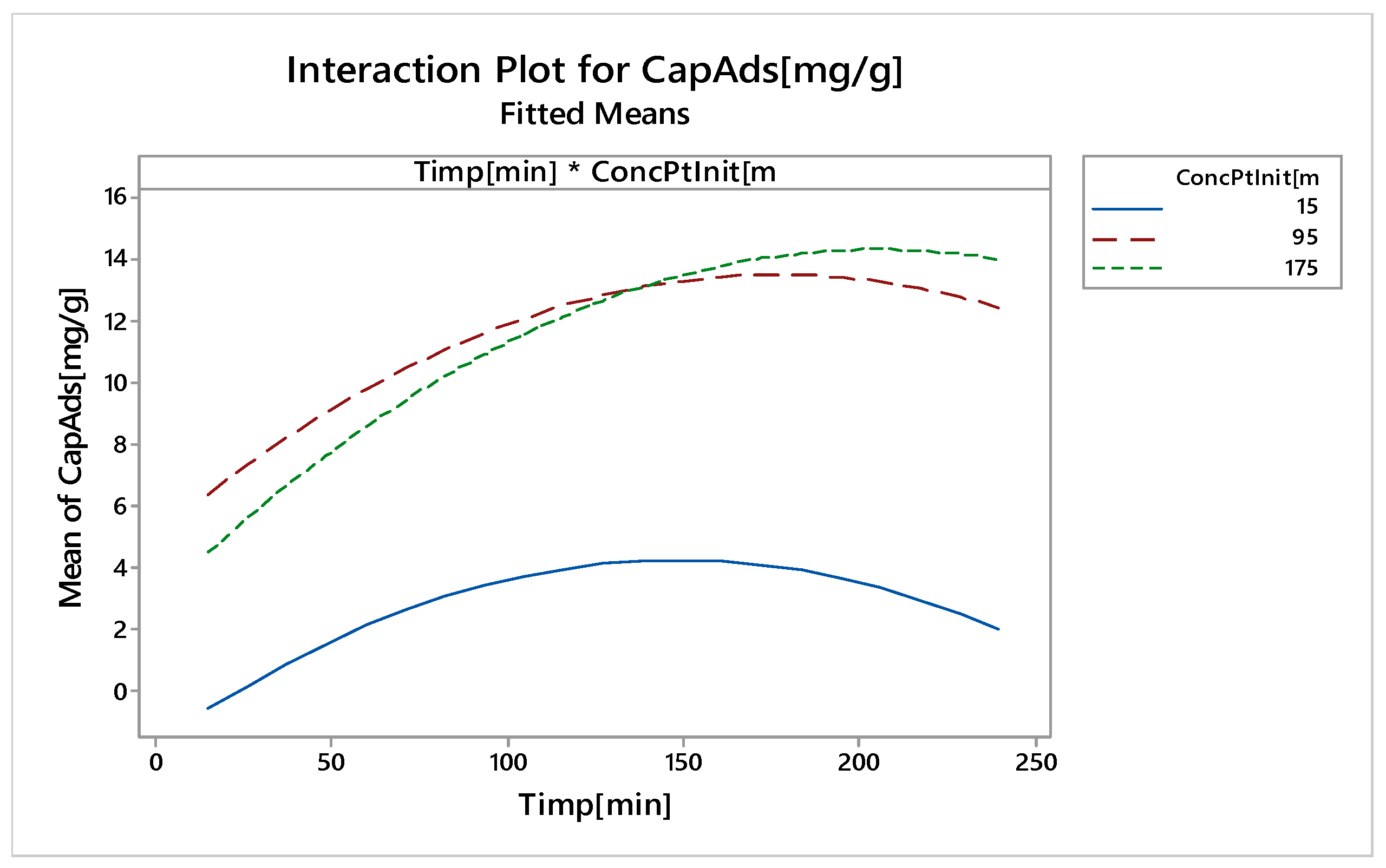
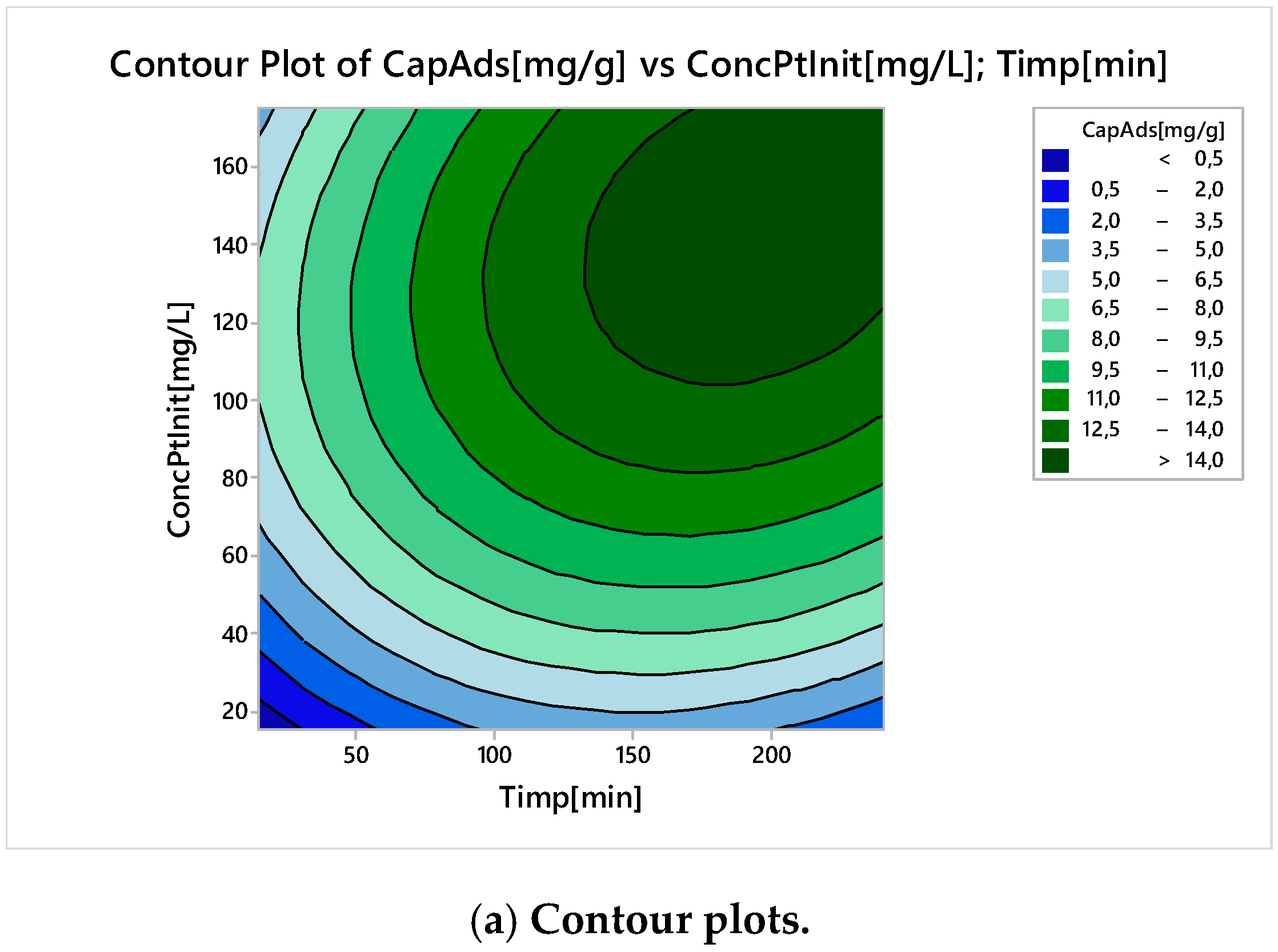
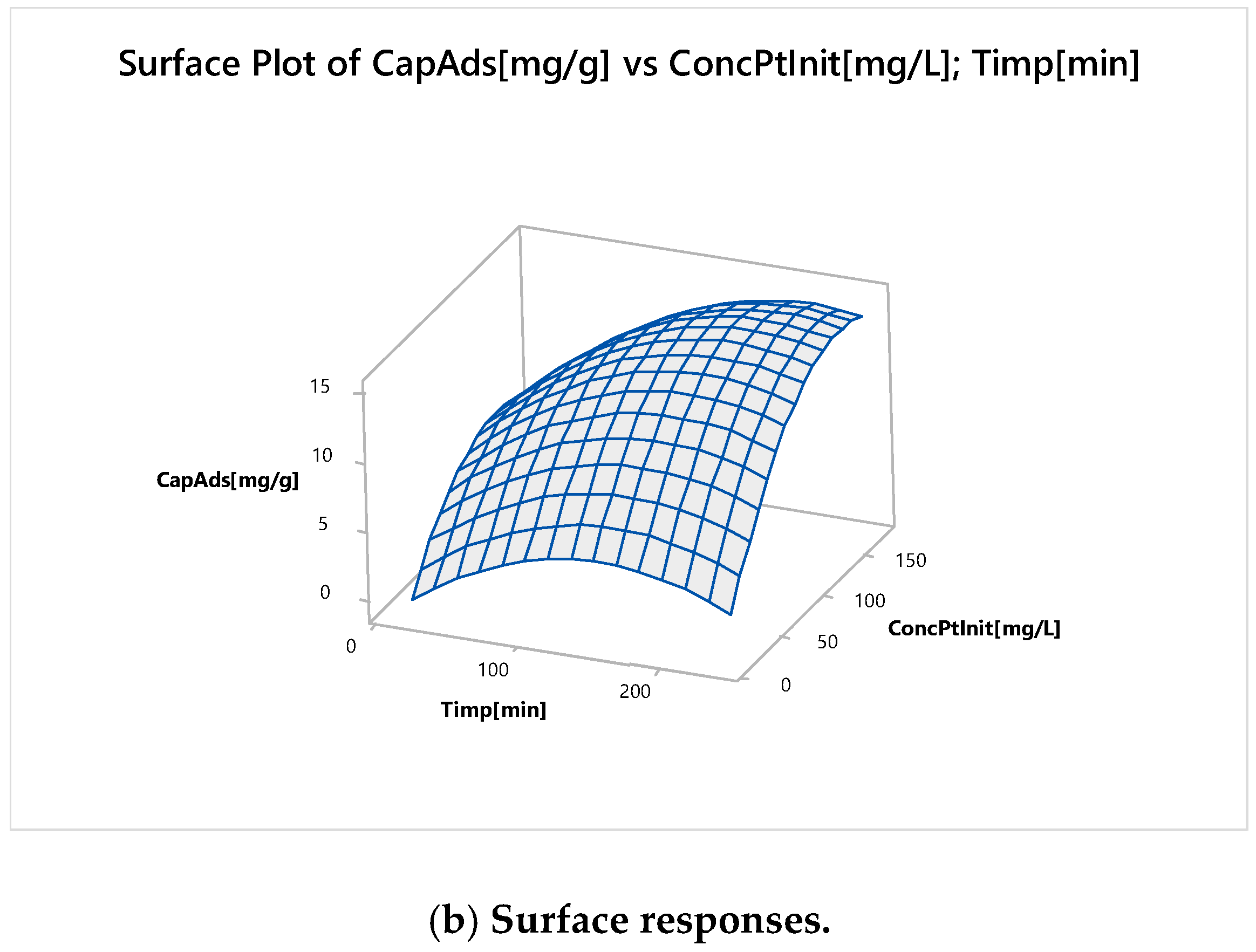
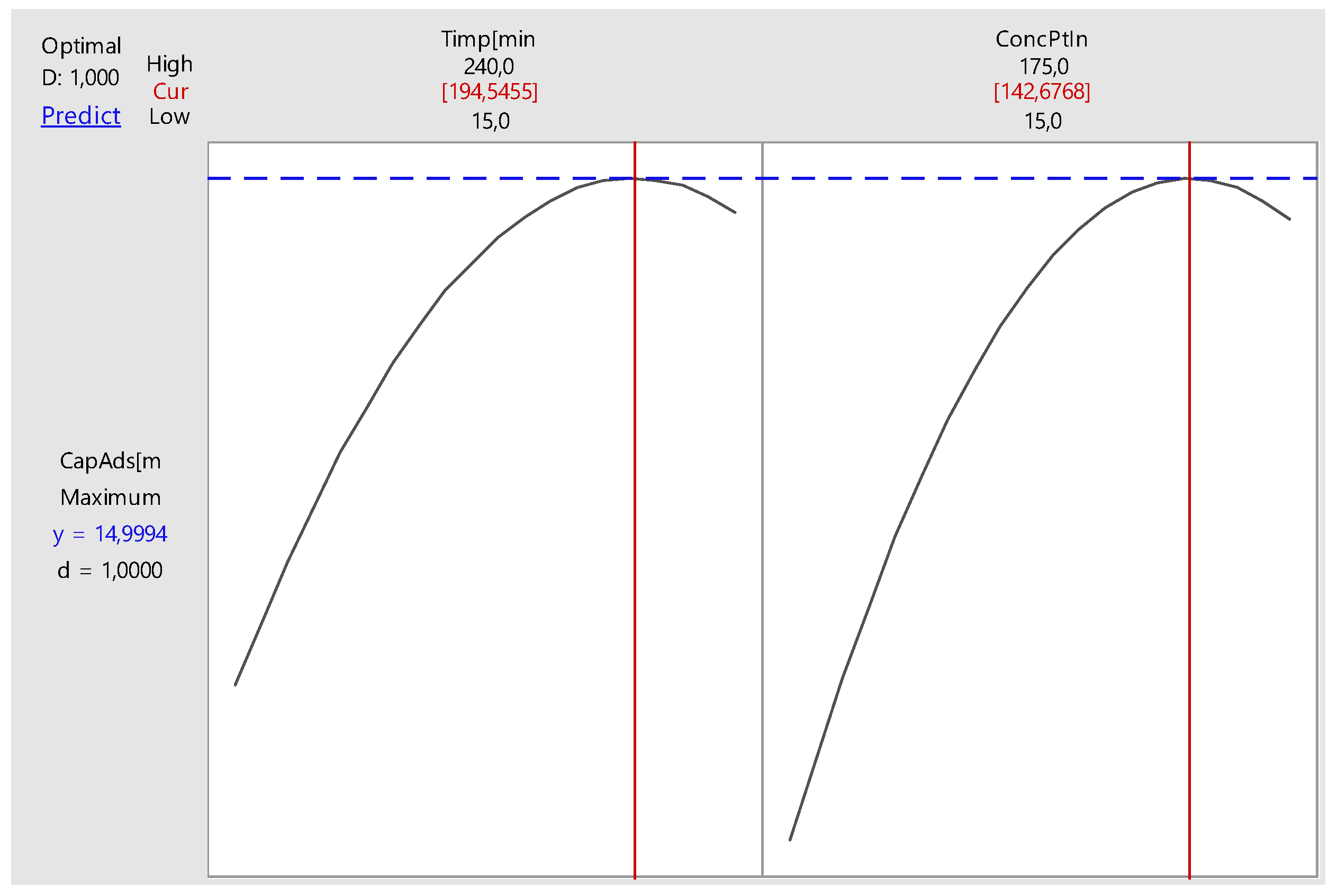
Nonlinear Experiments—Response Surface Design (RSD). Optimization of the Adsorption Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef]
- Liu, P.; Liu, G.-F.; Chen, D.-L.; Cheng, S.-Y.; Tang, N. Adsorption properties of Ag(I), Au(III), Pd(II) and Pt(IV) ions on commercial 717 anion-exchange resin. Trans. Nonferrous Met. Soc. China 2009, 19, 1509–1513. [Google Scholar] [CrossRef]
- Maruyama, T.; Matsushita, H.; Shimada, Y.; Kamata, I.; Sonokawa, S.; Kamiya, N.; Goto, M. Proteins and protein-rich biomass as environmentally friendly adsorbents selective for precious metal ions. Environ. Sci. Technol. 2007, 41, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Masllorens, J.; Roglans, A.; Anticó, E.; Fontàs, C. New applications of azamacrocyclic ligands in ion recognition, transport and preconcentration of palladium. Anal. Chim. Acta 2006, 560, 77–83. [Google Scholar] [CrossRef]
- Tu, Z.; Lu, S.; Chang, X.; Li, Z.; Hu, Z.; Zhang, L.; Tian, H. Selective solid-phase extraction and separation of trace gold, palladium and platinum using activated carbon modified with ethyl-3-(2-aminoethylamino)-2-chlorobut-2-enoate. Microchim. Acta 2011, 173, 231–239. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Min. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Goodman, P. Current and future uses of gold in electronics. Gold Bull. 2002, 35, 21–26. [Google Scholar] [CrossRef]
- Zheng, H.; Hu, D.; Zhang, L.; Ma, C.A.; Rufford, T. Thiol functionalized mesoporous silicas for selective adsorption of precious metals. Min. Eng. 2012, 35, 20–26. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Fleming, C.A. Hydrometallurgy of precious metals recovery. Hydrometallurgy 1992, 30, 127–162. [Google Scholar] [CrossRef]
- Lee, C.; Rhee, K.-I.; Sohn, H.-J. Recovery of gold from electronic scrap by hydrometallurgical process. J. Korean Inst. Resour. Recycl. 1997, 6, 36–40. [Google Scholar]
- Botz, M.M.; Mudder, T.I.; Akcil, A.U. Chapter 35—Cyanide Treatment: Physical, Chemical, and Biological Processes A2—Adams, Mike, D. In Gold Ore Processing, 2nd ed.; Elsevier: New York, NY, USA, 2016; pp. 619–645. [Google Scholar]
- Faramarzi, M.A.; Stagars, M.; Pensini, E.; Krebs, W.; Brandl, H. Metal solubilization from metal-containing solid materials by cyanogenic Chromobacterium violaceum. J. Biotechnol. 2004, 113, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Jal, P.K.; Patel, S.; Mishra, B.K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 62, 1005–1028. [Google Scholar] [CrossRef]
- Kang, T.; Park, Y.; Choi, K.; Lee, J.S.; Yi, J. Ordered mesoporous silica (SBA-15) derivatized with imidazole-containing functionalities as a selective adsorbent of precious metal ions. J. Mater. Chem. 2004, 14, 1043–1049. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Sanchez Marcano, J.; Macaskie, L.E.; Guibal, E. Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 2005, 76, 131–147. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Zhao, Y. Biosorption and reduction of Au (III) to gold nanoparticles by thiourea modified alginate. Carbohydr. Polym. 2017, 159, 108–115. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Funaoka, M. Recovery of Gold(III), Palladium(II), and Platinum(IV) by Aminated Lignin Derivatives. Ind. Eng. Chem. Res. 2006, 45, 6405–6412. [Google Scholar] [CrossRef]
- Parajuli, D.; Khunathai, K.; Adhikari, C.R.; Inoue, K.; Ohto, K.; Kawakita, H.; Funaoka, M.; Hirota, K. Total recovery of gold, palladium, and platinum using lignophenol derivative. Miner. Eng. 2009, 22, 1173–1178. [Google Scholar] [CrossRef]
- Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour. Technol. 2008, 99, 3801–3809. [Google Scholar] [CrossRef]
- Ciopec, M.; Davidescu, C.M.; Negrea, A.; Grozav, I.; Lupa, L.; Muntean, C.; Negrea, P.; Popa, A. Statististical optimization of chromium ions adsorption on dehpa-impregnated amberlite xad7. Environ. Eng. Manag. J. 2012, 11, 525–531. [Google Scholar] [CrossRef]
- Ciopec, M.; Davidescu, C.M.; Negrea, A.; Grozav, I.; Lupa, L.; Negrea, P.; Popa, A. Adsorption studies of Cr(III) ions from aqueous solutions by DEHPA impregnated onto Amberlite XAD7—Factorial design analysis. Chem. Eng. Res. Des. 2012, 90, 1660–1670. [Google Scholar] [CrossRef]
- Can, M.Y.; Yildiz, E. Phosphate removal from water by fly ash: Factorial experimental design. J. Hazard. Mater. 2006, 135, 65–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Luan, Z.; Peng, X.; Liang, Z.; Shi, L. Removal of phosphate from aqueous solution by red mud using a factorial design. J. Hazard. Mater. 2009, 165, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Behnajady, B.; Moghaddam, J. Selective leaching of zinc from hazardous As-bearing zinc plant purification filter cake. Chem. Eng. Res. Des. 2017, 117, 564–574. [Google Scholar] [CrossRef]
- Benamor, M.; Bouariche, Z.; Belaid, T.; Draa, M. Kinetic studies on cadmium ions by Amberlite XAD7 impregnated resins containing di(2-ethylhexyl) phosphoric acid as extractant. Sep. Purif. Technol. 2008, 59, 74–84. [Google Scholar] [CrossRef]
- Juang, R.-S. Preparation, properties and sorption behavior of impregnated resins containing acidic organophosphorus extractants. Proc. Natl. Sci. Counc. Repub. Chinapart A Phys. Sci. Eng. 1999, 23, 353–364. [Google Scholar]
- Gabor, A.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Grozav, I.; Negrea, P.; Duteanu, N. Optimizing the lanthanum adsorption process onto chemically modified biomaterials using factorial and response surface design. J. Env. Manag. 2017, 204, 839–844. [Google Scholar] [CrossRef]
- Katarina, R.K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Synthesis of a chitosan-based chelating resin and its application to the selective concentration and ultratrace determination of silver in environmental water samples. Anal. Chim. Acta 2006, 558, 246–253. [Google Scholar] [CrossRef]
- Tang, R.; Du, Y.; Fan, L. Dialdehyde starch-crosslinked chitosan films and their antimicrobial effects. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 993–997. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Dikio, C.W.; Wankasi, D.; Dikio, E.D.; Mtunzi, F.M. Sorption of Pb2+ from aqueous solution using polyethylene and polyvinylchloride wastes as adsorbents: A comparative study. Int. J. Environ. Stud. 2018, 75, 932–949. [Google Scholar] [CrossRef]
- Machedi, S.; Ejidike, I.P.; Mtunzi, F.M.; Pakade, V.E.; Klink, M.J. Chlorinated phenols sorption performance by macadamia activated carbon and grafted macadamia activated carbon: Characterization, kinetics, and thermodynamic studies. Orient. J. Chem. 2019, 35, 1469–1479. [Google Scholar] [CrossRef]
- Abasi, C.Y.; Ejidike, I.P.; Dikio, E.D. Synthesis, characterisation of ternary layered double hydroxides (LDH) for sorption kinetics and thermodynamics of Cd2+. Int. J. Environ. Stud. 2019, 76, 441–455. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F. Common data analysis errors in batch adsorption studies. Hydrometallurgy 2011, 105, 314–320. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Negrea, A.; Popa, A.; Ciopec, M.; Lupa, L.; Negrea, P.; Davidescu Corneliu, M.; Motoc, M.; Mînzatu, V. Phosphonium grafted styrene–divinylbenzene resins impregnated with Iron (III) and crown ethers for arsenic removal. Pure Appl. Chem. 2014, 86, 1729. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Woińska, S.; Godlewska-Żyłkiewicz, B. Determination of platinum and palladium in road dust after their separation on immobilized fungus by electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 522–528. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Mitomo, H.; Seko, N.; Tamada, M.; Yoshii, F. Platinum and palladium ions adsorption at the trace amounts by radiation crosslinked carboxymethylchitin and carboxymethylchitosan hydrogels. J. Appl. Polym. Sci. 2007, 104, 4015–4023. [Google Scholar] [CrossRef]
- Xiong, Y.; Adhikari, C.R.; Kawakita, H.; Ohto, K.; Inoue, K.; Harada, H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine. Bioresour. Technol. 2009, 100, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Q.; Guo, X.Y.; Liang, S.; Wang, C.; Tian, Q.H. Study on the adsorption behavior of modified persimmon powder biosorbent on Pt(IV). Int. J. Environ. Sci. Technol. 2015, 13, 47–54. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound are not available from the authors. |


| Temperature (K) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qe exp (mg g−1) | k1 (min−1) | qe, Kinetic Plot (mg g−1) | R2 | qe exp (mg g−1) | k2 (g mg−1·min−1) | qe Kinetic Plot (mg g−1) | R2 | |
| 298 | 3.26 | 0.0072 | 1.79 | 0.7154 | 3.26 | 2.15 | 3.62 | 0.994 |
| 308 | 3.37 | 0.0091 | 1.64 | 0.7930 | 3.37 | 3.00 | 3.95 | 0.9967 |
| 318 | 3.61 | 0.0153 | 1.27 | 0.7787 | 3.61 | 3.77 | 3.95 | 0.9955 |
| ΔH0 (kJ/mol−1) | ΔS0 (J/mol∙K) | ΔG0 (kJ/mol) | R2 | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| 5.72 | 19.21 | −6.4 | −6.9 | −7.3 | 0.9676 |
| Langmuir Isotherm | |||
| qm,exp (mg g−1) | KL (L mg−1) | qL (mg g−1) | R2 |
| 12.3 | 0.024 | 17.1 | 0.9085 |
| Freundlich Isotherm | |||
| KF(mg g−1) | 1/nF | R2 | |
| 1.28 | 0.484 | 0.8026 | |
| Sips Isotherm | |||
| KS | qS(mg g−1) | 1/nS | R2 |
| 1.0 × 10−2 | 12.5 | 1.2 | 0.9884 |
| Adsorbent | Adsorption Conditions | Adsorption Capacity [mg/g] | References |
| Fungus aspergillus sp. immobilized on Cellex-T | pH = 1; 298 K | 0.47 | [42] |
| Cross-linked carboxy methyl chitosan hydrogels | pH = 3.3; contact time 120 min; 298 K | 1.16 | [43] |
| Functionalized acrylic copolymers | pH = 1; time = 60 min, 303 K, | 1.10 | [44] |
| DMA persimmon waste gel (DMA-PW) | pH = 0.9; contact time = 24 h, 343 K, | 1.28 | [45] |
| Amberlite XAD-7- dibenzo-30-crown-10 ether | pH = 4; contact time = 120 min; 293 K | 12.3 | Present paper |
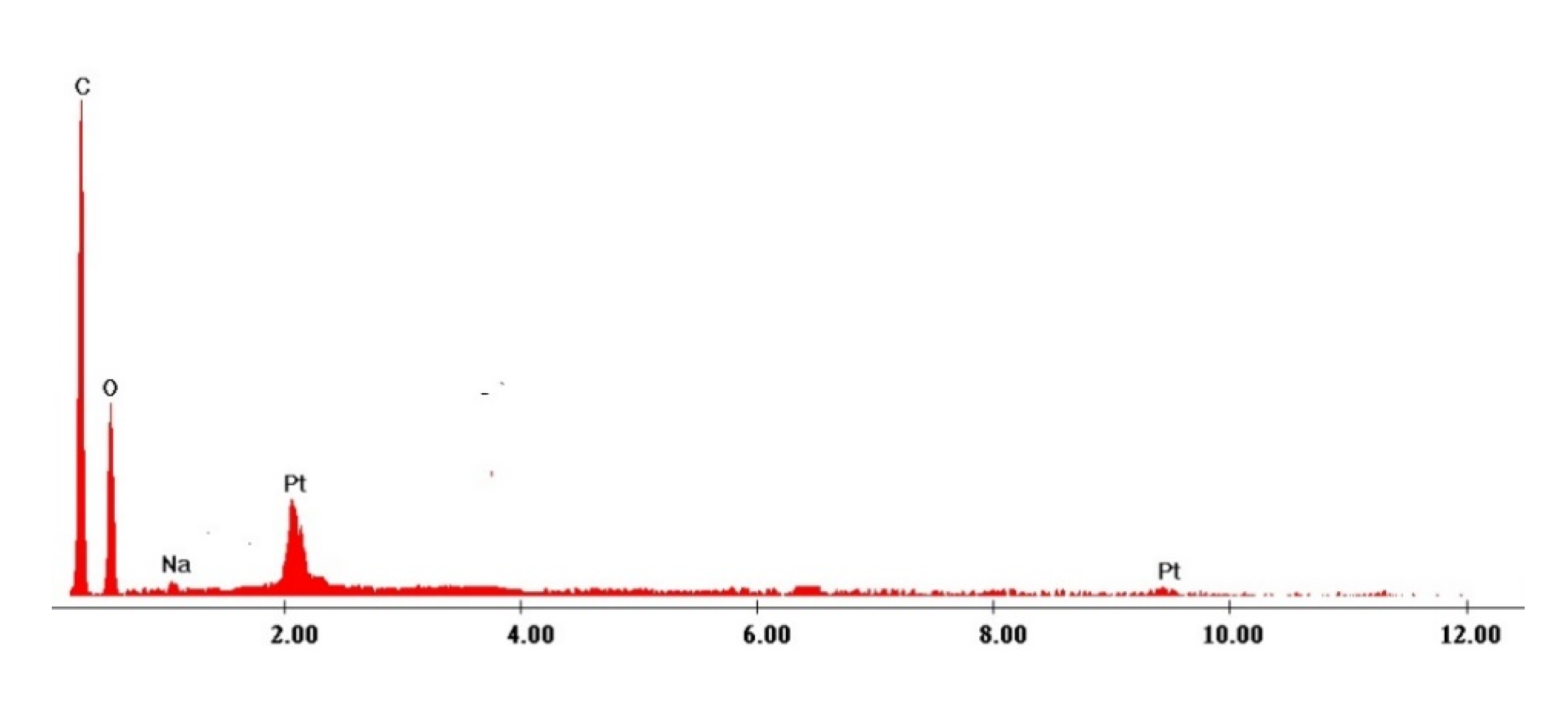
| Elem | Wt. % |
|---|---|
| C | 45.18 |
| O | 39.2 |
| Na | 6.39 |
| Pt | 9.23 |
| Total | 100 |
| Parameter | Minimum | Target |
|---|---|---|
| Adsorption capacity, mg g−1 | 10 | 14 |
| Global Solution | ||
| Time, minutes | 195 | |
| Initial Concentration, mg L−1 | 143 | |
| Predicted Response | ||
| Adsorption Capacity, mg g−1 | 15 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buriac, O.; Ciopec, M.; Duţeanu, N.; Negrea, A.; Negrea, P.; Grozav, I. Platinum (IV) Recovery from Waste Solutions by Adsorption onto Dibenzo-30-crown-10 Ether Immobilized on Amberlite XAD7 Resin–Factorial Design Analysis. Molecules 2020, 25, 3692. https://doi.org/10.3390/molecules25163692
Buriac O, Ciopec M, Duţeanu N, Negrea A, Negrea P, Grozav I. Platinum (IV) Recovery from Waste Solutions by Adsorption onto Dibenzo-30-crown-10 Ether Immobilized on Amberlite XAD7 Resin–Factorial Design Analysis. Molecules. 2020; 25(16):3692. https://doi.org/10.3390/molecules25163692
Chicago/Turabian StyleBuriac, Oana, Mihaela Ciopec, Narcis Duţeanu, Adina Negrea, Petru Negrea, and Ioan Grozav. 2020. "Platinum (IV) Recovery from Waste Solutions by Adsorption onto Dibenzo-30-crown-10 Ether Immobilized on Amberlite XAD7 Resin–Factorial Design Analysis" Molecules 25, no. 16: 3692. https://doi.org/10.3390/molecules25163692
APA StyleBuriac, O., Ciopec, M., Duţeanu, N., Negrea, A., Negrea, P., & Grozav, I. (2020). Platinum (IV) Recovery from Waste Solutions by Adsorption onto Dibenzo-30-crown-10 Ether Immobilized on Amberlite XAD7 Resin–Factorial Design Analysis. Molecules, 25(16), 3692. https://doi.org/10.3390/molecules25163692









