Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
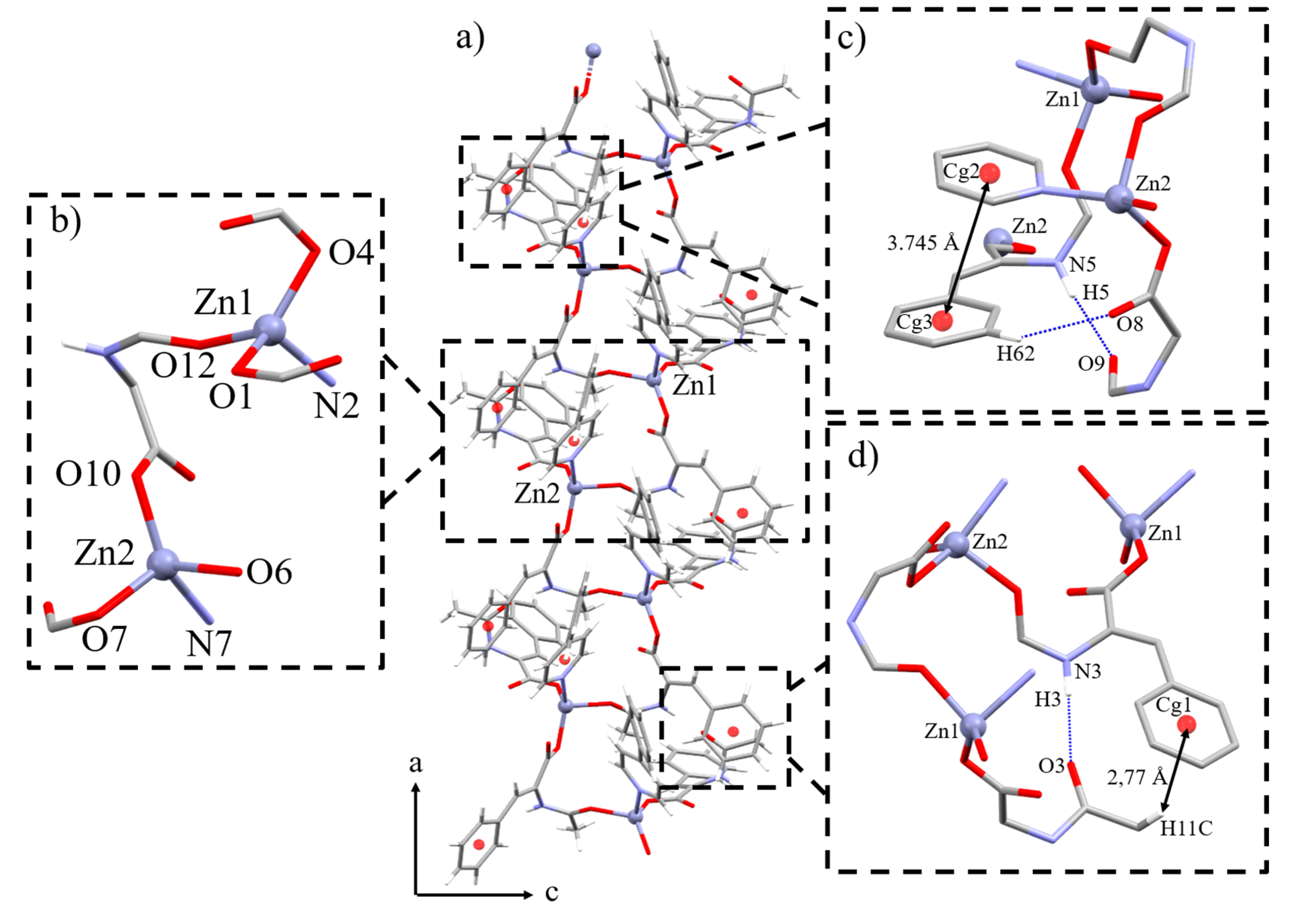
2.2. Crystal and Extended Structure of 1
2.3. Crystal Structures 2·4CH3CN and 3·4EtOH
2.4. Extended Structures of 2·4CH3CN and 3·4EtOH
2.5. Hirshfeld Surface Analysis
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthesis of [Zn2(µ-O,O’-ACA)2(ACA)2(4-Phpy)2]n (1)
4.3. Synthesis of [Zn3(µ-ACA)6(4-Phpy)2] (2)
4.4. Synthesis of [Zn3(µ-ACA)6(EtOH)2] (3)
5. X-ray Crystallographic Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.-P.; Chao, H.-Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-dimensional metal-organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincǎ, M. Grand challenges and future opportunities for metal-organic frameworks. Acs Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Ricco, R.; Pfeiffer, C.; Sumida, K.; Sumby, C.J.; Falcaro, P.; Furukawa, S.; Champness, N.R.; Doonan, C.J. Emerging applications of metal-organic frameworks. Cryst. Eng. Comm. 2016, 18, 6532–6542. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Lee, J.H.; Moon, H.R. Alterations to secondary building units of metal-organic frameworks for the development of new functions. Inorg. Chem. Front. 2020, 7, 12–27. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering-a new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Hubberstey, P.; Li, W.S.; Withersby, M.A.; Schröder, M. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 1999, 183, 117–138. [Google Scholar] [CrossRef]
- Wittmann, T.; Tschense, C.B.L.; Zappe, L.; Koschnick, C.; Siegel, R.; Stäglich, R.; Lotsch, B.V.; Senker, J. Selective host-guest interactions in metal-organic frameworks via multiple hydrogen bond donor-acceptor recognition sites. J. Mater. Chem. A 2019, 7, 10379–10388. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Rigamonti, L.; Carlino, S.; Halibi, Y.; Demartin, F.; Castellano, C.; Ponti, A.; Pievo, R.; Pasini, A. Copper 1D coordination polymers and dimers: Role of the carboxylate and the ammonium cation, crystal structures and magnetic studies. Polyhedron 2013, 53, 157–165. [Google Scholar] [CrossRef][Green Version]
- Lippard, S.J. Oxo-bridged polyiron centers in biology and chemistry. Angew. Chem. Int. Ed. Engl. 1988, 27, 344–361. [Google Scholar] [CrossRef]
- Reisner, E.; Telser, J.; Lippard, S.J. A planar carboxylate-rich tetrairon(II) complex and its conversion to linear triiron(II) and paddlewheel diiron(II) complexes. Inorg. Chem. 2007, 46, 10754–10770. [Google Scholar] [CrossRef]
- Wang, X.-W.; Chen, F.-P.; Chen, L.; Chen, J.-Z.; Jiang, W.-J.; Cai, T.-J.; Deng, Q. Crystal structures and fluorescent properties of two linear trinuclear zinc(II) complexes. J. Mol. Struct. 2007, 842, 75–80. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, D.-Y. Structure evolution and coordination modes of metal-carboxylate frameworks with robust linear trinuclear complexes as building units. Cryst. Eng. Comm. 2012, 14, 4567–4569. [Google Scholar] [CrossRef]
- Davies, R.P.; Less, R.J.; Lickiss, P.D.; White, A.J.P. Framework materials assembled from magnesium carboxylate building units. Dalton Trans. 2007, 2528–2535. [Google Scholar] [CrossRef]
- Rardin, R.L.; Bino, A.; Poganiuch, P.; Tolman, W.B.; Liu, S.; Lippard, S.J. Synthesis and characterization of the linear trinuclear complexes [M3II(O2CCH3)6(biphme)2], M = Mn, Fe. Angew. Chem Int. Ed. Engl. 1990, 29, 812–814. [Google Scholar] [CrossRef]
- Ménage, S.; Vitols, S.E.; Bergerat, P.; Codjovi, E.; Kahn, O.; Girerd, J.J.; Guillot, M.; Solans, X.; Calvet, T. Structure of the linear trinuclear complex MnII3(CH3CO2)6(bpy)2. determination of the J electron-exchange parameter through magnetic susceptibility and high-field magnetization measurements. Inorg. Chem. 1991, 30, 2666–2671. [Google Scholar] [CrossRef]
- Clegg, W.; Little, I.R.; Straughan, B.P. Zinc carboxylate complexes: Structural characterization of the mixed-metal linear trinuclear complexes MZn2(crot)6(base)2(M = Mn, Co, Ni, Zn, Cd, Mg, Ca, Sr; crot- = crotonate(l-); base = quinoline, 6-methylquinoline). Inorg. Chem. 1988, 27, 1916–1923. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.W.; Hu, B.; Chen, F.-P.; Chen, J.-Z.; Chen, L. Synthesis, characterization and crystal structure of a new linear trinuclear manganese(II) complex, [Mn3(PhCH=CHCO2)6(BPY)2]·H2O (BPY=2,2-bipyridine). J. Coord. Chem. 2007, 60, 2401–2408. [Google Scholar] [CrossRef]
- Wang, X.-W.; Chen, Y.; Chen, J.-Z.; Liu, J.-H.; Han, L. Structure and stability of a linear trinuclear cobalt(II) complex: Co3(PhCH=CHCO2)6(bpy)2. Z. Nat.. 2008, 63b, 129–133. [Google Scholar] [CrossRef]
- Vasile Scăeteanu, G.; Chifiriuc, M.C.; Bleotu, C.; Kamerzan, C.; Mărutescu, L.; Daniliuc, C.G.; Maxim, C.; Calu, L.; Olar, R.; Badea, M. Synthesis, structural characterization, antimicrobial activity, and in vitro biocompatibility of new unsaturated carboxylate complexes with 2,2′-bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Kaplanis, M.; Raptopoulou, C.P.; Perlepes, S.P.; Manessi-Zoupa, E.; Katsoulakou, E. Dinuclear versus trinuclear complex formation in zinc(II) benzoate/pyridyl oxime chemistry depending on the position of the oxime group. Polyhedron 2009, 28, 3243–3250. [Google Scholar] [CrossRef]
- Clegg, W.; Little, I.R.; Straughan, B.P. Zinc carboxylate complexes: structural characterisation of some binuclear and linear trinuclear complexes. J. Chem. Soc. Dalton Trans. 1986, 6, 1283–1288. [Google Scholar] [CrossRef]
- Tarushi, A.; Totta, X.; Raptopoulou, C.P.; Psycharis, V.; Psomas, G.; Kessissoglou, D.P. Structural features of mono- and tri-nuclear Zn(II) complexes with a non-steroidal anti-inflammatory drug as ligand. Dalton Trans. 2012, 41, 7082–7091. [Google Scholar] [CrossRef] [PubMed]
- Zeleňák, V.; Sabo, M.; Massa, W.; Llewellyn, P. Preparation, characterisation and crystal structure of two zinc(II) benzoate complexes with pyridine-based ligands nicotinamide and methyl-3-pyridylcarbamate. Inorg. Chim. Acta 2004, 357, 2049–2059. [Google Scholar] [CrossRef]
- Smolková, R.; Zeleňák, V.; Gyepes, R.; Sabolová, D.; Imrichová, N.; Hudecová, D.; Smolko, L. Synthesis, characterization, DNA binding, topoisomerase I inhibition and antimicrobial activity of four novel zinc(II) fenamates. Polyhedron 2018, 141, 230–238. [Google Scholar] [CrossRef]
- Smolková, R.; Zeleňák, V.; Smolko, L.; Sabolová, D.; Kuchár, J.; Gyepes, R. Novel Zn(II) complexes with non-steroidal anti-inflammatory ligand, flufenamic acid: Characterization, topoisomerase I inhibition activity, DNA and HSA binding studies. J. Inorg. Biochem. 2017, 177, 143–158. [Google Scholar] [CrossRef]
- Melnik, M.; Györyová, K.; Skorsepa, J.; Holloway, C.E. Zinc(II) compounds: Classification and analysis of crystallographic and structural data. J. Coord. Chem. 1995, 35, 179–279. [Google Scholar] [CrossRef]
- Song, W.; Cui, X.-Z.; Wang, X.-G.; Liang, L.; Yang, E.-C.; Zhao, X.-J. Structural diversity of Zn(II)/Cd(II) coordination polymers constructed from mixed ligand systems of conformationally flexible azo functionalized bis-imidazolate and dicarboxylates. Polyhedron 2017, 127, 266–277. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, Z.-Z.; Pan, J.; Li, J.-H.; Wang, G.-M. Dual ligand strategy for constructing a series of d10 coordination polymers: syntheses, structures, photoluminescence, and sensing properties. Cryst. Growth Des. 2018, 18, 1882–1890. [Google Scholar] [CrossRef]
- Qi, Y.-J.; Wang, Y.-J.; Li, X.-X.; Zhao, D.; Sun, Y.-Q.; Zheng, S.-T. Two d10 metal-organic frameworks as low-temperature luminescent molecular thermometers. Cryst. Growth Des. 2018, 18, 7383–7390. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, P.; Chen, H.; Wang, S.; Wang, H.; Guo, J.; Zhang, X.; Zhang, S.; Yan, J.; Xia, J.; et al. Assessment of the biocompatibility and biological effects of biodegradable pure Zinc material in the colorectum. Acs Biomater. Sci. Eng. 2018, 4, 4095–4103. [Google Scholar] [CrossRef]
- Tian, X.; Hussain, S.; de Pace, C.; Ruiz-Pérez, L.; Battaglia, G. ZnII complexes for bioimaging and correlated applications. Chem. Asian J. 2019, 14, 509–526. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Ayllón, J.A.; Pons, J. Modulating: P-hydroxycinnamate behavior as a ditopic linker or photoacid in copper(II) complexes with an auxiliary pyridine ligand. Dalton Trans. 2018, 47, 6479–6493. [Google Scholar] [CrossRef] [PubMed]
- Ejarque, D.; Sánchez-Férez, F.; Calvet, T.; Font-Bardia, M.; Pons, J. Exploring the reactivity of α-Acetamidocinnamic acid and 4-Phenylpyridine with Zn(II) and Cd(II). Inorg. Chim. Acta 2020, 509, 119695. [Google Scholar] [CrossRef]
- Rivera, V.M.; Ruelas-Leyva, J.P.; Fuentes, G.A. Pd and Ru complexes bearing axially chiral ligands for the asymmetric hydrogenation of C=C and C=O double bonds. Catal. Today 2013, 213, 109–114. [Google Scholar] [CrossRef]
- Bhaduri, S.; Lahiri, G.K.; Munshi, P. Hydrogenation of α-acetamidocinnamic acid with polystyrene-supported rhodium catalysts. J. Organomet. Chem. 2000, 606, 151–155. [Google Scholar] [CrossRef]
- Madarász, J.; Nánási, B.; Kovács, J.; Balogh, S.; Farkas, G.; Bakos, J. Immobilized phosphine–phosphite rhodium complexes: Highly active and enantioselective catalysts for asymmetric hydrogenation under continuous flow conditions. Mon. Chem. 2018, 149, 19–25. [Google Scholar] [CrossRef]
- Inoue, M.; Ohta, K.; Ishizuka, N.; Enomoto, S. Asymmetric hydrogenation of α-Acetamidocinnamic acid with chiral Rhodium complexes of DIOP and BPPM on charcoal. Chem. Pharm. Bull. 1983, 31, 3371–3376. [Google Scholar] [CrossRef][Green Version]
- Gheorghiu, C.C.; Machado, B.F.; Salinas-Martínez De Lecea, C.; Gouygou, M.; Román-Martínez, M.C.; Serp, P. Chiral rhodium complexes covalently anchored on carbon nanotubes for enantioselective hydrogenation. Dalton Trans. 2014, 43, 7455–7463. [Google Scholar] [CrossRef]
- Nakamichi, K.; Nabe, K.; Yamada, S.; Tosa, T.; Chibata, I. L-Phenylalanine formation from acetamidocinnamic acid by newly isolated bacteria. Appl. Microbiol. Biotechnol. 1984, 19, 100–105. [Google Scholar] [CrossRef]
- Nishida, Y.; Nakamichi, K.; Nabe, K.; Tosa, T. Continuous production of L-phenylalanine from acetamidocinnamic acid using co-immobilized cells of Corynebacterium sp. and Paracoccus denitrificans. Appl. Biochem. Biotechnol. 1987, 9, 479–483. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley Interscience: Hoboken, NJ, USA, 2000. [Google Scholar]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 5th ed.; McGrawHill: London, UK, 1995. [Google Scholar]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.C.M.; Salvagni, E.; Parsons, S. Investigating the effect of hydrogen bonding environments in amide cleavage reactions at zinc(II) complexes with intramolecular amide oxygen co-ordination. Dalton Trans. 2004, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Girma, K.B.; Lorenz, V.; Blaurock, S.; Edelmann, F.T. Coordination chemistry of acrylamide. 5. Crystal structures of complexes of metal(II) perchlorates and tetrafluoroborates with acrylamide. Z. Anorg. Allg. Chem. 2006, 632, 1874–1878. [Google Scholar] [CrossRef]
- Pal Chaudhuri, U.; Whiteaker, L.R.; Mondal, A.; Klein, E.L.; Powell, D.R.; Houser, R.P. Substituted pyridylmethylamide ligands and their zinc complexes. Inorg. Chim. Acta 2007, 360, 3610–3618. [Google Scholar] [CrossRef]
- Zeleňák, V.; Císařová, I.; Llewellyn, P. Diversity of carboxylate coordination in two novel zinc(II) cinnamate complexes. Inorg. Chem. Commun. 2007, 10, 27–32. [Google Scholar] [CrossRef]
- Chakraborty, G.; Mandal, S.K. Neutral luminescent metal-organic frameworks: Structural diversification, photophysical properties, and sensing applications. Inorg. Chem. 2017, 56, 14556–14566. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Morse, P.M.; Girolami, G.S. Are d0 ML6 complexes always octahedral? The X-ray structure of trigonal-prismatic [Li(tmed)]2[ZrMe6]. J. Am. Chem. Soc. 1989, 111, 4114–4116. [Google Scholar] [CrossRef]
- Friese, J.C.; Krol, A.; Puke, C.; Kirschbaum, K.; Giolando, D.M. Trigonal prismatic vs octahedral coordination geometry: Syntheses and structural characterization of hexakis(arylthiolato) zirconate complexes. Inorg. Chem. 2000, 39, 1496–1500. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17.5; University of Western, Australia: Crawley, Australia, 2017. [Google Scholar]
- Jayatilaka, D.; Grimwood, D.J. Tonto: A fortran based object-oriented system for quantum chemistry and crystallography. Comput. Sci.-Iccs 2003, 4, 142–151. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0 — new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Persistence of Vision Pty. Ltd. Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2004. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |









| Bond Lengths (Å) | |||||
|---|---|---|---|---|---|
| Zn(1)-O(1) | 1.949(4) | Zn(2)-O(7) | 1.979(3) | ||
| Zn(1)-O(4) | 1.977(3) | Zn(2)-O(10)#1 | 2.005(5) | ||
| Zn(1)-O(12) | 2.008(3) | Zn(2)-O(6) | 2.010(3) | ||
| Zn(1)-N(2) | 2.072(4) | Zn(2)-O(6) | 2.054(4) | ||
| Bond Angles (°) | |||||
| O(1)-Zn(1)-O(4) | 129.1(1) | O(7)-Zn(2)-O(10)#1 | 106.6(2) | ||
| O(1)-Zn(1)-O(12) | 101.9(1) | O(7)-Zn(2)-O(6) | 101.6(1) | ||
| O(4)-Zn(1)-O(12) | 116.7(1) | O(10)#1-Zn(2)-O(6) | 103.8(1) | ||
| O(1)-Zn(1)-N(2) | 116.0(2) | O(7)-Zn(2)-N(7) | 108.2(2) | ||
| O(4)-Zn(1)-N(2) | 97.1(1) | O(10)#1-Zn(2)-N(7) | 139.1(2) | ||
| O(12)-Zn(1)-N(2) | 89.4(1) | O(6)-Zn(2)-N(7) | 89.6(1) | ||
| Intermolecular Interactions (Å) | |||||
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | >D-H···A (°) | |
| N(1)-H(1)···O(2) | 0.86 | 2.02 | 2.870(5) | 173 | |
| N(4)-H(4)···O(8) | 0.86 | 2.09 | 2.899(6) | 157 | |
| C(46)-H(46A)···O(9) | 0.96 | 2.53 | 3.429(7) | 155 | |
| C(46)-H(46C)···O(8) | 0.96 | 2.49 | 3.204(8) | 131 | |
| C(31)-H(31)···Cg(4) | 0.93 | 2.67 | 3.516(5) | 151 | |
| C(40)-H(40)···Cg(4) | 0.93 | 2.79 | 3.571(5) | 142 | |
| C(64)-H(64)···Cg(1) | 0.93 | 2.97 | 3.693(8) | 135 | |
| #1: x + 1,y,z; Cg(1) = C(29) C(30) C(31) C(32) C(33) C(34); Cg(2) = N(7) C(19) C(47) C(48) C(49) C(50); Cg(3) = C(60) C(61) C(62) C(63) C(64) C(65); Cg(4) = C(51) C(52) C(53) C(54) C(55) C(56) | |||||
| Bond Lengths (Å) | ||||
|---|---|---|---|---|
| Zn(1)-O(1) | 2.1028(15) | Zn(2)-O(2) | 1.9465(14) | |
| Zn(1)-O(4) | 2.1644(15) | Zn(2)-O(8) | 1.9673(15) | |
| Zn(1)-O(7) | 2.0861(15) | Zn(2)-O(5) | 1.9254(15) | |
| Zn(2)-N(4) | 2.0358(17) | |||
| Bond Angles (°) | ||||
| O(1)-Zn(1)-O(4) | 90.39(6) | O(1)-Zn(1)-O(4)#1 | 89.61(6) | |
| O(7)-Zn(1)-O(1) | 90.00(6) | O(7)-Zn(1)-O(4) | 93.10(6) | |
| O(7)#1-Zn(1)-O(4) | 86.90(6) | O(1)#1-Zn(1)-O(1) | 180.00(7) | |
| O(4)-Zn(1)-O(4)#1 | 180.00(4) | O(7)#1-Zn(1)-O(7) | 180.0 | |
| O(2)-Zn(2)-N(4) | 101.22(7) | O(5)-Zn(2)-N(4) | 103.85(7) | |
| O(8)-Zn(2)-N(4) | 99.97(7) | O(5)-Zn(2)-O(2) | 119.84(7) | |
| O(2)-Zn(2)-O(8) | 117.26(7) | O(5)-Zn(2)-O(8) | 110.86(7) | |
| Twist Angles (°) | ||||
| O(1)-Cg(1)-Cg(1)#1-O(4) | 60.32 | O(4)-Cg(1)-Cg(1)#1-O(7) | 57.80 | |
| O(7)-Cg(1)-Cg(1)#1-O(1) | 61.88 | |||
| Intermolecular Interactions (Å) | ||||
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | >D-H···A (°) |
| C(44)-H(44)···N(1W) | 0.95 | 2.60 | 3.496(4) | 157 |
| C(4W)-H(4W)A···O(9) | 0.98 | 2.30 | 3.279(3) | 177 |
| C(4W)-H(4W)C···O(3) | 0.98 | 2.46 | 3.428(4) | 172 |
| C(22)-H(22A)···Cg(2) | 0.98 | 2.69 | 3.553(3) | 148 |
| C(27)-H(27)···Cg(3) | 0.95 | 2.88 | 3.608(3) | 134 |
| Cg(3)···Cg(4) | 3.8858(14) | |||
| #1: -x + 1,-y + 1,-z + 1; Cg(1) = O(1) O(4) O(7); Cg(2) = C(26) C(27) C(28) C(29) C(30) C(31); Cg(3) = C(4) C(5) C(6) C(7) C(8) C(9); Cg(4) = N(4) C(34) C(35) C(36) C(37) C(38) | ||||
| Bond Lengths (Å) | |||||
|---|---|---|---|---|---|
| Zn(1)-O(1) | 2.172(3) | Zn(1)-O(4) | 2.173(3) | ||
| Zn(1)-O(7) | 2.159(3) | Zn(1)-O(10) | 2.172(3) | ||
| Zn(1)-O(13) | 2.150(3) | Zn(1)-O(16) | 2.161(3) | ||
| Zn(2)-O(2) | 1.952(2) | Zn(2)-O(5) | 1.967(2) | ||
| Zn(2)-O(8) | 1.934(2) | Zn(2)-O(19) | 1.985(2) | ||
| Zn(3)-O(11) | 1.9617(18) | Zn(3)-O(14) | 1.940(2) | ||
| Zn(3)-O(17) | 1.948(2) | Zn(3)-O(20) | 1.985(2) | ||
| Bond Angles (°) | |||||
| O(1)-Zn(1)-O(4) | 87.06(9) | O(7)-Zn(1)-O(1) | 89.52(9) | ||
| O(7)-Zn(1)-O(4) | 90.31(9) | O(7)-Zn(1)-O(10) | 90.45(9) | ||
| O(7)-Zn(1)-O(16) | 89.71(9) | O(10)-Zn(1)-O(4) | 92.96(9) | ||
| O(13)-Zn(1)-O(1) | 89.69(9) | O(13)-Zn(1)-O(4) | 89.75(9) | ||
| O(13)-Zn(1)-O(10) | 90.35(9) | O(13)-Zn(1)-O(16) | 90.23(9) | ||
| O(16)-Zn(1)-O(1) | 92.74(9) | O(16)-Zn(1)-O(10) | 87.23(8) | ||
| O(1)-Zn(1)-O(10) | 179.96(11) | O(13)-Zn(1)-O(7) | 179.20(10) | ||
| O(16)-Zn(1)-O(4) | 179.80 (11) | O(2)-Zn(2)-O(5) | 114.81(10) | ||
| O(2)-Zn(2)-O(19) | 99.26(11) | O(5)-Zn(2)-O(19) | 105.43(11) | ||
| O(8)-Zn(2)-O(2) | 122.91(10) | O(8)-Zn(2)-O(5) | 115.08(9) | ||
| O(8)-Zn(2)-O(19) | 92.69(10) | O(11)-Zn(3)-O(20) | 99.53(10) | ||
| O(14)-Zn(3)-O(11) | 123.27(10) | O(14)-Zn(3)-O(17) | 113.34(10) | ||
| O(14)-Zn(3)-O(20) | 97.72(10) | O(17)-Zn(3)-O(11) | 115.20(9) | ||
| O(17)-Zn(3)-O(20) | 107.00(10) | ||||
| Twist Angles (°) | |||||
| O(1)-Cg(1)-Cg(2)-O(13) | 59.10 | O(7)-Cg(1)-Cg(2)-O(16) | 58.88 | ||
| O(4)-Cg(1)-Cg(2)-O(10) | 61.49 | ||||
| Intermolecular Interactions (Å) | |||||
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | >D-H···A (°) | |
| O(19)-H(19)O···O(3W) | 0.845(19) | 1.861(19) | 2.536(4) | 135.8(17) | |
| O(20)-H(20)O···O(2W) | 0.83(2) | 1.76(2) | 2.585(4) | 174(4) | |
| O(3W)-H(3WO)···O(4W) | 0.84 | 1.91 | 2.669(4) | 149 | |
| O(2W)-H(2WO)···O(1W) | 0.84 | 2.01 | 2.683(3) | 136 | |
| O(4W)-H(4WO)···O(6) | 0.84 | 1.85 | 2.685(4) | 175 | |
| O(1W)-H(1WO)···O(18) | 0.84 | 1.88 | 2.712(4) | 172 | |
| C(31)-H(31)···O(4W) | 0.95 | 2.54 | 3.481(5) | 172 | |
| C(53)-H(53)···O(1W) | 0.95 | 2.52 | 3.460(5) | 172 | |
| C(4W)-H(4WA)···O(20) | 0.98 | 2.61 | 3.418(7) | 140 | |
| C(4W)-H(4WB)···O(2) | 0.98 | 2.63 | 3.502(6) | 148 | |
| C(7W)-H(7WB)···Cg(3) | 0.99 | 2.97 | 3.954(7) | 172 | |
| C(20)-H(20)···Cg(4) | 0.95 | 2.88 | 3.674(4) | 142 | |
| C(60)-H(60)···Cg(5) | 0.95 | 2.90 | 3.705(4) | 143 | |
| C(69)-H(69B)···Cg(4) | 0.99 | 2.69 | 3.611(4) | 155 | |
| C(67)-H(67A)···Cg(5) | 0.99 | 2.85 | 3.703(4) | 145 | |
| Cg(1) = O(1) O(4) O(7); Cg(2) = O(10) O(13) O(16); Cg(3) = C(15) C(16) C(17) C(18) C(19) C(20); Cg(4) = C(4) C(5) C(6) C(7) C(8) C(9); Cg(5) = C(37) C(38) C(39) C(40) C(41) C(42) | |||||
| Complex | D-H···A | Number of Interactions | % Hirshfeld Surface Implied |
|---|---|---|---|
| 2·4CH3CN | C-H···Nsolvent | 2 | 6.0 |
| C-H···OC=O | 4 | 7.9 | |
| C-H···π | 4 | 28.1 | |
| π ··· π | 4 | 2.9 | |
| 3·4EtOH | O-H···Osolvent | 2 | 12.3 |
| OC=O···H-Osolvent | 2 | ||
| C-H···Osolvent | 2 | ||
| C-Hsolvent···OCOO | 1 | ||
| C-Hsolvent···Ocoordinated EtOH | 1 | ||
| C-H··· π | 5 | 20.4 |
| 1 | 2·4CH3CN | 3·4EtOH | |
| Empirical Formula | C66H58N6O12Zn2 | C96H90N12O18Zn3 | C78H96N6O24Zn3 |
| Formula weight | 1257.92 | 1895.90 | 1697.71 |
| T (K) | 293(2) | 100(2) | 100(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| System, space group | Monoclinic, P21/c | Monoclinic, P21/c | Monoclinic, P21 |
| Unit cell dimensions | |||
| a (Å) | 10.1338(8) | 15.7748(10) | 13.2110(4) |
| b (Å) | 24.469(2) | 23.7227(15) | 24.9706(9) |
| c (Å) | 25.598(2) | 13.1019(8) | 13.3721(4) |
| β (°) | 111.681(4) | 112.111(2) | 113.6880(10) |
| V (Å3) | 5898.3(8) | 4542.4(5) | 4039.6(2) |
| Z | 4 | 2 | 2 |
| Dcalc (mg/m3) | 1.417 | 1.386 | 1.396 |
| µ (mm−1) | 0.883 | 0.861 | 0.962 |
| F (000) | 2608 | 1968 | 1776 |
| Crystal size (mm−3) | 0.141×0.052×0.032 | 0.150×0.368×0.398 | 0.142×0.252×0.505 |
| hkl ranges | −11 <= h <= 14, −34 <= k <= 34, −36 <= l <= 36 | −21 <= h <= 22, −33 <= k <= 33, −18 <= l <= 17 | −19 <= h <= 19, −36 <= k <= 36, −19 <= l <= 19 |
| 2θ range (°) | 2.176 to 30.705 | 2.211 to 30.554 | 2.452 to 30.972 |
| Reflections collected/ unique/[Rint] | 80518/18035/ 0.2023 | 141282/13913/ 0.0604 | 25525/25525/ 0.0421 |
| Completeness to θ (%) | 98.4 | 99.8 | 99.7 |
| Absorption correction | Semi-empirical | Semi-empirical | Semi-empirical |
| Max. and min. transmission | 0.7461 and 0.6289 | 0.7461 and 0.6411 | 0.7461 and 0.6349 |
| Refinement method | Full-matrix least-square on F2 | Full-matrix least-square on F2 | Full-matrix least-square on F2 |
| Data/Restrains/Parameters | 18035/0/780 | 13913/0/588 | 25525/17/1016 |
| Goodness-on-fit on F2 | 1.038 | 1.043 | 1.032 |
| Final R indices [I > 2σ(I)] | R1 = 0.0756 wR2 = 0.1335 | R1 = 0.0442 wR2 = 0.1155 | R1 = 0.0348 wR2 = 0.0823 |
| R indices (all data) | R1 = 0.2135 wR2 = 0.1958 | R1 = 0.0639 wR2 = 0.1259 | R1 = 0.0473 wR2 = 0.0885 |
| Extinction coefficient | n/a | n/a | n/a |
| Largest Diff. peak and hole (e. Å−3) | 0.986 and −1.239 | 0.538 and −2.492 | 0.726 and −1.656 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine. Molecules 2020, 25, 3615. https://doi.org/10.3390/molecules25163615
Ejarque D, Calvet T, Font-Bardia M, Pons J. Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine. Molecules. 2020; 25(16):3615. https://doi.org/10.3390/molecules25163615
Chicago/Turabian StyleEjarque, Daniel, Teresa Calvet, Mercè Font-Bardia, and Josefina Pons. 2020. "Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine" Molecules 25, no. 16: 3615. https://doi.org/10.3390/molecules25163615
APA StyleEjarque, D., Calvet, T., Font-Bardia, M., & Pons, J. (2020). Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine. Molecules, 25(16), 3615. https://doi.org/10.3390/molecules25163615






