Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation
Abstract
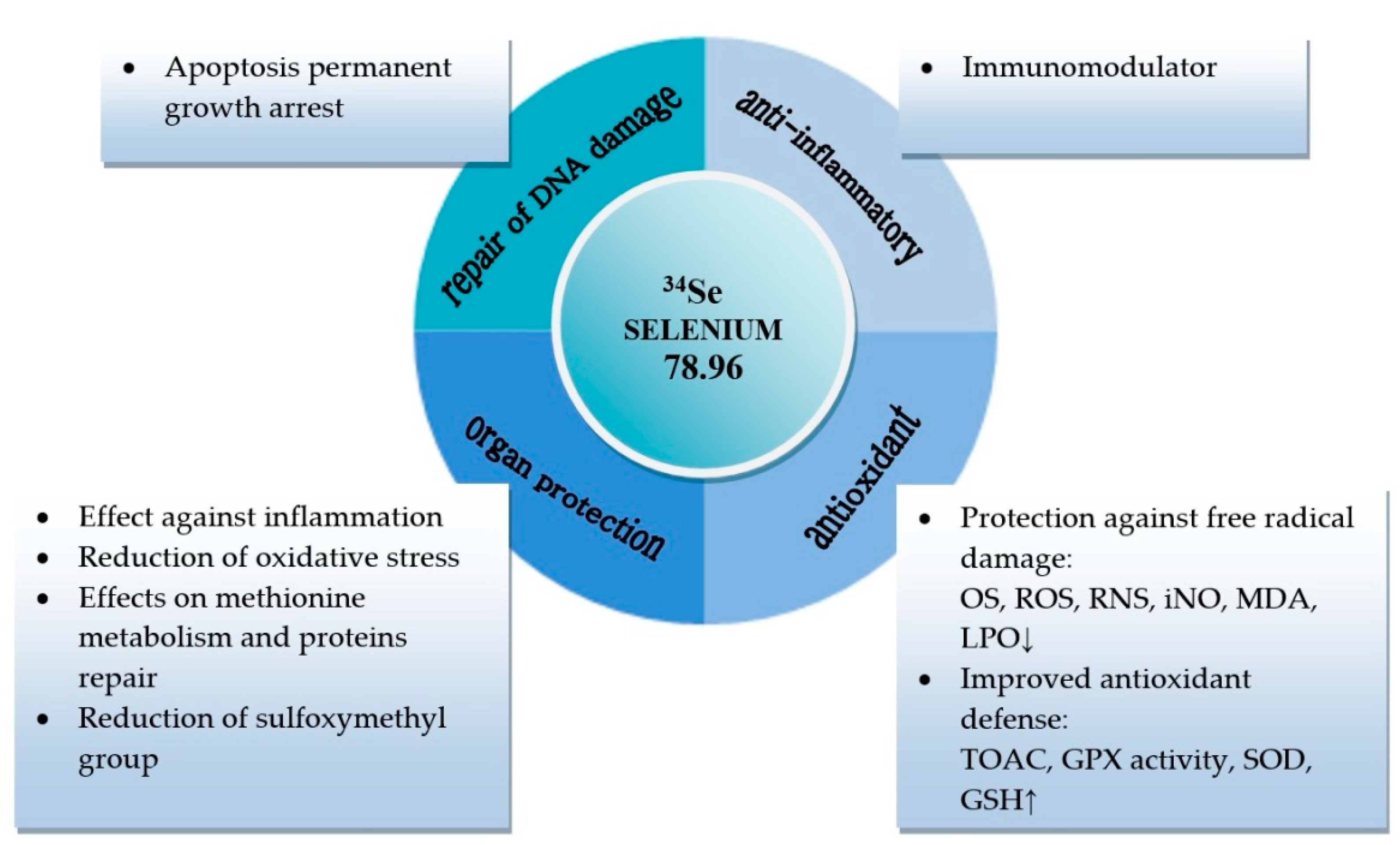
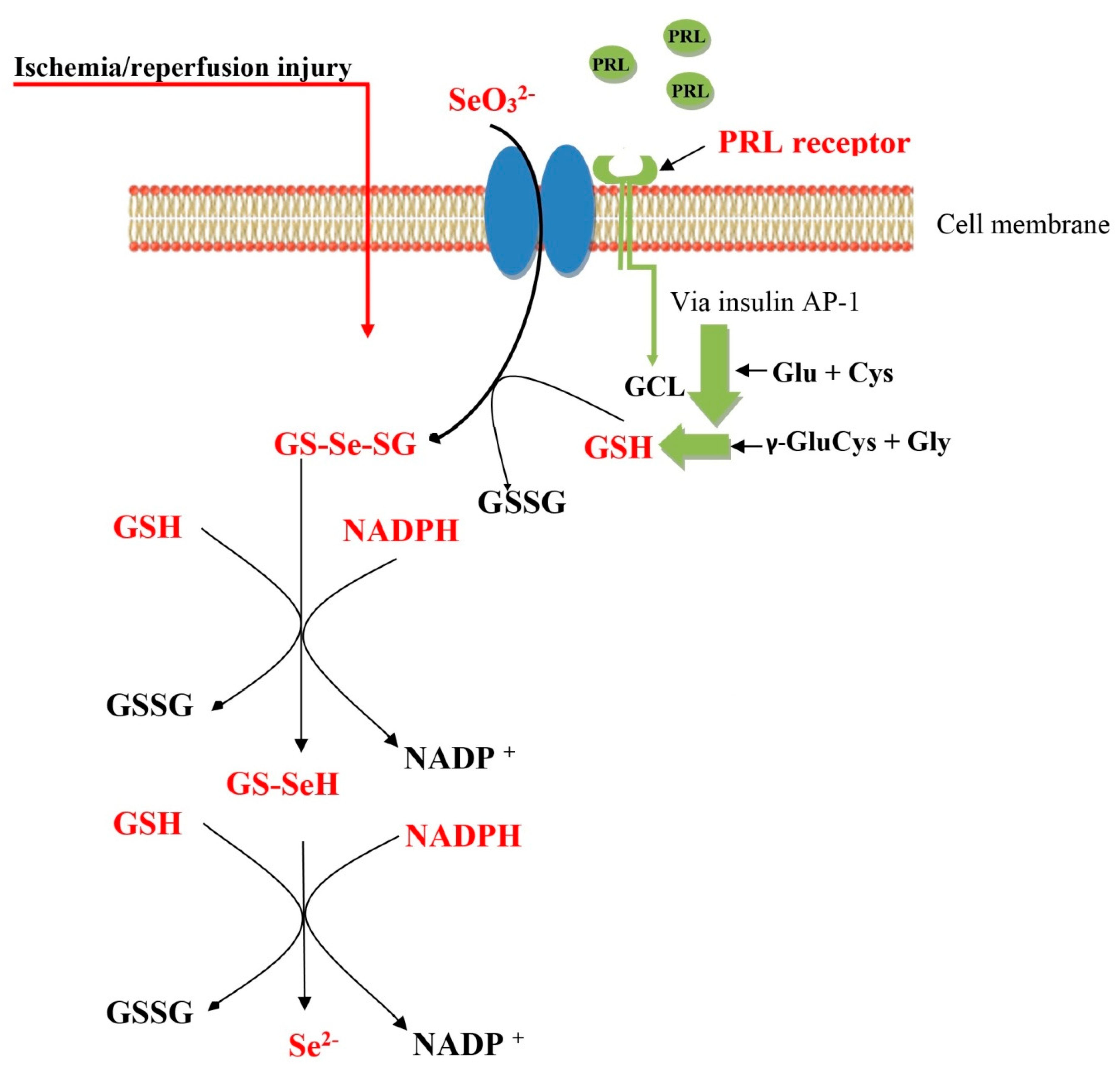
1. Introduction
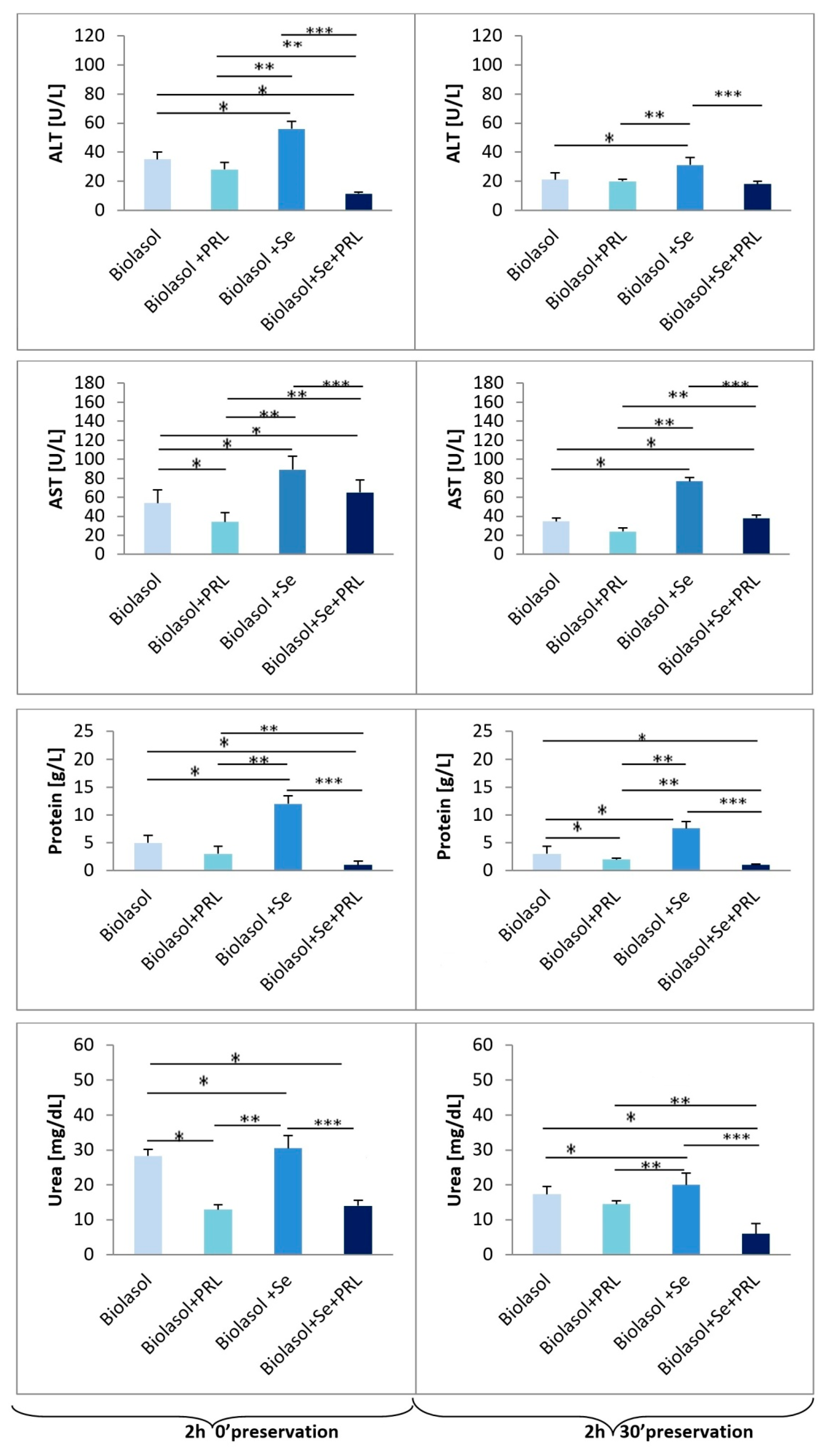
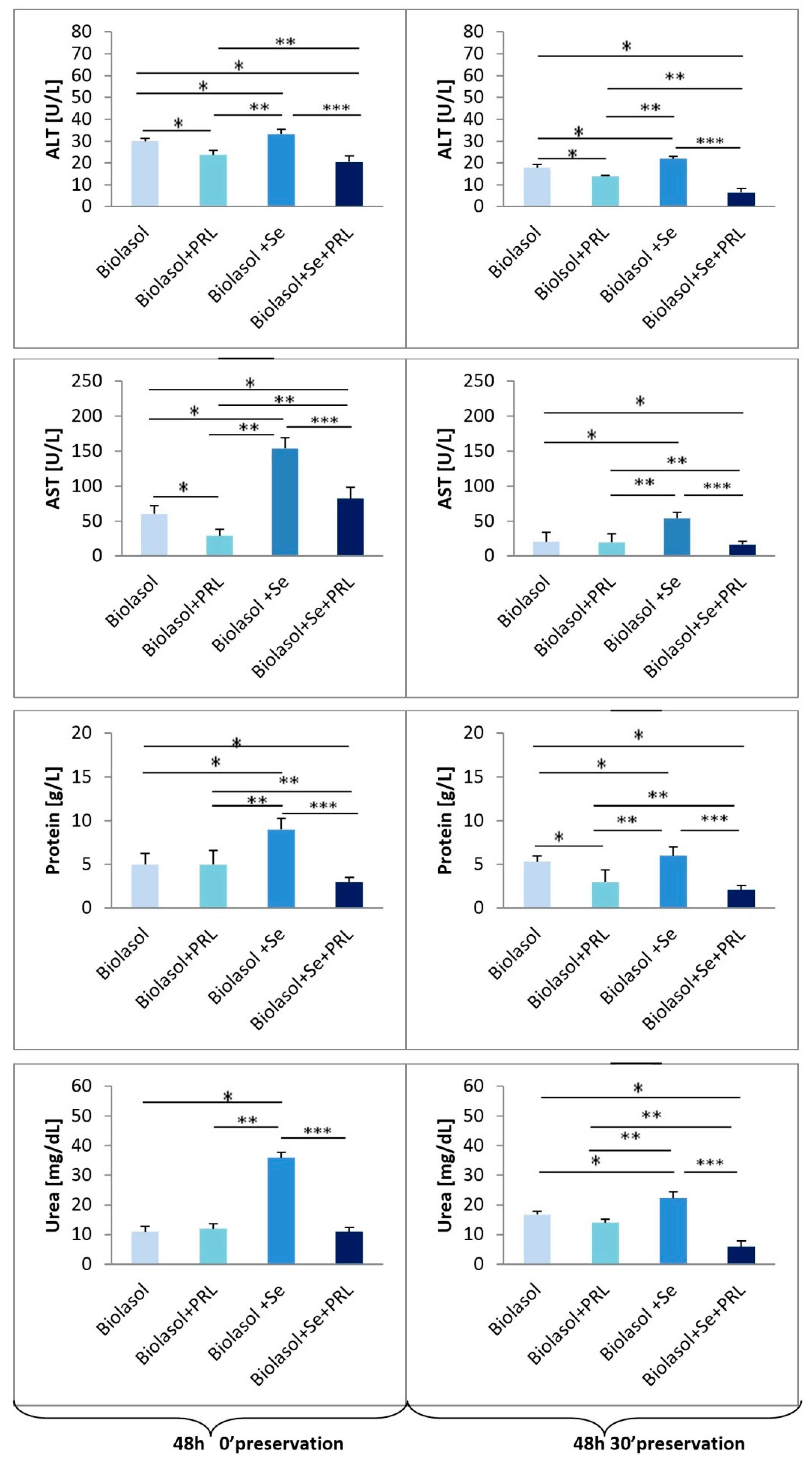
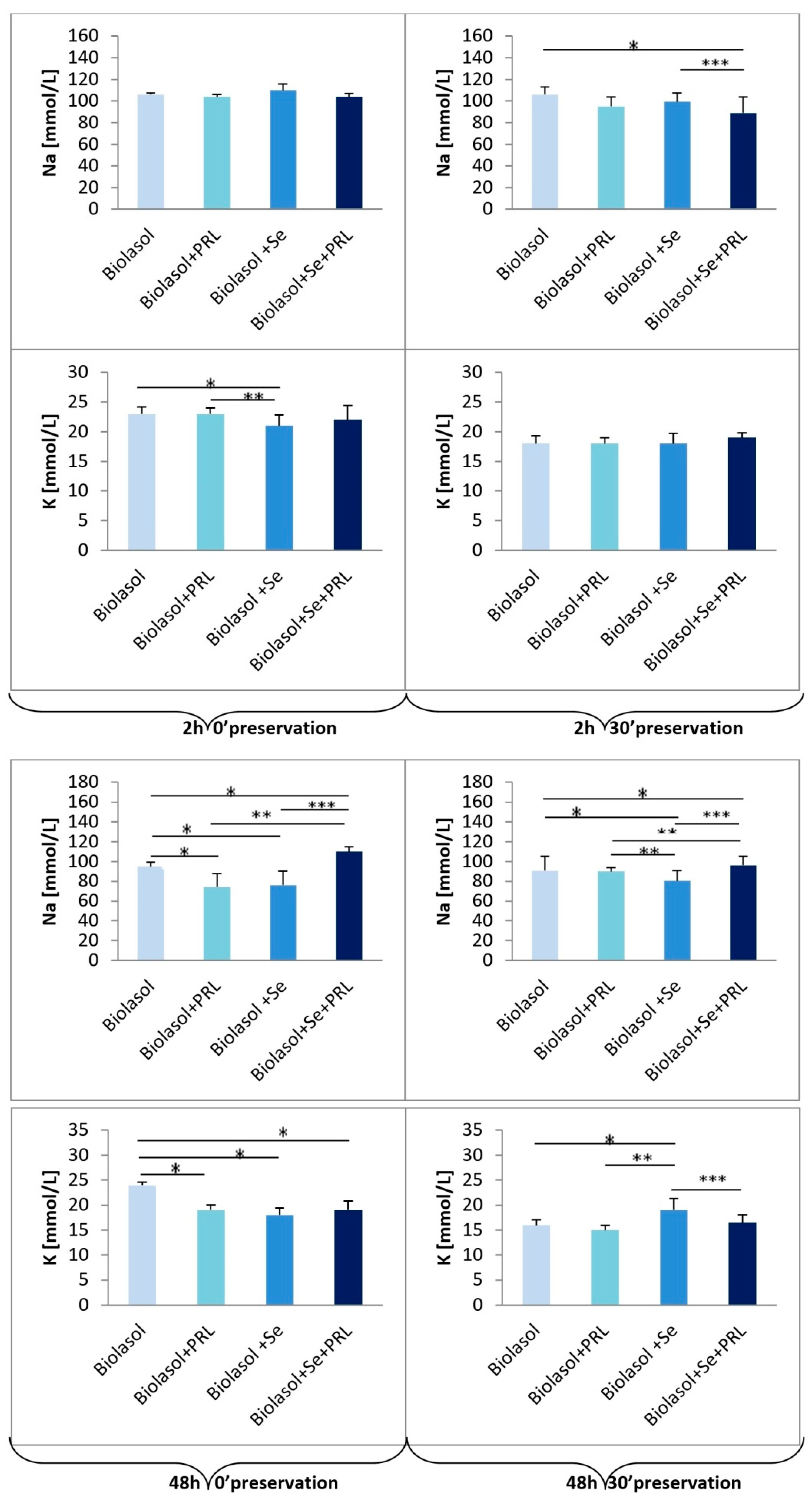
2. Results
3. Discussion
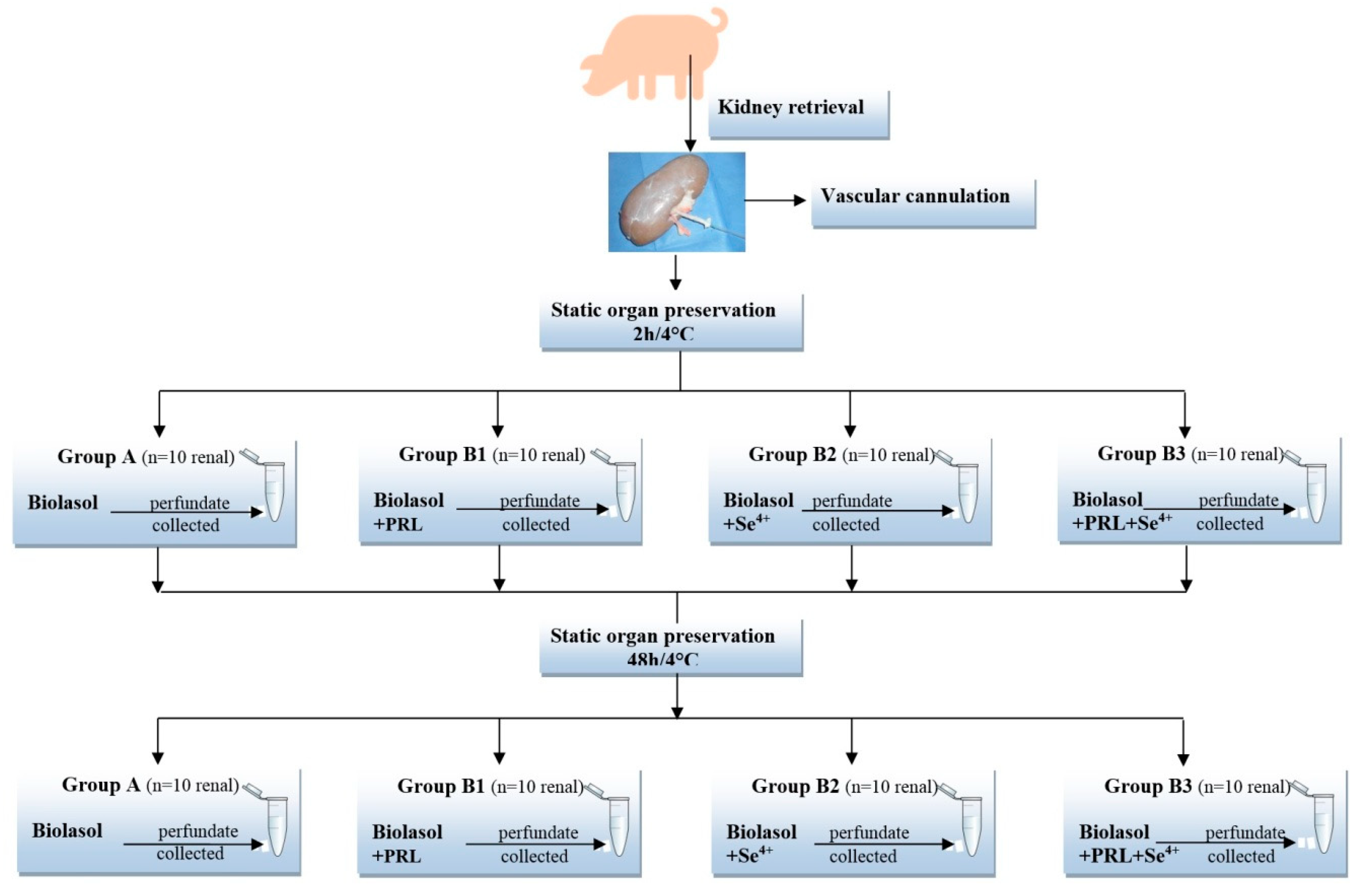
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Experimental Groups
4.4. Surgical Procedure
4.5. Perfusion and Preservation Technique
4.6. Instruments
4.7. Biochemical Determinants
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, A.; Wilkaniec, A.; Wroczyński, P.; Adamczyk, A. Selenium in the therapy of neurological diseases. Where is it going? Curr. Neuropharmacol. 2016, 14, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yoon, C.; Johnston, T.V.; Ku, S.; Ji, G.E. Production of selenomethionine-enriched bifidobacterium bifidum BGN4 via sodium selenite biocatalysis. Molecules 2018, 23, 2860. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, L.; Zeng, H.; Wu, R.T.; Wu, T.-L.; Cheng, W.-H. Analyses of selenotranscriptomes and selenium concentrations in response to dietary selenium deficiency and age reveal common and distinct patterns by tissue and sex in telomere-dysfunctional mice. J. Nutr. 2017, 147, 1858–1866. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Sher, L. Role of thyroid hormones in the effects of selenium on mood, behavior, and cognitive function. Med. Hypotheses 2001, 57, 480–483. [Google Scholar] [CrossRef]
- Youn, H.-S.; Lim, H.J.; Choi, Y.J.; Lee, J.Y.; Lee, M.-Y.; Ryu, J.-H. Selenium suppresses the activation of transcription factor NF-κB and IRF3 induced by TLR3 or TLR4 agonists. Int. Immunopharmacol. 2008, 8, 495–501. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Kim, Y.M.; Surh, Y.-J.; Baik, H.W.; Lee, S.K.; Ha, J.; Park, O.J. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res. 2006, 66, 10057–10063. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartín, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and therapeutic potential of selenazo compounds. J. Med. Chem. 2019, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S.J.; O’Reilly, D.S.J. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2011, 95, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Reinhart, K.; Bloos, F.; Marx, G.; Russwurm, S.; Bauer, M.; Brunkhorst, F.M. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br. J. Anaesth. 2007, 98, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Toufektsian, M.C.; Boucher, F.; Pucheu, S.; Tanguy, S.; Ribuot, C.; Sanou, D.; Tresallet, N.; de Leiris, J. Effects of selenium deficiency on the response of cardiac tissue to ischemia and reperfusion. Toxicology 2000, 148, 125–132. [Google Scholar] [CrossRef]
- Poltronieri, R.; Cevese, A.; Sbarbati, A. Protective effect of selenium in cardiac ischemia and reperfusion. Cardioscience 1992, 3, 155–160. [Google Scholar]
- Tanguy, S.; Boucher, F.; Besse, S.; Ducros, V.; Favier, A.; De Leiris, J. Trace elements and cardioprotection: Increasing endogenous glutathione peroxidase activity by oral selenium supplementation in rats limits reperfusion-induced arrhythmias. J. Trace Elements Med. Boil. 1998, 12, 28–38. [Google Scholar] [CrossRef]
- Zapletal, C.; Heyne, S.; Breitkreutz, R.; Gebhard, M.-M.; Golling, M. The influence of selenium substitution on microcirculation and glutathione metabolism after warm liver ischemia/reperfusion in a rat model. Microvasc. Res. 2008, 76, 104–109. [Google Scholar] [CrossRef]
- Hasanvand, A.; Abbaszadeh, A.; Darabi, S.; Nazari, A.; Gholami, M.; Kharazmkia, A. Evaluation of selenium on kidney function following ischemic injury in rats; protective effects and antioxidant activity. J. Ren. Inj. Prev. 2016, 6, 93–98. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Babaeenezhad, E.; Nayeri, H.; Nezhad, Z.Z. Selenium effects on antioxidant and inflammatory indices in renal ischemia-reperfusion injury in rats. J. Ren. Inj. Prev. 2018, 8, 71–77. [Google Scholar] [CrossRef]
- Kim, H.-Y. The methionine sulfoxide reduction system: Selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxidants Redox Signal. 2013, 19, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.; Meng, Q.; Han, H.-B.; et al. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Ryszka, F.; Dolińska, B.; Czyż, K.; Jelińska, M.; Strabel, A.; Bocheńska, J. Effect of recombinant human prolactin addition to biolasol solution on biochemical indicators in perfundates of porcine kidneys. Transplant. Proc. 2016, 48, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. The effect of modified biolasol solution on the efficacy of storing isolated porcine kidneys. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B. The Role of hormones and trophic factors as components of preservation solutions in protection of renal function before transplantation: A review of the literature. Molecules 2020, 25, 2185. [Google Scholar] [CrossRef] [PubMed]
- Cierpka, L.; Ryszka, F.; Dolińska, B.; Smorąg, Z.; Słomski, R.; Wiaderkiewicz, R.; Caban, A.; Budziński, G.; Oczkowicz, G.; Wieczorek, J. Biolasol: Novel perfusion and preservation solution for kidneys. Transplant. Proc. 2014, 46, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Ryszka, F.; Dolińska, B.; Ostróżka-Cieślik, A.; Caban, A.; Cierpka, L. Comparing the effect of Biolasol® and HTK solutions on maintaining proper homeostasis, indicating the kidney storage efficiency prior to transplantation. Ann. Transplant. 2012, 17, 74–78. [Google Scholar] [CrossRef]
- Almalki, A.M.; Ajarem, J.; Altoom, N.; Al-Otaibi, F.S.; Maodaa, S.N.; Allam, A.A.; Mahmoud, A.M. Effects of mining activities on Gerbillus nanus in Saudi Arabia: A biochemical and histological study. Animals 2019, 9, 664. [Google Scholar] [CrossRef]
- Uslu, G.A.; Uslu, H.; Adalı, Y. Hepatoprotective and nephroprotective effects of Trigonella foenum-graecum L. (Fenugreek) seed extract against sodium nitrite toxicity in rats. Biomed. Res. Ther. 2019, 6, 3142–3150. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Effect of lutropin concentration on the efficiency of isolated porcine kidney storage in modified biolasol solution. Transplant. Proc. 2020, 1–4. [Google Scholar] [CrossRef]
- Siwek, B.; Bahbouth, E.; Serra, M.; Sabbioni, E.; De Pauw-Gillet, M.-C.; Bassleer, R. Effect of selenium compounds on murine B16 melanoma cells and pigmented cloned pB16 cells. Arch. Toxicol. 1994, 68, 246–254. [Google Scholar] [CrossRef]
- Björnstedt, M.; Kumar, S.; Holmgren, A. Selenodiglutathione is a highly efficient oxidant of reduced thioredoxin and a substrate for mammalian thioredoxin reductase. J. Boil. Chem. 1992, 267, 8030–8034. [Google Scholar]
- Twardoch, M.; Lodwich, M.; Mazur, B. Allergy and oxidative stress. Ann. Acad. Med. Silesiensis 2016, 70, 15–23. [Google Scholar] [CrossRef]
- Meister, A. Selective modification of glutathione metabolism. Science 1983, 220, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Lee, P.H.; Chen, C.F.; Ma, M.C.; Lai, M.K.; Hsu, S.M. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J. Am. Soc. Nephrol. 2001, 12, 973–982. [Google Scholar] [PubMed]
- Shang, Y.; Siow, Y.L.; Isaak, C.K. Downregulation of glutathione biosynthesis contributes to oxidative stress and liver dysfunction in acute kidney injury. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Grattan, D.R. The eyes have it! Protective role of prolactin in the retina. EBioMedicine 2016, 8, 26–27. [Google Scholar] [CrossRef][Green Version]
- Mountjoy, K.; Cowden, E.A.; Dobbie, J.W.; Ratcliffe, J.G. Prolactin receptors in the rat kidney. J. Endocrinol. 1980, 87, 47. [Google Scholar] [CrossRef]
- Sakai, Y.; Hiraoka, Y.; Ogawa, M.; Takeuchi, Y.; Aiso, S. The prolactin gene is expressed in the mouse kidney. Kidney Int. 1999, 55, 833–840. [Google Scholar] [CrossRef]
- Thébault, S. Potential mechanisms behind the antioxidant actions of prolactin in the retina. Exp. Eye Res. 2017, 160, 56–61. [Google Scholar] [CrossRef]
- Goffin, V.; Touraine, P. The prolactin receptor as a therapeutic target in human diseases: Browsing new potential indications. Expert Opin. Ther. Targets 2015, 19, 1229–1244. [Google Scholar] [CrossRef]
- García, R.M.; Zamarripa, D.A.; Arnold, E.; Ruiz-Herrera, X.; Imm, R.N.; Cruz, G.B.; Adan, N.; Binart, N.; Riesgo-Escovar, J.; Goffin, V.; et al. Prolactin protects retinal pigment epithelium by inhibiting sirtuin 2-dependent cell death. EBioMedicine 2016, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Wellinger, R.E. Yeast as a model system to study metabolic impact of selenium compounds. Microb. Cell 2015, 2, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Letavayová, L.; Vlčková, V.; Brozmanová, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Acute renal failure. Lancet 2005, 365, 417–430. [Google Scholar] [CrossRef]
- Třeška, V.; Kuntscher, V.; Molacek, J.; Kobr, J.; Racek, J.; Trefil, L. Can the ischemia-reperfusion syndrome in transplanted kidneys procured from non-heart-beating donors be influenced by adding selenium into the reperfusion solution? An experimental study. Transplant. Proc. 2003, 35, 1584–1586. [Google Scholar] [CrossRef]
- Třeška, V.; Kuntscher, V.; Molacek, J.; Kobr, J.; Racek, J.; Trefil, L. Can ischemia-reperfusion syndrome in transplanted kidneys procured from non-heart-beating donors be influenced by adding selenium into the reperfusion solution? An experimental study. Transplant. Proc. 2003, 35, 3125–3127. [Google Scholar] [CrossRef]
- Darago, A.; Sapota, A.; Nasiadek, M.; Klimczak, M.; Bruchajzer, E.; Kilanowicz, A. The influence of subchronic zinc and/or selenium supplementation in Wistar rats on homeostasis of these bioelements in the Sidney. Bromat. Chem. Toksykol. 2017, 50, 80–88. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Author, Year of Publication | Species | Preservation Solution Modification/Cold Ischemia | Outcome Measures, (Intervention, I/control, C) | Selenium Dose | Effects of Selenium |
|---|---|---|---|---|---|
| Treśka et al. 2003 [48] | Piglets | HTK /24 h, 4 °C/SCS | I: HTK + Se C: HTK | 200 µg | Decreased level of MDA Reduced the production of FOR Higher levels of AOC |
| Treśka et al. 2003 [49] | Piglets | HTK /24 h, 4 °C/SCS | I: HTK + Se C:HTK | 200 µg | Decreased level of MDA Reduced the production of FOR Higher levels of AOC Decreased the intensity of IRS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation. Molecules 2020, 25, 3592. https://doi.org/10.3390/molecules25163592
Ostróżka-Cieślik A, Dolińska B, Ryszka F. Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation. Molecules. 2020; 25(16):3592. https://doi.org/10.3390/molecules25163592
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Barbara Dolińska, and Florian Ryszka. 2020. "Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation" Molecules 25, no. 16: 3592. https://doi.org/10.3390/molecules25163592
APA StyleOstróżka-Cieślik, A., Dolińska, B., & Ryszka, F. (2020). Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation. Molecules, 25(16), 3592. https://doi.org/10.3390/molecules25163592







