Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase
Abstract
1. Introduction
2. Results
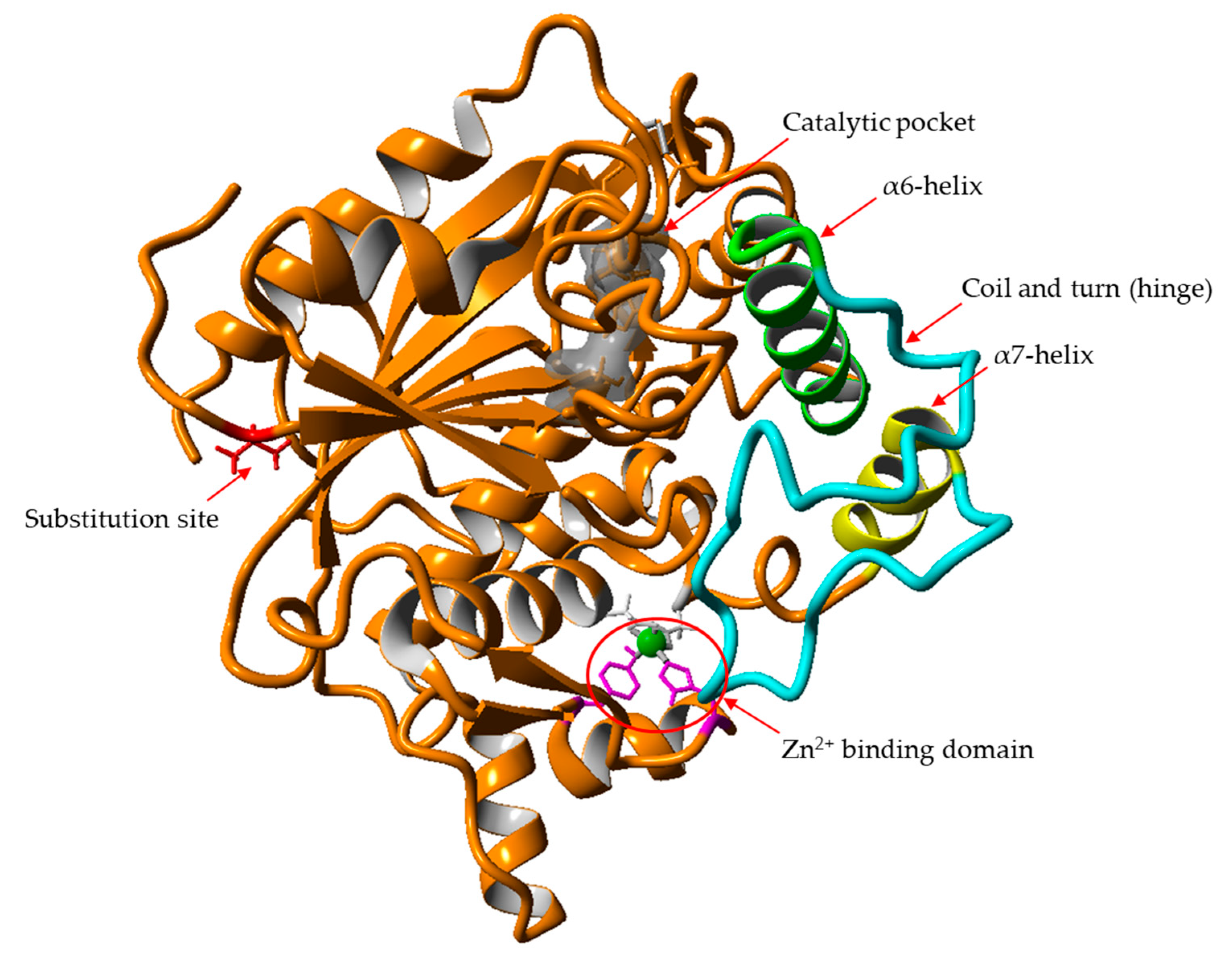
2.1. Screening for N-terminal Mutation of wt-L2 Lipase
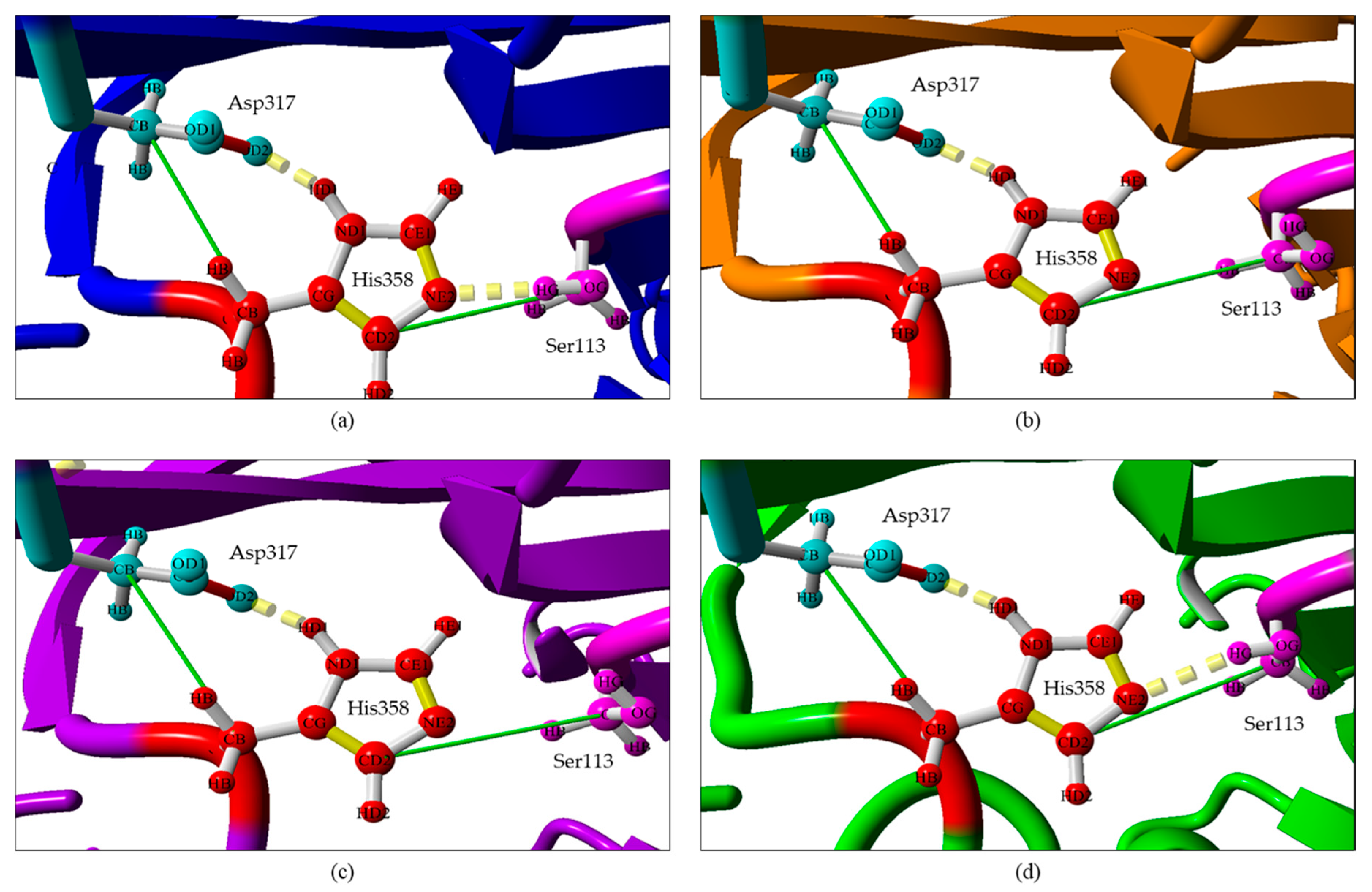
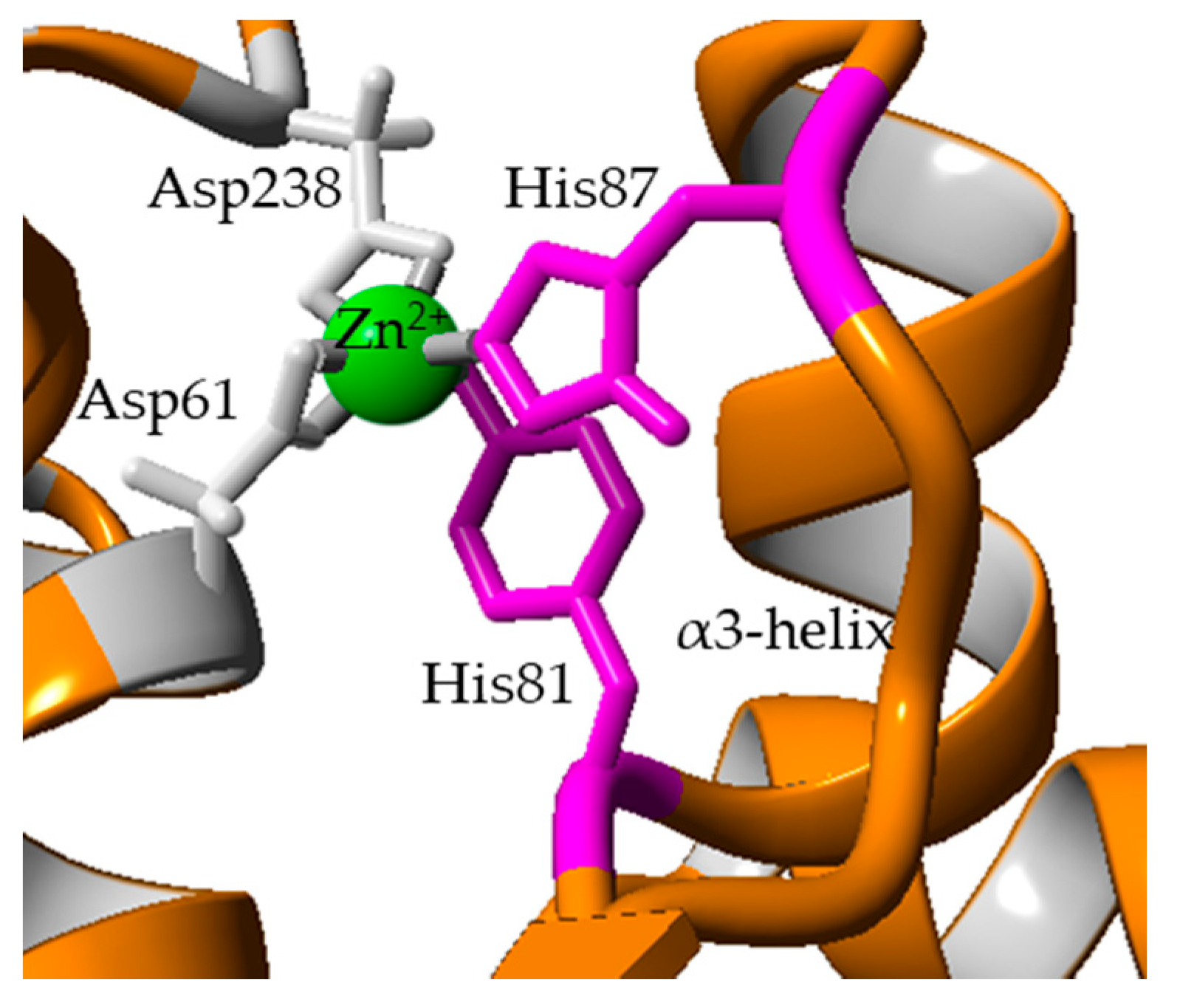
2.2. Comparison of Modelled Structures of wt-L2 and Mutant Lipases
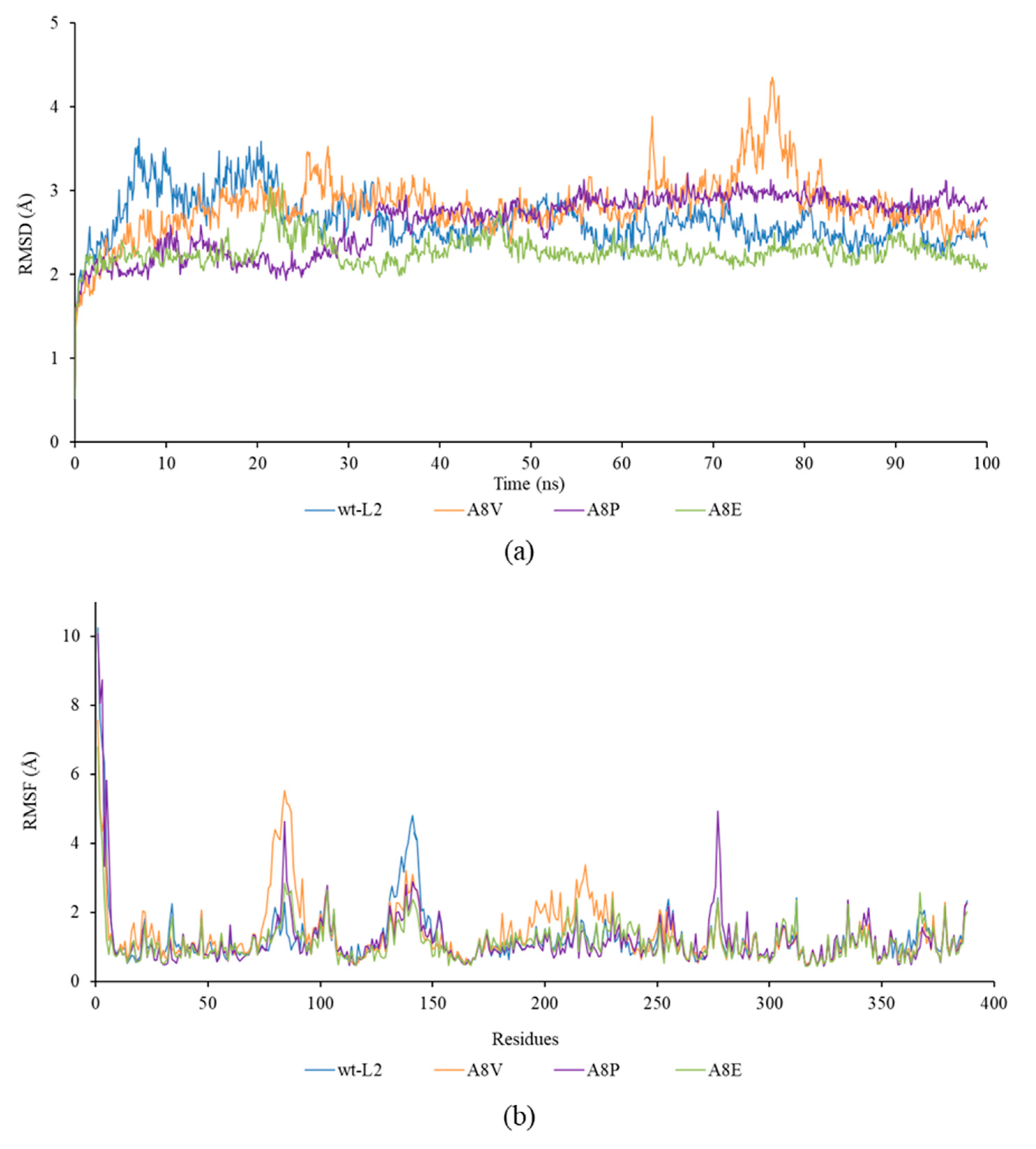
2.3. Molecular Dynamics Simulation Analysis for RMSD, RMSF, SASA and Rgyration
2.4. Temperature Stability Characterisation of Mutant Lipases
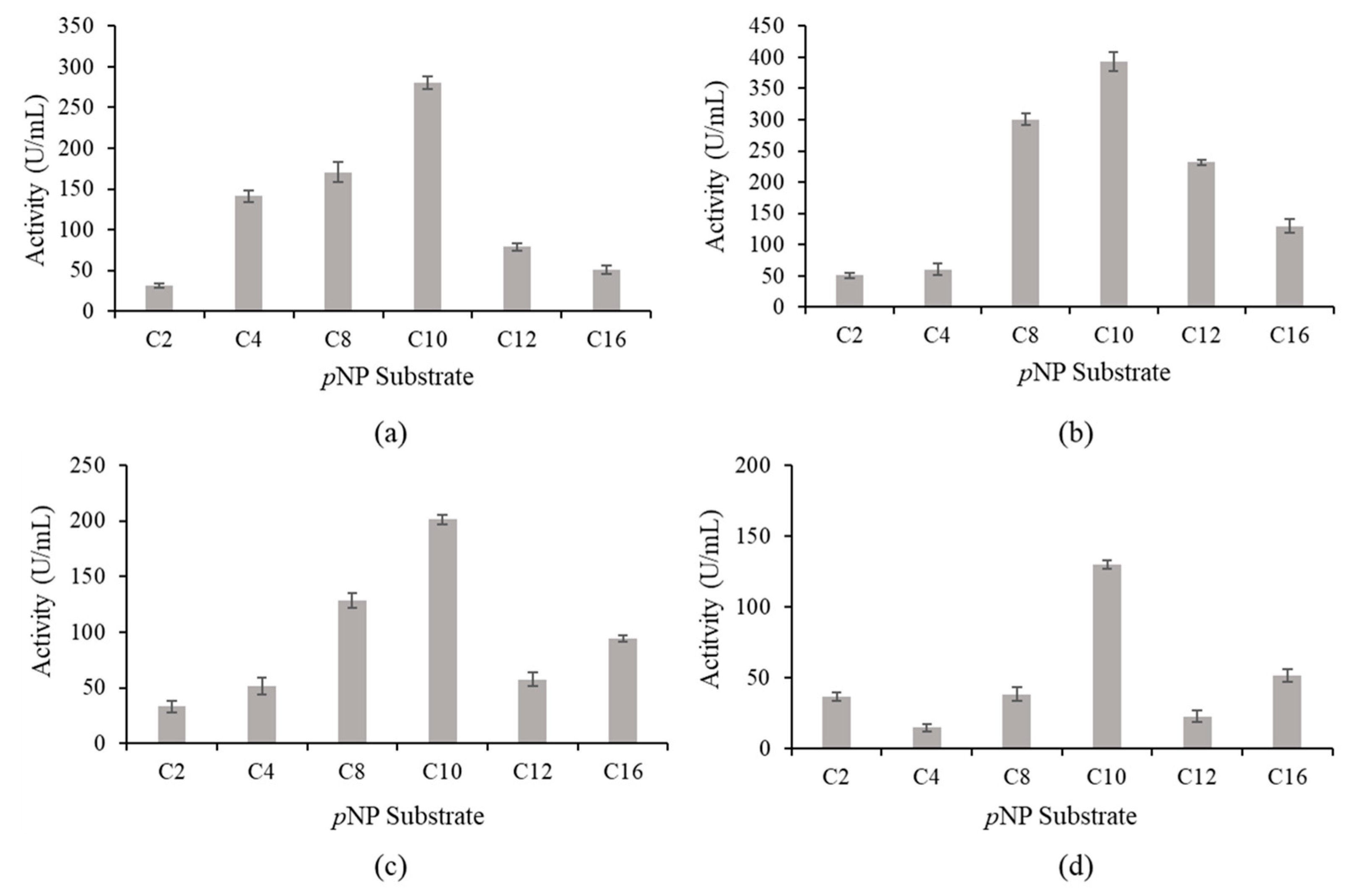
2.5. Substrate Specificity and Kinetic Constants of wt-L2 and Mutant Lipases
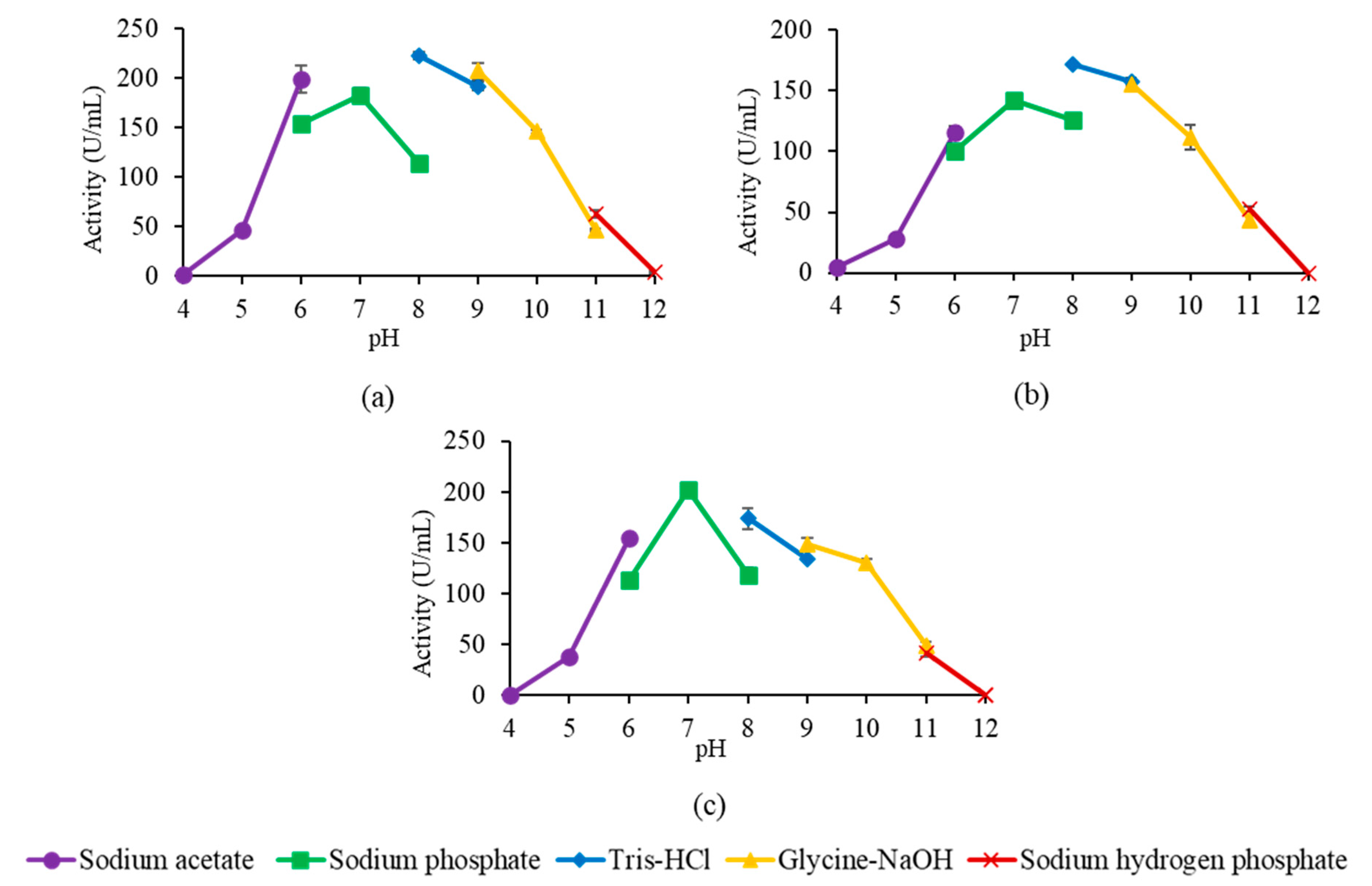
2.6. Optimum pH and pH Stability of Mutant Lipases
3. Discussion
4. Materials and Methods
4.1. Rational Design for N-Terminal Mutant Lipases
4.2. Remodeling of wt-L2, Homology Modelling and Molecular Dynamics Simulation of Mutant Lipases
4.3. Construction of Mutant Lipases
4.4. Expression and Purification of wt-L2 and Mutant Lipases
4.5. Characterisation of wt-L2 and Mutant Lipases
4.5.1. Effects of Temperature on wt-L2 and Mutant Lipases
4.5.2. Substrate Specificity of wt-L2 and Mutant Lipases
4.5.3. Kinetic Constants of wt-L2 and Mutant Lipases
4.5.4. Optimum pH and pH Stability of Mutant Lipases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sangeetha, R.; Arulpandi, I.; Geetha, A. Bacterial Lipases as Potential Industrial Biocatalysts: An Overview. Res. J. Microbiol. 2011, 6, 1–24. [Google Scholar] [CrossRef]
- Harwood, J.L. The versatility of lipases for industrial uses. Trends Biochem. Sci. 1989, 14, 125–126. [Google Scholar] [CrossRef]
- Jaeger, K. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Verma, N.; Thakur, S.; Bhatt, A.K. Microbial lipases: Industrial applications and properties (a review). Int. Res. J. Biol. Sci. 2012, 1, 88–92. [Google Scholar]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.H.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Boil. 2018, 132, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Z.; Huang, H.; Zhang, H.; Yao, B.; Xiong, H.; Turunen, O. Improved thermal performance of Thermomyces laniginosus GH11 xylanase by engineering of an N-terminal disulfide bridge. Bioresour. Technol. 2012, 112, 275–279. [Google Scholar] [CrossRef]
- Kumar, V.; Marín-Navarro, J.; Shukla, P. Thermostable microbial xylanases for pulp and paper industries: Trends, applications and further perspectives. World J. Microbiol. Biotechnol. 2016, 32, 34. [Google Scholar] [CrossRef]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Review: Engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018, 2, 011501. [Google Scholar] [CrossRef]
- Ruslan, R.; Rahman, R.N.Z.R.A.; Leow, T.C.; Ali, M.S.M.; Basri, M.; Salleh, A.B. Improvement of Thermal Stability via Outer-Loop Ion Pair Interaction of Mutated T1 Lipase from Geobacillus zalihae Strain T1. Int. J. Mol. Sci. 2012, 13, 943–960. [Google Scholar] [CrossRef]
- Goomber, S.; Kumar, R.; Singh, R.; Mishra, N.; Kaur, J. Point mutation Gln121-Arg increased temperature optima of Bacillus lipase (1.4 subfamily) by fifteen degrees. Int. J. Boil. Macromol. 2016, 88, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Veno, J.; Rahman, R.N.Z.R.A.; Masomian, M.; Ali, M.S.M.; Leow, T.C. Insight into Improved Thermostability of Cold-Adapted Staphylococcal Lipase by Glycine to Cysteine Mutation. Molecules 2019, 24, 3169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tsai, C.-J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng. 2000, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Leelavathi, S.; Mazumdar-Leighton, S.; Ghosh, A.; Ramakumar, S.; Reddy, V.S. The Critical Role of N- and C-Terminal Contact in Protein Stability and Folding of a Family 10 Xylanase under Extreme Conditions. PLoS ONE 2010, 5, e11347. [Google Scholar] [CrossRef]
- Shih, T.-W.; Pan, T.-M. Substitution of Asp189 residue alters the activity and thermostability of Geobacillus sp. NTU 03 lipase. Biotechnol. Lett. 2011, 33, 1841–1846. [Google Scholar] [CrossRef]
- Le, Q.A.T.; Joo, J.C.; Yoo, Y.J.; Kim, Y.H. Development of thermostable Candida antarctica lipase B through novel in silico design of disulfide bridge. Biotechnol. Bioeng. 2011, 109, 867–876. [Google Scholar] [CrossRef]
- Han, Z.-L.; Han, S.-Y.; Zheng, S.-P.; Lin, Y. Enhancing thermostability of a Rhizomucor miehei lipase by engineering a disulfide bond and displaying on the yeast cell surface. Appl. Microbiol. Biotechnol. 2009, 85, 117–126. [Google Scholar] [CrossRef]
- Lu, J.; Deng, L.; Nie, K.; Wang, F.; Tan, T. Stability of immobilized Candida sp. 99–125 lipase for biodiesel production. Chem. Eng. Technol. 2012, 25, 2120–2124. [Google Scholar] [CrossRef]
- Gao, C.; Lan, D.; Liu, L.; Zhang, H.; Yang, B.; Wang, Y. Site-directed mutagenesis studies of the aromatic residues at the active site of a lipase from Malassezia globosa. Biochimie 2014, 102, 29–36. [Google Scholar] [CrossRef]
- Lehmann, M.; Wyss, M. Engineering proteins for thermostability: The use of sequence alignments versus rational design and directed evolution. Curr. Opin. Biotechnol. 2001, 12, 371–375. [Google Scholar] [CrossRef]
- Godoy, C.A.; Klett, J.; Di Geronimo, B.; Hermoso, J.A.; Guisan, J.M.; Carrasco-López, C. Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2. Int. J. Mol. Sci. 2019, 20, 5245. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, G.; Zhang, Z.; Wang, S.; Chen, H. Terminal Amino Acids Disturb Xylanase Thermostability and Activity*. J. Boil. Chem. 2011, 286, 44710–44715. [Google Scholar] [CrossRef] [PubMed]
- Shariff, F.M.; Zaliha, R.N.; Basri, M.; Salleh, A.B. A Newly Isolated Thermostable Lipase from Bacillus sp. Int. J. Mol. Sci. 2011, 12, 2917–2934. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.N.Z.R.A.; Shariff, F.M.; Basri, M.; Salleh, A.B. 3D Structure Elucidation of Thermostable L2 Lipase from Thermophilic Bacillus sp. L2. Int. J. Mol. Sci. 2012, 13, 9207–9217. [Google Scholar] [CrossRef]
- Anobom, C.D.; Pinheiro, A.S.; De-Andrade, R.A.; Aguieiras, É.C.; Andrade, G.; Moura, M.V.; Almeida, R.V.; Freire, D.M. From Structure to Catalysis: Recent Developments in the Biotechnological Applications of Lipases. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 1271. [Google Scholar] [CrossRef]
- Sinchaikul, S.; Sookkheo, B.; Phutrakul, S.; Wu, Y.-T.; Pan, F.-M.; Chen, S.-T. Structural Modeling and Characterization of a Thermostable Lipase from Bacillus stearothermophilus P1. Biochem. Biophys. Res. Commun. 2001, 283, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-T.; Kim, H.-K.; Kim, S.-J.; Chi, S.-W.; Pan, J.-G.; Oh, T.-K.; Ryu, S.-E. Novel Zinc-binding Center and a Temperature Switch in the Bacillus stearothermophilus L1 Lipase. J. Boil. Chem. 2002, 277, 17041–17047. [Google Scholar] [CrossRef]
- Aris, S.N.A.M.; Chor, A.L.T.; Ali, M.S.M.; Basri, M.; Salleh, A.B.; Rahman, R.N.Z.R.A. Crystallographic Analysis of Ground and Space Thermostable T1 Lipase Crystal Obtained via Counter Diffusion Method Approach. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Zha, D.; Zhang, H.; Zhang, H.; Xu, L.; Yan, Y. N-terminal transmembrane domain of lipase LipA from Pseudomonas protegens Pf-5: A must for its efficient folding into an active conformation. Biochim. 2014, 105, 165–171. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sepehrizadeh, Z.; Ebrahim-Habibi, A.; Shahverdi, A.R.; Faramarzi, M.A.; Setayesh, N. Bacterial expression and characterization of an active recombinant lipase A from Serratia marcescens with truncated C-terminal region. J. Mol. Catal. B: Enzym. 2015, 120, 84–92. [Google Scholar] [CrossRef]
- Tyndall, J.D.A.; Sinchaikul, S.; Fothergill-Gilmore, L.A.; Taylor, P.; Walkinshaw, M.D. Crystal structure of a thermostable lipase from Bacillus stearothermophilus P1. J. Mol. Boil. 2002, 323, 859–869. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Godoy, C.; Rivas, B.D.L.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R.; Martinez-Ripoll, M.; Hermoso, J.A. Activation of Bacterial Thermoalkalophilic Lipases Is Spurred by Dramatic Structural Rearrangements. J. Boil. Chem. 2008, 284, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Sani, H.A.; Shariff, F.M.; Rahman, R.N.Z.R.A.; Leow, T.C.; Salleh, A.B. The Effects of One Amino Acid Substitutions at the C-Terminal Region of Thermostable L2 Lipase by Computational and Experimental Approach. Mol. Biotechnol. 2017, 60, 1–11. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, J.G. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Boil. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Q.; Jiang, S.; Zhang, G.; Ma, Y. Truncation of the unique N-terminal domain improved the thermos-stability and specific activity of alkaline α-amylase Amy703. Sci. Rep. 2016, 6, 22465. [Google Scholar] [CrossRef]
- Choi, W.-C.; Kim, M.H.; Ro, H.-S.; Ryu, S.R.; Oh, T.-K.; Lee, J.-K. Zinc in lipase L1 from Geobacillus stearothermophilus L1 and structural implications on thermal stability. FEBS Lett. 2005, 579, 3461–3466. [Google Scholar] [CrossRef]
- Yu, H.; Yan, Y.; Zhang, C.; Dalby, P.A. Two strategies to engineer flexible loops for improved enzyme thermostability. Sci. Rep. 2017, 7, 41212. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Nie, J.; McNeil, B.; Jeffrey, L.; Yang, Y.; Bai, Z. Protein engineering of Bacillus acidopullulyticus pullulanase for enhanced thermostability using in silico data driven rational design methods. Enzym. Microb. Technol. 2015, 78, 74–83. [Google Scholar] [CrossRef]
- Li, H.; Robertson, A.D.; Jensen, J.H. Very fast empirical prediction and rationalization of protein pKa values. Proteins: Struct. Funct. Bioinform. 2005, 61, 704–721. [Google Scholar] [CrossRef]
- Tan, K.P.; Nguyen, T.B.; Patel, S.; Varadarajan, R.; Madhusudhan, M.S. Depth: A web server to compute depth, cavity sizes, detect potential small-molecule ligand-binding cavities and predict the pKa of ionizable residues in proteins. Nucleic Acids Res. 2013, 41, W314–W321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Huang, L.; Gui, S.; Zheng, N.; Jia, L.; Fu, Y.; Lu, F. Improvement in thermostability of an alkaline lipase I from Penicillium cyclopium by directed evolution. RSC Adv. 2017, 7, 38538–38548. [Google Scholar] [CrossRef]
- Miller, S.R. An appraisal of the enzyme stability-activity trade-off. Evolution 2017, 71, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef]
- Tina, K.G.; Bhadra, R.; Srinivasan, N. PIC: Protein Interactions Calculator. Nucleic Acids Res. 2007, 35, W473–W476. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Rhee, J.S. A simple and rapid colorimetric method for determination of free fatty acids for lipase assay. J. Am. Oil Chem. Soc. 1986, 63, 89–92. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Position | No. of Potential Residues | No. of Interaction | Total Interactions | ||
|---|---|---|---|---|---|
| Hydrophobic | Main chain–Main Chain | Main Chain–Side Chain | |||
| Ala1 | 3 | 0 | 0 | 0 | 0 |
| Ser2 | 14 | 0 | 0 | 0 | 0 |
| Ala5 | 6 | 0 | 1 | 1 | 2 |
| Asn6 | 9 | 0 | 1 | 1 | 2 |
| Asp7 | 6 | 0 | 0 | 0 | 0 |
| →Ala8 | 5 | 2 | 1 | 3 | 6 |
| His14 | 7 | 0 | 0 | 2 | 2 |
| Gly15 | 1 | 0 | 1 | 2 | 3 |
| Thr17 | 6 | 0 | 1 | 1 | 2 |
| Gly18 | 11 | 0 | 0 | 1 | 1 |
| Gly20 | 11 | 0 | 0 | 1 | 1 |
| Substituent | Stability Change | RI Score |
|---|---|---|
| →Val | Increase | 5 |
| Leu | Decrease | 3 |
| Ile | Increase | 2 |
| Met | Increase | 1 |
| Phe | Decrease | 3 |
| Trp | Decrease | 4 |
| Tyr | Decrease | 4 |
| Gly | Decrease | 5 |
| →Pro | Increase | 6 |
| Ser | Decrease | 7 |
| Thr | Decrease | 7 |
| Cys | Increase | 1 |
| His | Decrease | 5 |
| Arg | Decrease | 1 |
| Lys | Decrease | 6 |
| Gln | Decrease | 6 |
| →Glu | Increase | 3 |
| Asn | Decrease | 4 |
| Asp | Decrease | 1 |
| Interaction | Total Interactions | ||
|---|---|---|---|
| A8V | A8P | A8E | |
| Hydrogen Bonds | 5 | 5 | 8 |
| Hydrophobic Interaction | 18 | 23 | 25 |
| Lipase | Vmax (µmol/min/mL) | KM (mM) | kcat (min−1) | Catalytic Efficiency (s−1/mM) |
|---|---|---|---|---|
| wt-L2 | 105.26 | 1.08 | 10,526 | 162.43 |
| A8V | 81.30 | 0.52 | 8,130 | 206.57 |
| A8P | 76.33 | 1.34 | 7,633 | 94.93 |
| A8E | 71.91 | 4.38 | 7,194 | 27.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhari, N.; Leow, A.T.C.; Abd Rahman, R.N.Z.R.; Mohd Shariff, F. Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase. Molecules 2020, 25, 3433. https://doi.org/10.3390/molecules25153433
Bukhari N, Leow ATC, Abd Rahman RNZR, Mohd Shariff F. Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase. Molecules. 2020; 25(15):3433. https://doi.org/10.3390/molecules25153433
Chicago/Turabian StyleBukhari, Noramirah, Adam Thean Chor Leow, Raja Noor Zaliha Raja Abd Rahman, and Fairolniza Mohd Shariff. 2020. "Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase" Molecules 25, no. 15: 3433. https://doi.org/10.3390/molecules25153433
APA StyleBukhari, N., Leow, A. T. C., Abd Rahman, R. N. Z. R., & Mohd Shariff, F. (2020). Single Residue Substitution at N-Terminal Affects Temperature Stability and Activity of L2 Lipase. Molecules, 25(15), 3433. https://doi.org/10.3390/molecules25153433