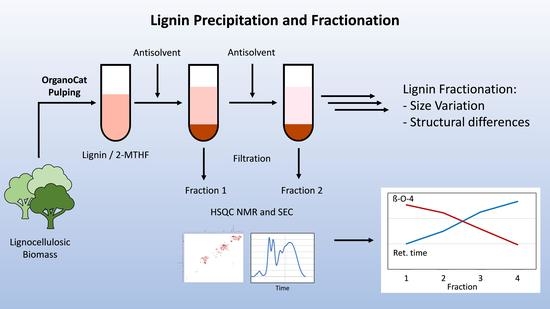
Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition
Abstract
1. Introduction
2. Results and Discussion
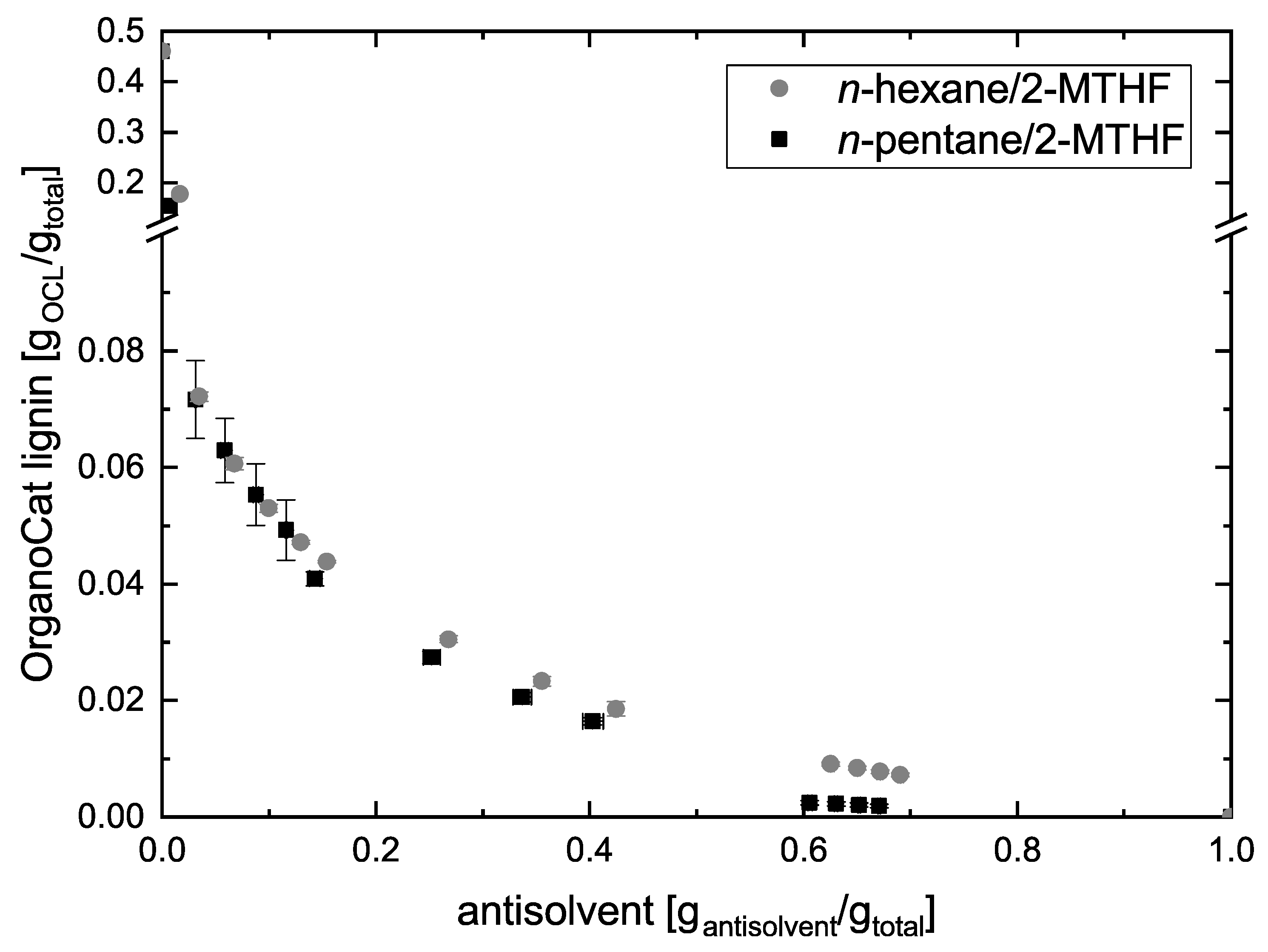
2.1. Solubility of Lignin and Furfural in n-Hexane/2-MTHF and n-Pentane/2-MTHF
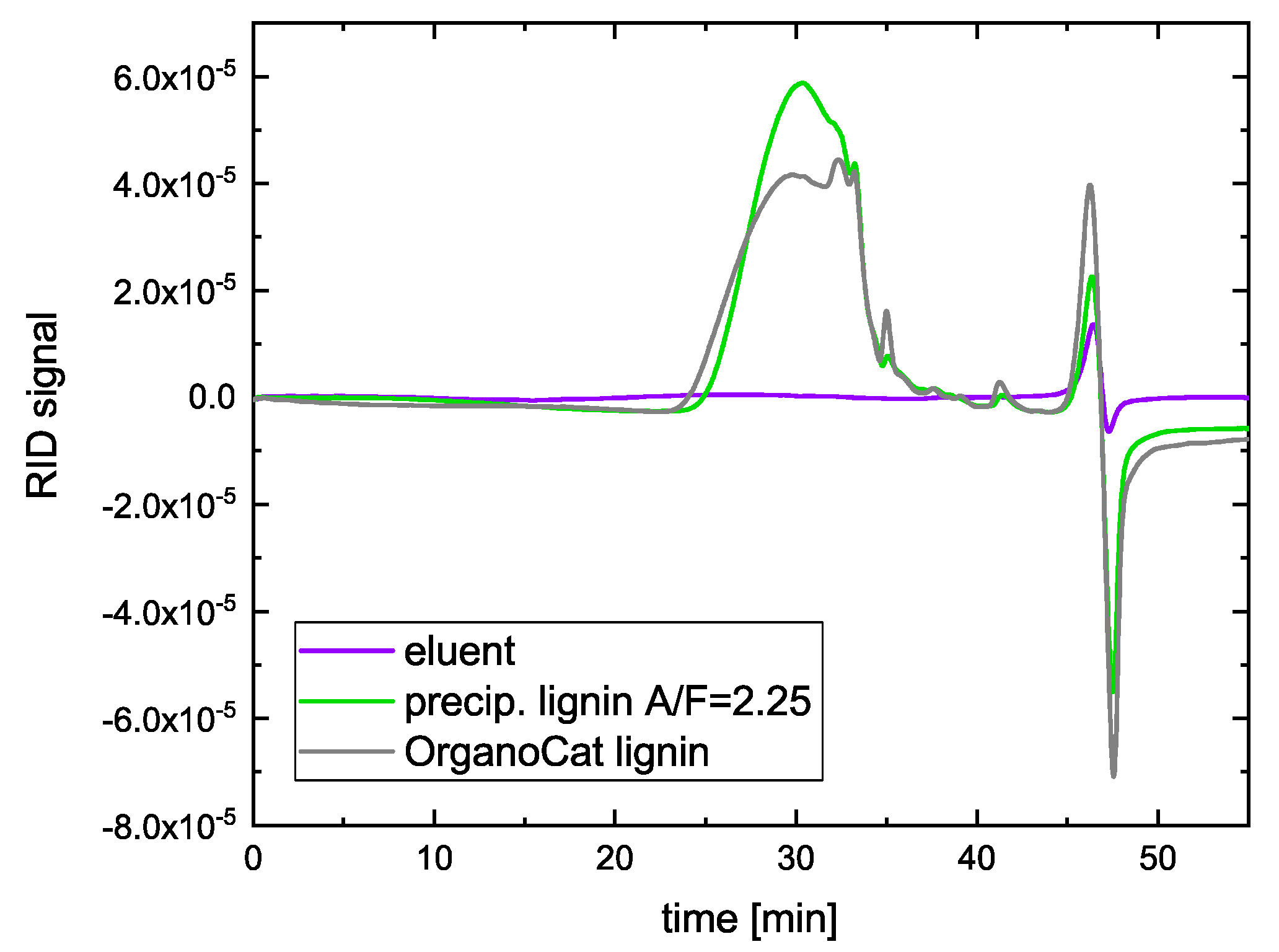
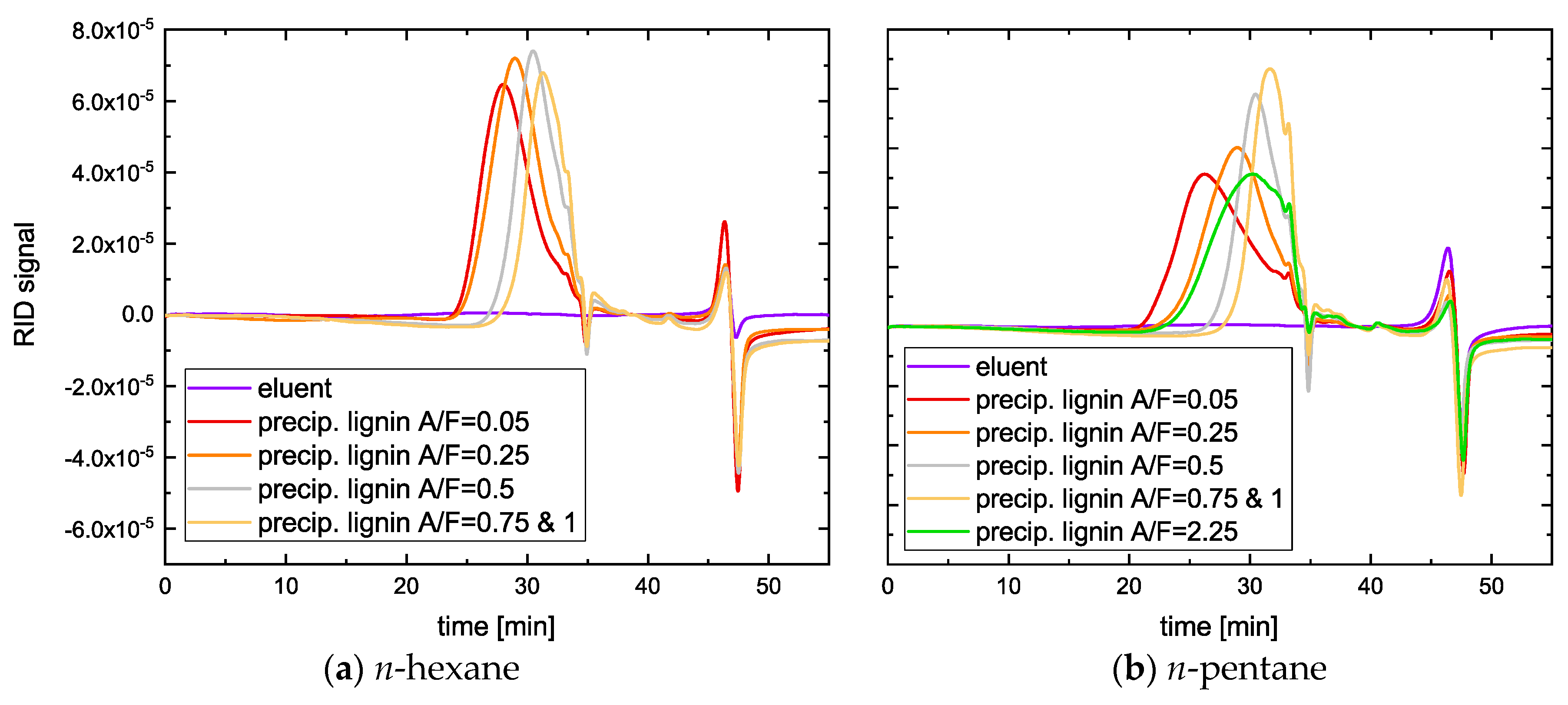
2.2. Size Distribution of Lignin Fractions
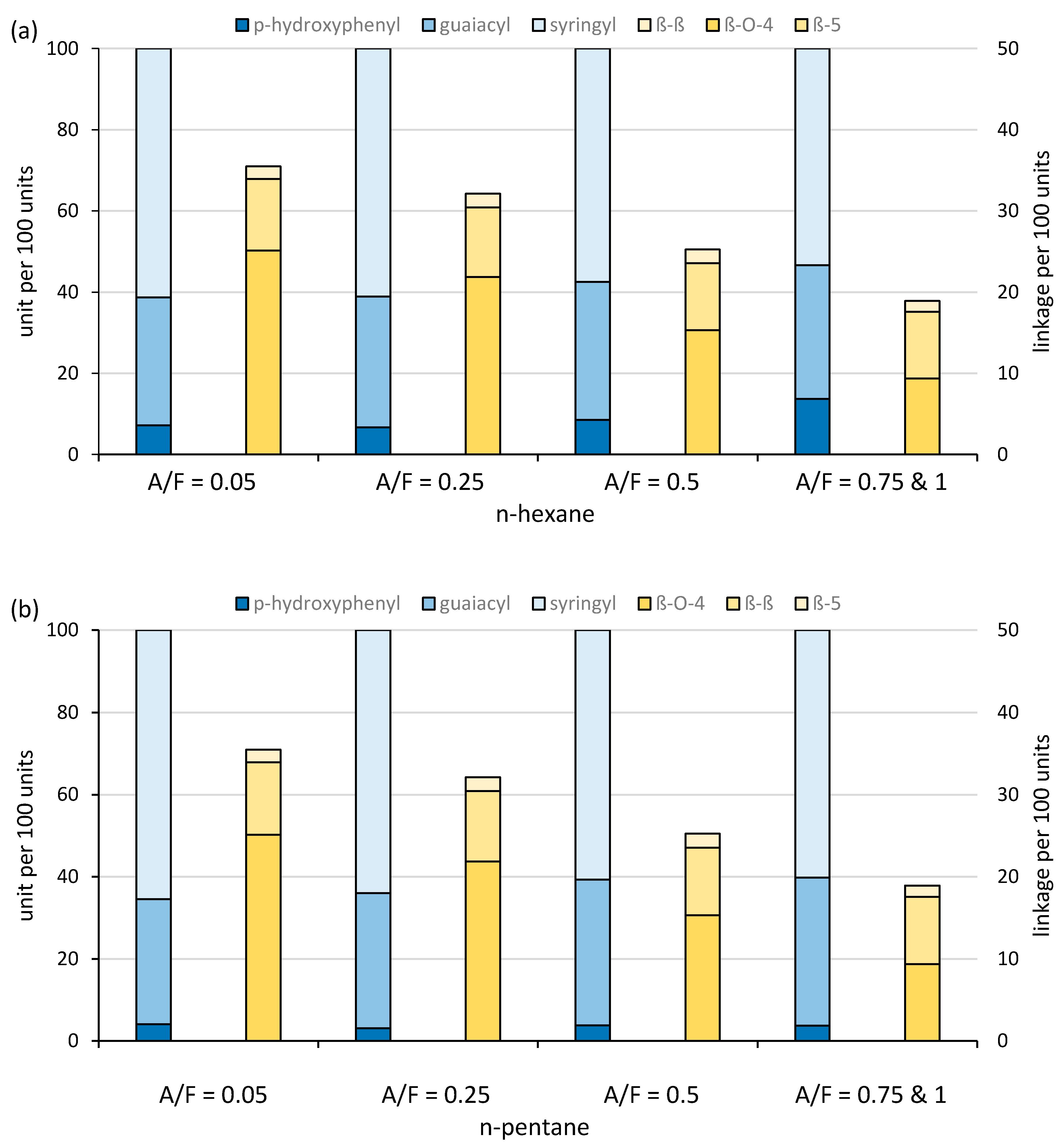
2.3. Chemical Composition of Lignin Fractions
3. Materials and Methods
3.1. Materials
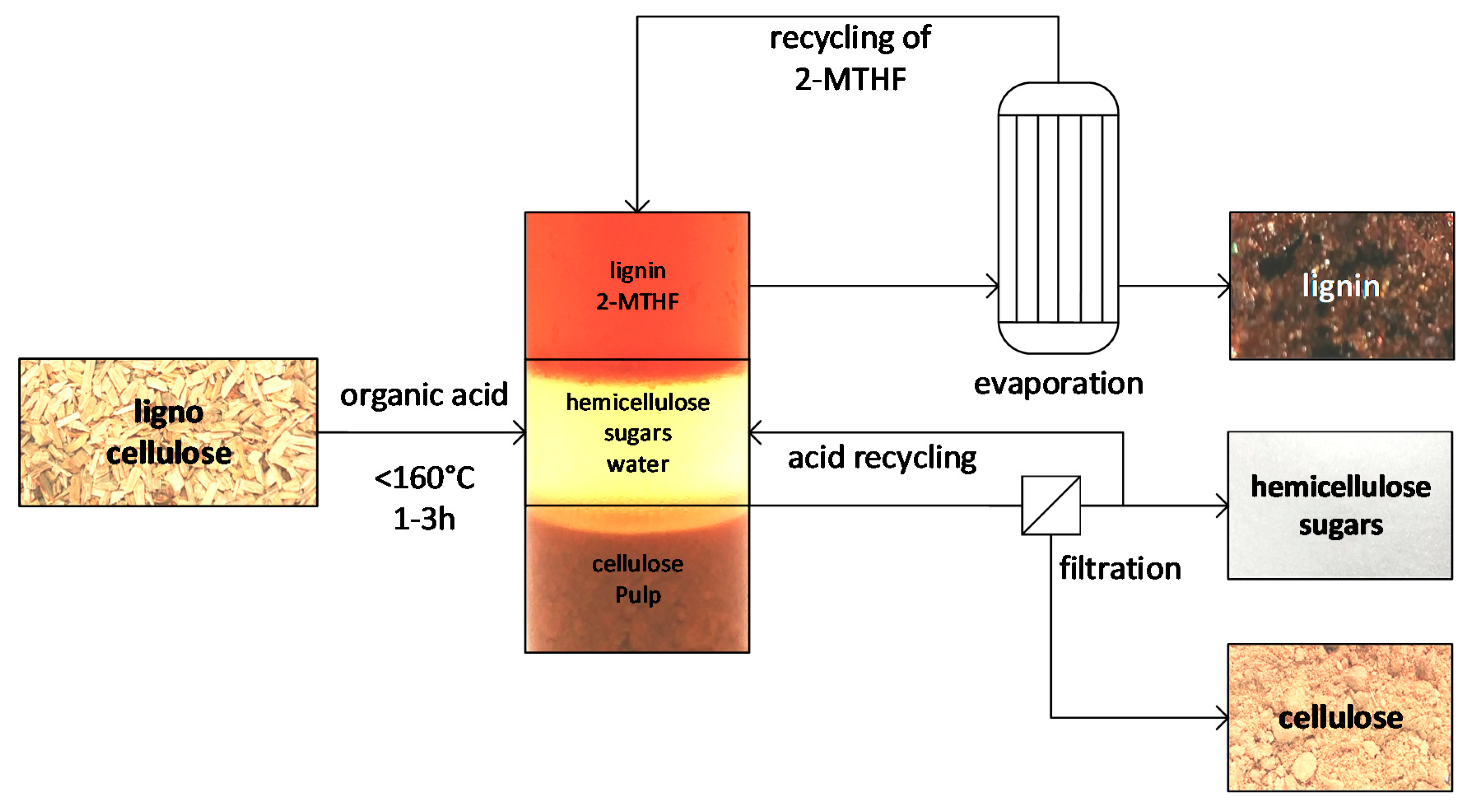
3.2. Procedure for OrganoCat Pulping
3.3. Procedure for Lignin Fractionation
3.4. Lignin Analysis (NMR spectroscopy)
3.5. Lignin Analysis (SEC)
3.6. Furfural Quantification (GC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lago, C.; Caldés, N.; Lechón, Y. The Role of Bioenergy in the Emerging Bioeconomy: Resources, Technologies, Sustainability and Policy; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128130575. [Google Scholar]
- Denny, K.S.; Kok, S.; Rex, T.L. Integrated Biorefineries; Elsevier: Amsterdam, The Netherland, 2017; ISBN 9780128047927. [Google Scholar]
- Laure, S.; Leschinsky, M.; Fröhling, M.; Schultmann, F.; Unkelbach, G. Assessment of an Organosolv Lignocellulose Biorefinery Concept Based on a Material Flow Analysis of a Pilot Plant. Cellul. Chem. Technol. 2014, 48, 793–798. [Google Scholar]
- vom Stein, T.; Grande, P.M.; Kayser, H.; Sibilla, F.; Leitner, W.; Domínguez de María, P. From biomass to feedstock: one-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system. Green Chem. 2011, 13, 1772–1777. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Jedvert, K.; Saltberg, A.; Theliander, H.; Wang, Y.; Henriksson, G.; Lindström, M.E. BIOREFINERY: Mild steam explosion: A way to activate wood for enzymatic treatment, chemical pulping and biorefinery processes. Nord. Pulp Pap. Res. J. 2012, 27, 828–835. [Google Scholar] [CrossRef]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Liquid Hot Water Pretreatment of Cellulosic Biomass. In Biofuels Methods and Protocol; Springer International Publishing: Cham, Switzerland, 2009; pp. 93–102. [Google Scholar]
- Weidener, D.; Klose, H.; Leitner, W.; Schurr, U.; Usadel, B.; Domínguez de María, P.; Grande, P.M. One-Step Lignocellulose Fractionation by using 2,5-Furandicarboxylic Acid as a Biogenic and Recyclable Catalyst. ChemSusChem 2018, 11, 2051–2056. [Google Scholar] [CrossRef]
- Grande, P.M.; Viell, J.; Theyssen, N.; Marquardt, W.; Domínguez de María, P.; Leitner, W. Fractionation of lignocellulosic biomass using the OrganoCat process. Green Chem. 2015, 17, 3533–3539. [Google Scholar] [CrossRef]
- Morone, A.; Pandey, R.A.; Chakrabarti, T. Evaluation of OrganoCat process as a pretreatment during bioconversion of rice straw. Ind. Crop. Prod. 2017, 99, 7–18. [Google Scholar] [CrossRef]
- Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Scott, M.; Deuss, P.J.; De Vries, J.G.; Prechtl, M.H.G.; Barta, K. New insights into the catalytic cleavage of the lignin β-O-4 linkage in multifunctional ionic liquid media. Catal. Sci. Technol. 2016, 6, 1882–1891. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Yoo, C.G.; Yang, X.; Lin, X.; Ralph, J.; Pan, X. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Wang, G.; Chen, H. Fractionation and characterization of lignin from steam-exploded corn stalk by sequential dissolution in ethanol-water solvent. Sep. Purif. Technol. 2013, 120, 402–409. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Jonas Ragauskas, A. Fractionation of Organosolv Lignin Using Acetone:Water and Properties of the Obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Holtz, A.; Weidener, D.; Leitner, W.; Klose, H.; Grande, P.M.; Jupke, A. Process development for separation of lignin from OrganoCat lignocellulose fractionation using antisolvent precipitation. Sep. Purif. Technol. 2020, 236, 116295. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Tachibana, Y.; Yamahata, M.; Kasuya, K.I. Synthesis and characterization of a renewable polyester containing oxabicyclic dicarboxylate derived from furfural. Green Chem. 2013, 15, 1318–1325. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Jääskeläinen, A.-S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crop. Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fiţigău, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterisation of isolated fractions. Ind. Crop. Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent screening for the fractionation of industrial kraft lignin. Holzforschung 2016, 70, 11–20. [Google Scholar] [CrossRef]
- Martineau, E.; Akoka, S.; Boisseau, R.; Delanoue, B.; Giraudeau, P. Fast quantitative 1H-13C two-dimensional NMR with very high precision. Anal. Chem. 2013, 85, 4777–4783. [Google Scholar] [CrossRef]
- Cheng, K.; Sorek, H.; Zimmermann, H.; Wemmer, D.E.; Pauly, M. Solution-state 2D NMR spectroscopy of plant cell walls enabled by a dimethylsulfoxide-d6/1-ethyl-3-methylimidazolium acetate solvent. Anal. Chem. 2013, 85, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.O.; Bicke, C.; Faix, O.; Tuszynski, W.; Angermann, R.; Karas, M.; Strupat, K. Matrix-Assisted Laser Desorption Mass Spectrometry of Lignins**. Angew. Chem. Int. Ed. Engl. 1992, 31, 762–764. [Google Scholar] [CrossRef]
- Grande, P.M.; Weidener, D.; Dietrich, S.; Dama, M.; Bellof, M.; Maas, R.; Pauly, M.; Leitner, W.; Klose, H.; Domínguez de María, P. OrganoCat Fractionation of Empty Fruit Bunches from Palm Trees into Lignin, Sugars, and Cellulose-Enriched Pulp. ACS Omega 2019, 4, 14451–14457. [Google Scholar] [CrossRef]
- Reichardt, C. Empirical Parameters of Solvent Polarity as Linear Free-Energy Relationships. Angew. Chem. Int. Ed. Engl. 1979, 18, 98–110. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidener, D.; Holtz, A.; Klose, H.; Jupke, A.; Leitner, W.; Grande, P.M. Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition. Molecules 2020, 25, 3330. https://doi.org/10.3390/molecules25153330
Weidener D, Holtz A, Klose H, Jupke A, Leitner W, Grande PM. Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition. Molecules. 2020; 25(15):3330. https://doi.org/10.3390/molecules25153330
Chicago/Turabian StyleWeidener, Dennis, Arne Holtz, Holger Klose, Andreas Jupke, Walter Leitner, and Philipp M. Grande. 2020. "Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition" Molecules 25, no. 15: 3330. https://doi.org/10.3390/molecules25153330
APA StyleWeidener, D., Holtz, A., Klose, H., Jupke, A., Leitner, W., & Grande, P. M. (2020). Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition. Molecules, 25(15), 3330. https://doi.org/10.3390/molecules25153330