Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Polyphenol and Total Flavonoid Content
2.2. LC-MS and GS-MS
2.3. Quantification of Marker and Metal Compounds
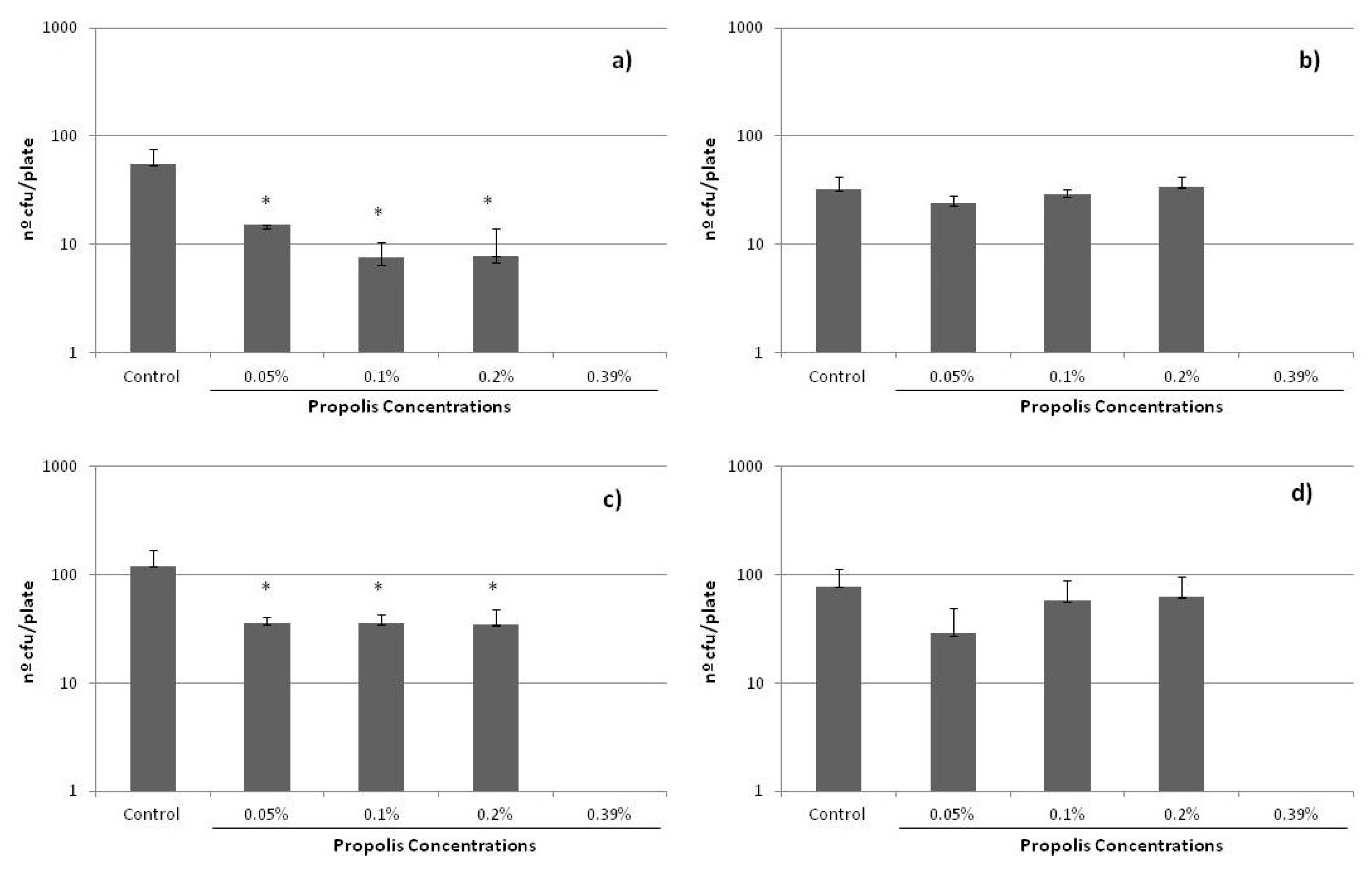
2.4. Antimicrobial Activity
3. Materials and Methods
3.1. Propolis Samples Preparation
3.2. Determination of Total Polyphenol and Flavonoid Contents
3.3. Liquid Chromatography Mass Spectrometry Analysis (LC–MS)
3.4. Gas Chromatography Mass Spectrometry Analysis (GC–MS)
3.5. Quantification of Marker Compounds
3.6. Detection of Metal Ions/Complexes in SPEE
3.7. Antibacterial Activity of Propolis
3.8. Statistical Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| Cfu | Colony formation unit |
| CLSI | Clinical and Laboratory Standard Institute |
| GAE | Gallic acid equivalents |
| GC-MS | Gas chromatography coupled to mass spectrometry |
| LC-MS | Liquid chromatographic mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MBC | Minimum bactericidal concentrations |
| MIC | Minimum inhibitory concentrations |
| p-HPEA-EA | p-HPEA-Elenolic acid mono-Aldehyde |
| QE | Quercetin equivalent |
| S. epidermidis | Staphylococcus epidermidis |
| SEEP | Spanish ethanolic extracts of propolis |
| TFC | Total flavonoid content |
| TPC | Total polyphenol content |
| TSB | Trypticase Soy Broth |
| WDXRF | Wavelength dispersive X ray fluorescence |
| 3,4-DHPEA-EDA | dialdehydic form of elenolic acid linked to hydroxytyrosol |
References
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids. 2017, 207, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramah, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi. J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- de Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography–tandem mass spectrometry. J. Pharmaceut. Biomed. 2007, 45, 390–399. [Google Scholar] [CrossRef]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Popova, M.P.; Graikou, K.; Chinou, I.; Bankova, V.S. GC-MS Profiling of Diterpene Compounds in Mediterranean Propolis from Greece. J. Agric. Food Chem. 2010, 58, 3167–3176. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In vitro Evaluation of the Potential Use of Propolis as a Multitarget Therapeutic Product: Physicochemical Properties, Chemical Composition, and Immunomodulatory, Antibacterial, and Anticancer Properties. BioMed. Res. Int. 2019, 4836378. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar]
- García-Viguera, C.; Greenaway, W.; Whatley, F.R. Composition of Propolis from Two Different Spanish Regions. Z. Nat. C 1992, 47, 634. [Google Scholar]
- Bankova, V.S.; Christov, R.; Tejera, A.D. Lignans and other constituents of propolis from the canary islands. Phytochemistry 1998, 49, 1411–1415. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Gutiérrez, A.L. Antioxidant Activity and Total Phenolics of Propolis from the Basque Country (Northeastern Spain). J. Am. Oil Chem. Soc. 2011, 88, 1387–1395. [Google Scholar] [CrossRef]
- Kumazawa, S.; Bonvehí, J.S.; Torres, C.; Mok-Ryeon, A.; Bermejo, F.J.O. Chemical and Functional Characterisation of Propolis Collected from East Andalusia (Southern Spain). Phytochem. Anal. 2013, 24, 608–615. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borras, M. Standardizing the analysis of phenolic profile in propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Gutierrez, A.L. The antimicrobial effects of propolis collected in different regions in the Basque Country (Northern Spain). World J. Microbiol. Biotechnol. 2012, 28, 1351–1358. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis-the ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Pantavou, K.; Lykoudis, S.; Nikolopoulou, M.; Tsiros, I.X. Thermal sensation and climate: A comparison of UTCI and PET thresholds in different climates. Int. J. Biometeorol. 2018, 62, 1695–1708. [Google Scholar] [CrossRef]
- Ambi, A.; Bryan, J.; Borbon, K.; Centeno, D.; Liu, T.; Chen, T.P.; Cattabiani, T.; Traba, C. Are Russian propolis ethanol extracts the future for the prevention of medical and biomedical implant contaminations? Phytomedicine 2017, 30, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Garcia, A.; Garcia, P.; Rios, J.J.; Garrido, A. Phenolic compounds in Spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef]
- Brenes, M.; Hidalgo, F.J.; García, A.; Rios, J.J.; García, P.; Zamora, R.; Garrido, A. Pinoresinol and 1-acetoxypinoresinol, two new phenolic compounds identified in olive oil. J. Am. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Fernandes, J.; Santos, V.; Silva, L.; Borges, F.; Rocha, S.; Belo, L.; Santos-Silva, A. Powerful protective role of 3,4-dihydroxyphenylethanol-elenolic acid dialdehyde against erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2010, 58, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Karaosmanoglu, H.; Soyer, F.; Ozen, B.; Tokatli, F. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J. Agric. Food Chem. 2010, 58, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Koo, M.H.; Abreu, J.A.; Ikegaki, M.; Cury, J.A.; Rosalen, P.L. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol. 1998, 36, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Chemical and biological properties of propolis from the western countries of the Mediterranean basin and Portugal. Int. J. Pharm. Pharm. Sci. 2013, 5, 403–409. [Google Scholar]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.-G. Chemical Composition of European Propolis: Expected and Unexpected Results. Z. Nat. C 2002, 57, 530. [Google Scholar] [CrossRef] [PubMed]
- Siseon, L.; Ajay, K.M.; Robert, J.M. Biological activities of lignin hydrolysate-related compounds. BMB Rep. 2012, 45, 265–274. [Google Scholar]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goni, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study. PLoS ONE 2017, 12, e0170077. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Shaik, Y.B.; Castellani, M.L.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; De Lutiis, M.A.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents. 2006, 20, 47–52. [Google Scholar] [PubMed]
- Isla, M.I.; Paredes-Guzman, J.F.; Nieva-Moreno, M.I.; Koo, H.; Park, Y.K. Some Chemical Composition and Biological Activity of Northern Argentine Propolis. J. Agric. Food Chem. 2005, 53, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Dusan, M.; Vesna, K. Investigation of metal–flavonoid chelates and the determination of flavonoids via metal–flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Oncag, O.; Cogulu, D.; Gencay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Boisard, S.; Le Ray, A.M.; Landreau, A.; Kempf, M.; Cassisa, V.; Flurin, C.; Richomme, P. Antifungal and antibacterial metabolites from a French poplar type propolis. Evid Based Complement. Alternat Med. 2015, 2015, 319240. [Google Scholar] [CrossRef]
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. R. Soc. Med. 1990, 83, 159–160. [Google Scholar] [CrossRef]
- Scazzocchio, F.; D’Auria, F.D.; Alessandrini, D.; Pantanella, F. Multifactorial aspects of antimicrobial activity of propolis. Microbiol. Res. 2006, 161, 327–333. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Švabić-Vlahović, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-haghi, A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. Iran. Biomed. J. 2018, 22, 50–65. [Google Scholar] [PubMed]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hatano, A.; Yoshino, M.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ahn, M.R.; Tazawa, S.; Araki, Y.; Kumazawa, S. Identification of the phenolic compounds contributing to antibacterial activity in ethanol extracts of Brazilian red propolis. Nat. Prod. Res. 2014, 28, 1293–1296. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wasik, T.J. In Vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis against Biofilm Forming Staphylococcus epidermidis Strains. Evid. Based Complementary Altern. Med. 2013, 2013, 590703. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Medica 2004, 70, 515–519. [Google Scholar] [CrossRef]
- Papachroni, D.; Graikou, K.; Kosalec, I.; Damianakos, H.; Ingram, V.; Chinou, I. Phytochemical Analysis and Biological Evaluation of Selected African Propolis Samples from Cameroon and Congo. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Campos, J.F.; Dos Santos, U.P.; da Rocha, P.S.; Damiao, M.J.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jatai). Evid Based Complement. Alternat Med. 2015, 2015, 296186. [Google Scholar] [CrossRef]
- Gonçalves, G.; Santos, N.; Srebernich, S. Antioxidant and antimicrobial activities of propolis and açai (Euterpe oleracea Mart) extracts. Rev. de Cienc. Farm. Basica e Apl. 2012, 32, 349–356. [Google Scholar]
- Carrillo, M.L.; Castillo, L.N.; Mauricio, R. Evaluación de la Actividad Antimicrobiana de Extractos de Propóleos de la Huasteca Potosina (México). Inf. Tecnológica 2011, 22, 21–28. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2017, 31, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Frozza, C.O.; Garcia, C.S.; Gambato, G.; de Souza, M.D.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Campos, J.F.; dos Santos, U.P.; Macorini, L.F.; de Melo, A.M.; Balestieri, J.B.; Paredes-Gamero, E.J.; Cardoso, C.A.; de Picoli Souza, K.; dos Santos, E.L. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem. Toxicol. 2014, 65, 374–380. [Google Scholar] [CrossRef]
- Alencar, S.M.; Oldoni, T.L.; Castro, M.L.; Cabral, I.S.; Costa-Neto, C.M.; Cury, J.A.; Rosalen, P.L.; Ikegaki, M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007, 113, 278–283. [Google Scholar] [CrossRef]
- Christensen, G.D.; Barker, L.P.; Mawhinney, T.P.; Baddour, L.M.; Simpson, W.A. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect. Immun. 1990, 58, 2906–2911. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard–Ninth Edition. CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
Sample Availability: Samples of SEEP are available from the authors. |
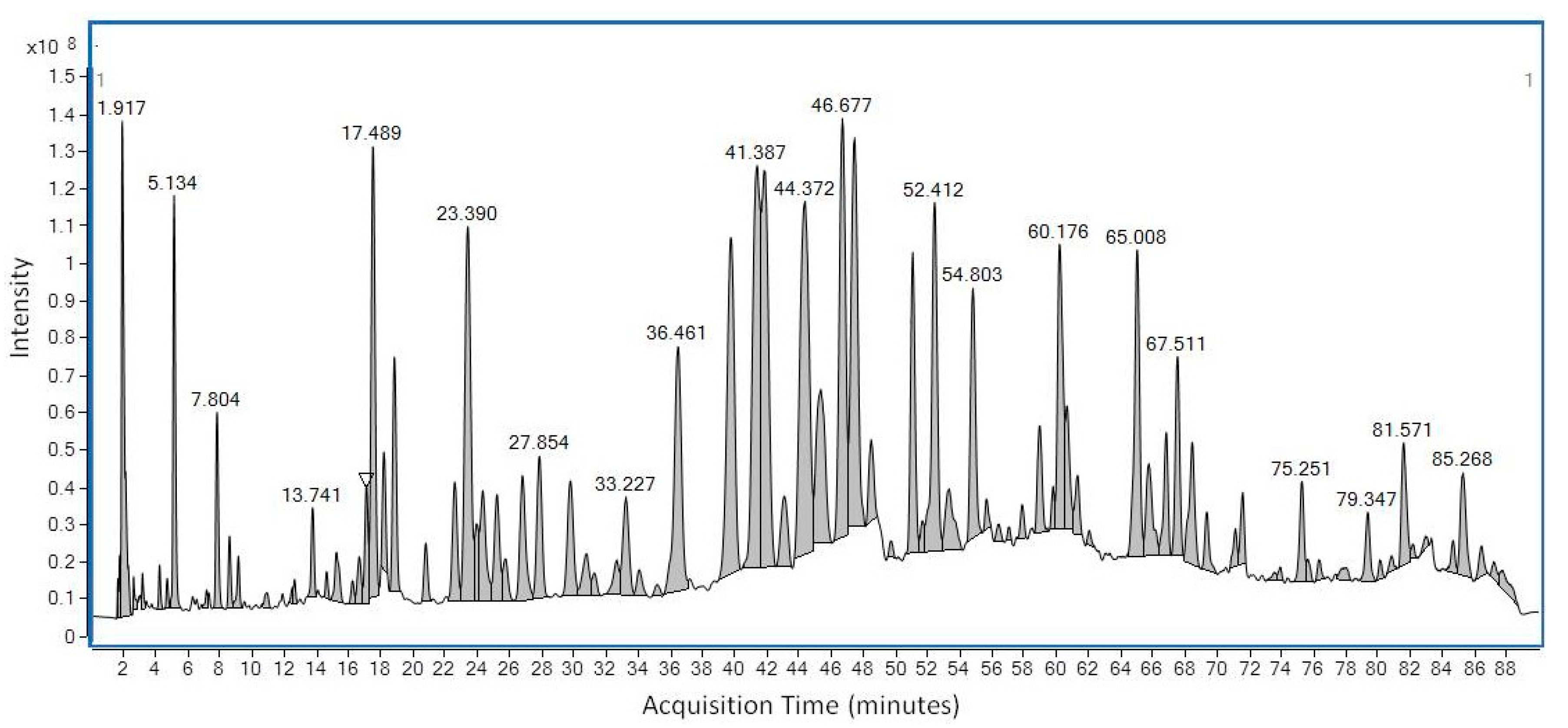
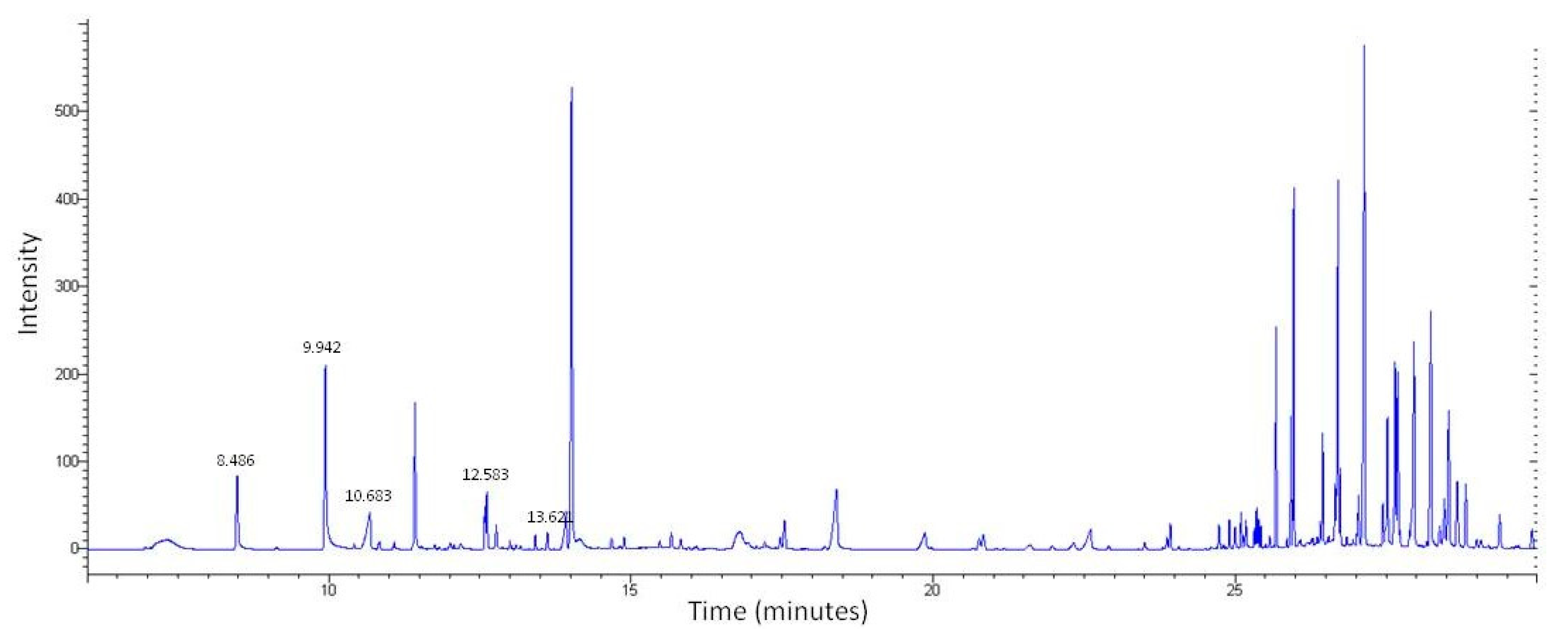
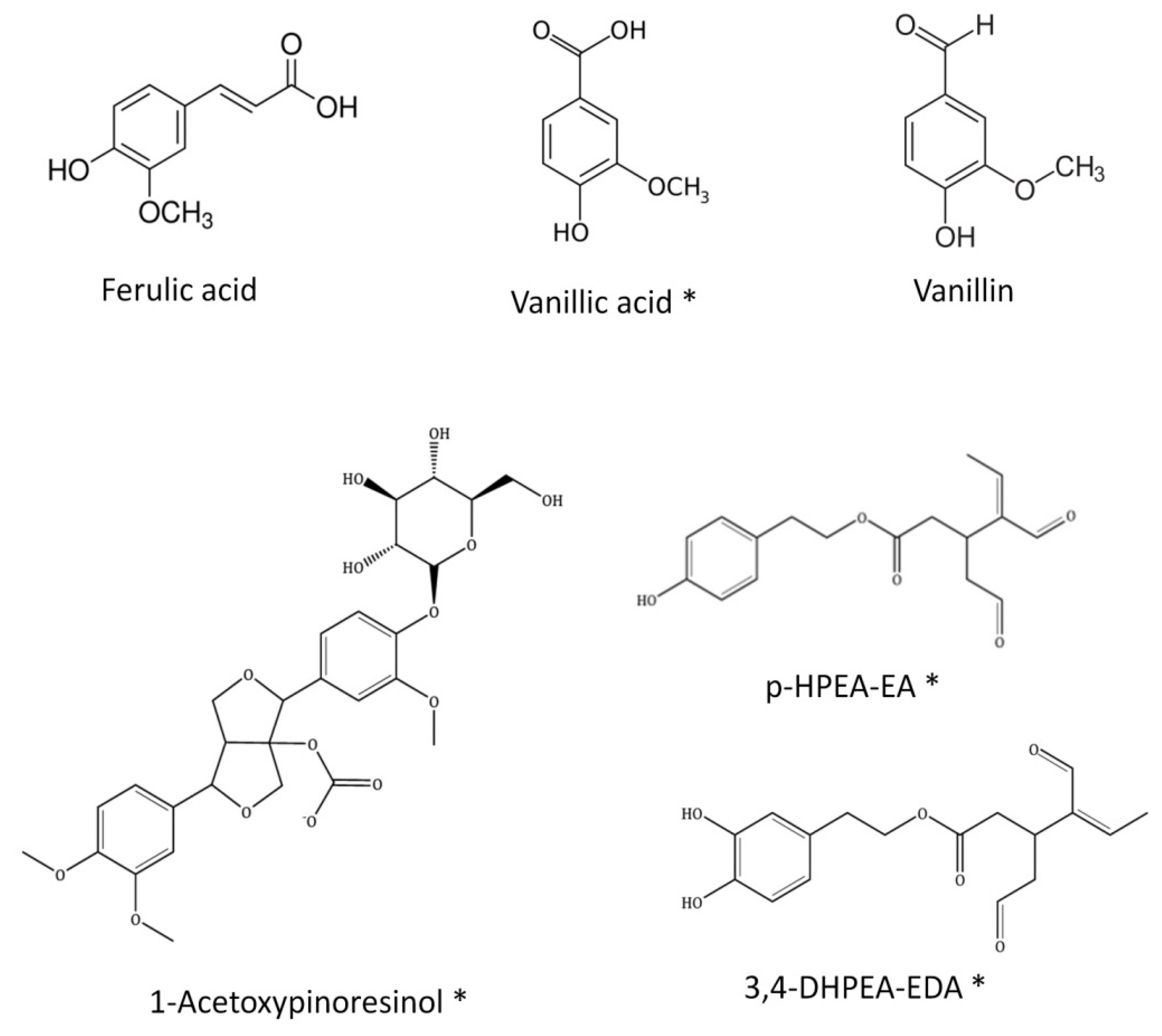
| Compounds Identified by LC-MS | ||||
|---|---|---|---|---|
| RT | Proposed Structure | Formula | Mw | m/z |
| Flavonoids | ||||
| 1.9 | 3-Methoxynobiletin | C22 H24 O9 | 432.142 | 432.143 |
| 2.0 | Nobiletin | C21 H22 O8 | 402.1315 | 402.132 |
| 2.0 | Quercetin-dimethyl ether-O-rutinoside | C29 H34 O16 | 638.1847 | 638.1877 |
| 2.2 | Quercetin-dimethyl ether-O-glucuronide | C22 H20 O13 | 492.0904 | 492.088 |
| 5.1 | Cirsimaritin | C17H14 O6 | 314.079 | 314.0781 |
| 4.86 | Epigallocatechin | C15 H14 O7 | 306.074 | 306.0754 |
| 6.4 | Quercetin 3-O-rhamnosylrhamnosyl-glucoside | C33 H40 O20 | 756.2113 | 756.2096 |
| 6.4 | Quercetin 3-O-rutinoside | C27 H30 O16 | 610.1534 | 610.1511 |
| 6.9 | Kaempferol-3-O-rutinoside | C27 H30 O15 | 594.1585 | 594.1559 |
| 7.0 | Isorhamnetin 3-O-glucoside7-O-rhamnoside | C28 H32 O16 | 624.169 | 624.1659 |
| 7.5 | Quercetin 4′-O-glucoside | C21 H20 O12 | 464.0955 | 464.0935 |
| 7.7 | Quercetin 3-O-glucuronide | C21 H18 O13 | 478.0747 | 478.0747 |
| 7.8 | Luteolin 7-O-glucuronide | C21 H18 O12 | 462.0798 | 462.0801 |
| 7.8 | Isorhamnetin-3-O-glucuronide | C21 H18 O12 | 462.0798 | 462.0775 |
| 9.1 | Dihydroquercetin | C15 H12 O7 | 304.0583 | 304.0569 |
| 9.5 | Quercetin-3-O-rhamnoside | C21 H20 O11 | 448.1006 | 448.0985 |
| 12.6 | Apigenin 6-C-glucoside | C21 H20 O10 | 432.1056 | 432.1039 |
| 13.7 | Naringenin | C15 H12 O5 | 272.0685 | 272.069 |
| 14.6 | Hispidulin | C16 H12 O6 | 300.0634 | 300.0629 |
| 15.1 | Daidzin | C21 H20 O9 | 416.1107 | 416.1089 |
| 15.2 | Quercetin-dimethyl ether | C17 H14 O7 | 330.074 | 330.0725 |
| 15.3 | Sakuranetin | C16 H14 O5 | 286.0841 | 286.0827 |
| 16.6 | Kaempferol | C15 H10 O6 | 286.0477 | 286.0481 |
| 18.2 | Chrysoeriol 7-O-glucoside | C22 H22 O11 | 462.1162 | 462.1139 |
| 20.8 | Formononetin | C16 H12 O4 | 268.0736 | 268.0723 |
| 24.7 | Hesperetin | C16 H14 O6 | 302.079 | 302.0776 |
| 29.8 | Eriodictyol | C15 H12 O6 | 288.0634 | 288.0621 |
| 33.2 | Rhamnetin | C16 H12 O7 | 316.0583 | 316.0588 |
| 33.2 | 7,3′,4′-Trihydroxyflavone | C15 H10 O5 | 270.0528 | 270.0534 |
| 41.4 | Chrysin | C15 H10 O4 | 254.0579 | 254.0568 |
| 53.2 | Caffeic acid phenylethyl ester (CAPE) | C17 H16 O4 | 284.1049 | 284.1035 |
| 56.4 | Arbutin | C12 H16 O7 | 272.0896 | 272.0904 |
| 58.2 | Chrysoeriol7-O-(6″-malonyl-apiosyl-glucoside) | C30 H32 O18 | 680.1589 | 680.1556 |
| Phenolic Acids | ||||
| 3.1 | Caffeic acid 4-Oglucoside | C15 H18 O9 | 342.0951 | 342.0937 |
| 3.2 | Hydroxycaffeic acid | C9 H8 O5 | 196.0372 | 196.0362 |
| 5.5 | p-Coumaroyl tartaric acid | C13H12O8 | 296.0532 | 296.0521 |
| 6.4 | Ferulic acid | C10 H10 O4 | 194.0579 | 194.0584 |
| 8.5 | Vanillic acid * | C8 H8 O4 | 168.0423 | 168.0422 |
| 12.6 | 5-8′-Dehydrodiferulic acid | C20 H18 O8 | 386.1002 | 386.1007 |
| 15.5 | Cinnamic acid | C9 H8 O2 | 148.0524 | 148.0518 |
| 19.0 | p-Coumaric acid methyl ester | C10 H10 O3 | 178.063 | 178.0622 |
| 19.1 | Hydroxyphenyl propionate | C9 H10 O3 | 166.063 | 166.0622 |
| 21.9 | p-Coumaric acid isoprenyl ester | C14 H14 O3 | 230.0943 | 230.0932 |
| 27.1 | p-Coumaric acid ethyl ester | C11 H12 O3 | 192.0786 | 192.0779 |
| 30.6 | Cinnamyliden acetic acid | C11 H10 O2 | 174.0681 | 174.0674 |
| 41.4 | p-Coumaroyl tyrosine | C18 H17 N O5 | 327.1107 | 327.1102 |
| 52.4 | Caffeic acid cinnamyl ester | C18 H16 O4 | 296.1049 | 296.1035 |
| 60.7 | Cinnamoyl glucose | C15 H18 O7 | 310.1053 | 310.1052 |
| Lignans | ||||
| 15.1 | Episesaminol | C20 H18 O7 | 370.1053 | 370.1035 |
| 18.2 | 1-Acetoxypinoresinol * | C22 H24 O8 | 416.1471 | 416.1452 |
| Others Polyphenols | ||||
| 4.2 | Sinapaldehyde | C11 H12 O4 | 208.0736 | 208.0726 |
| 7.0 | p-HPEA-EA * | C19 H22 O7 | 362.1366 | 362.1349 |
| 67.3 | Demethoxycurcumin | C20 H18 O5 | 338.1154 | 338.1138 |
| 7.8 | Coumarin | C9 H6 O2 | 146.0368 | 146.0363 |
| 34.5 | 3,4-DHPEA-EDA * | C17 H20 O6 | 320.126 | 320.1245 |
| Compounds Identified by GC-MS | ||||
| RT (min) | Match Result | Compound | ||
| 8.486 | 937 | Benzyl Alcohol | ||
| 9.942 | 947 | Phenylethyl Alcohol | ||
| 10.683 | 929 | Benzoic acid | ||
| 12.583 | 836 | Benzenepropanal | ||
| 13.621 | 938 | Vanillin | ||
| Compound | Range | Calibration Curve | R2 | Quantity (ppm) |
|---|---|---|---|---|
| Vanillic acid | 5–200 | y = 23263x − 58213 | 0.9991 | 5.2 |
| Trans-ferulic acid | 10–300 | y = 120462x + 627419 | 0.9974 | 250 |
| Quercetin | 10–200 | y = 196425x + 2 × 106 | 0.9817 | 23.5 |
| Formula | Z | Concentration | Line 1 | Calc. Concentration | Stat. Error |
|---|---|---|---|---|---|
| K2O | 19 | 135 ppm | K KA1-HR-Tr | 0.0134 | 4.38% |
| P2O5 | 15 | 44.8 ppm | P KA1-HR-Tr | 0.004 | 19.20% |
| ZnO | 30 | 10.9 ppm | Zn KA1-HR-Tr | 0.001 | 6.93% |
| CuO | 29 | 5.42 ppm | Cu KA1-HR-Tr | 0.001 | 14.60% |
| Staphylococcus epidermidis (n) | Propolis Origin | MIC/Range (μg/mL) | MBC/Range (μg/mL) | Methodology | References |
|---|---|---|---|---|---|
| 4 | Extremadura (Southwest of Spain) | 240 | 480 | MIC—Agar dilution MBC—Microdilution in broth and subcultured on agar | Present work |
| 2 | Poplar Type propolis (France) | >100 | - | Agar dilution | [48] |
| 1 | Lyon (France) | 3000 | - | Agar dilution | [49] |
| 1 | Germany, Ireland and Czech Republic | 600 | 1200 | MIC—Microdilution in broth MBC—Subcultured on blood agar | [56] |
| 63 | Italy | 620–2500 | - | Agar dilution | [50] |
| 11 | Poland | 780–1560 | - | Microdilution in broth | [57] |
| 2 | Serbia | 780–6300 | - | Agar dilution | [51] |
| 1 | Cretan propolis (Greece) | 50 | - | Microdilution in broth | [46] |
| 1 | Greek propolis (Northwest Greece) | 750 | - | Microdilution in broth | [58] |
| 1 | Anatolian propolis (Turkey) | 8–32 * | - | Macrodilution in broth | [47] |
| 1 | Cameroon and Congo propolis (Africa) | 10850–20000 * | - | Microdilution in broth | [59] |
| 2 | Brazil | 770–880 | 1750–1920 | MIC—Microdilution in broth MBC—TCC staining lecture and subcultured on NB agar | [60] |
| 1 | Brazil | 10700 | - | Macrodilution tube | [61] |
| 1 | Huasteca Potosina (México) | - | 1870–30000 | MBC—Macrodilution tube and subcultured on agar | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Calderón, M.C.; Navarro-Pérez, M.L.; Blanco-Roca, M.T.; Gómez-Navia, C.; Pérez-Giraldo, C.; Vadillo-Rodríguez, V. Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids. Molecules 2020, 25, 3318. https://doi.org/10.3390/molecules25153318
Fernández-Calderón MC, Navarro-Pérez ML, Blanco-Roca MT, Gómez-Navia C, Pérez-Giraldo C, Vadillo-Rodríguez V. Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids. Molecules. 2020; 25(15):3318. https://doi.org/10.3390/molecules25153318
Chicago/Turabian StyleFernández-Calderón, María Coronada, María Luisa Navarro-Pérez, María Teresa Blanco-Roca, Carolina Gómez-Navia, Ciro Pérez-Giraldo, and Virgina Vadillo-Rodríguez. 2020. "Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids" Molecules 25, no. 15: 3318. https://doi.org/10.3390/molecules25153318
APA StyleFernández-Calderón, M. C., Navarro-Pérez, M. L., Blanco-Roca, M. T., Gómez-Navia, C., Pérez-Giraldo, C., & Vadillo-Rodríguez, V. (2020). Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids. Molecules, 25(15), 3318. https://doi.org/10.3390/molecules25153318







