Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes
Abstract
1. Introduction
2. Results
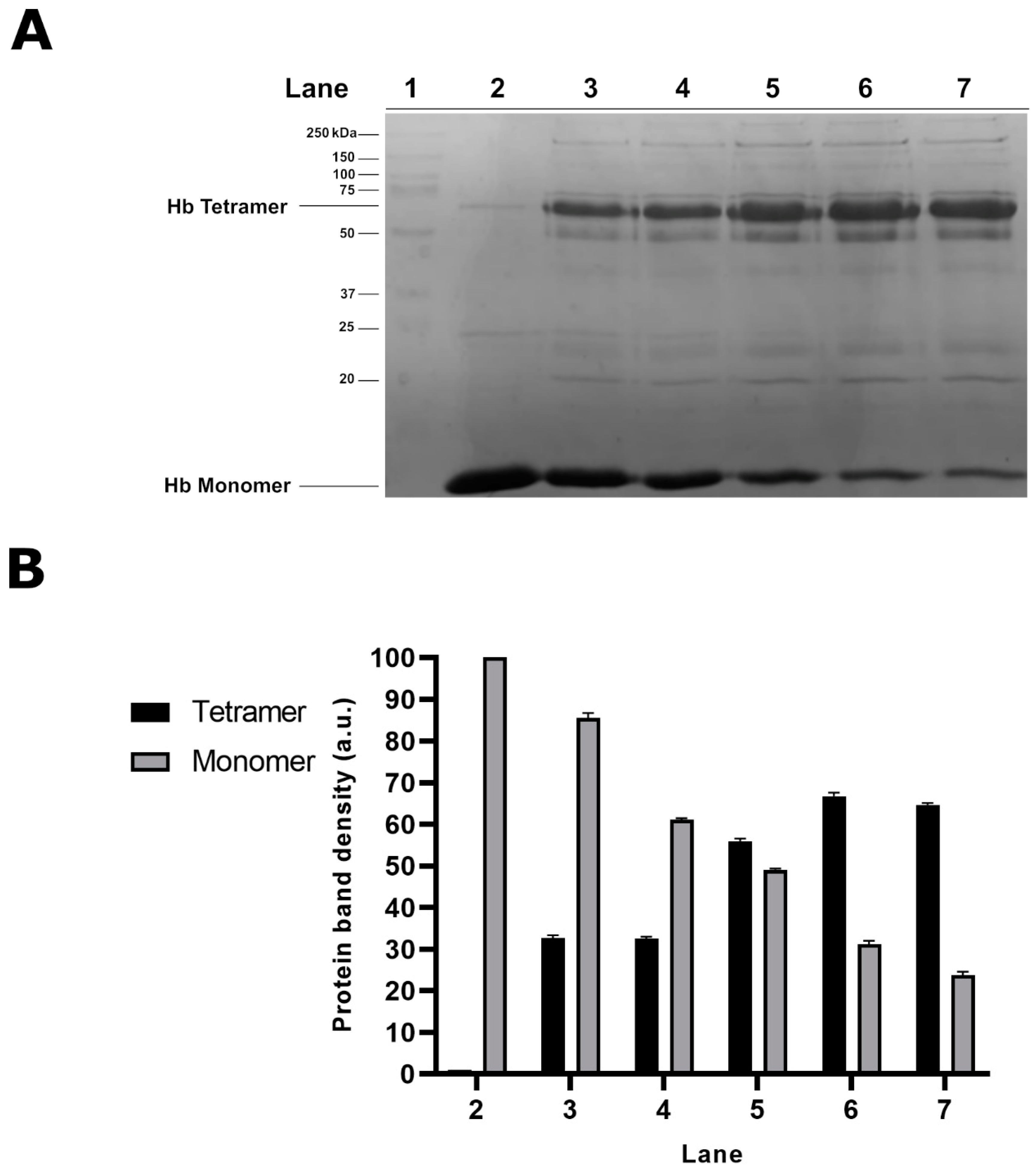
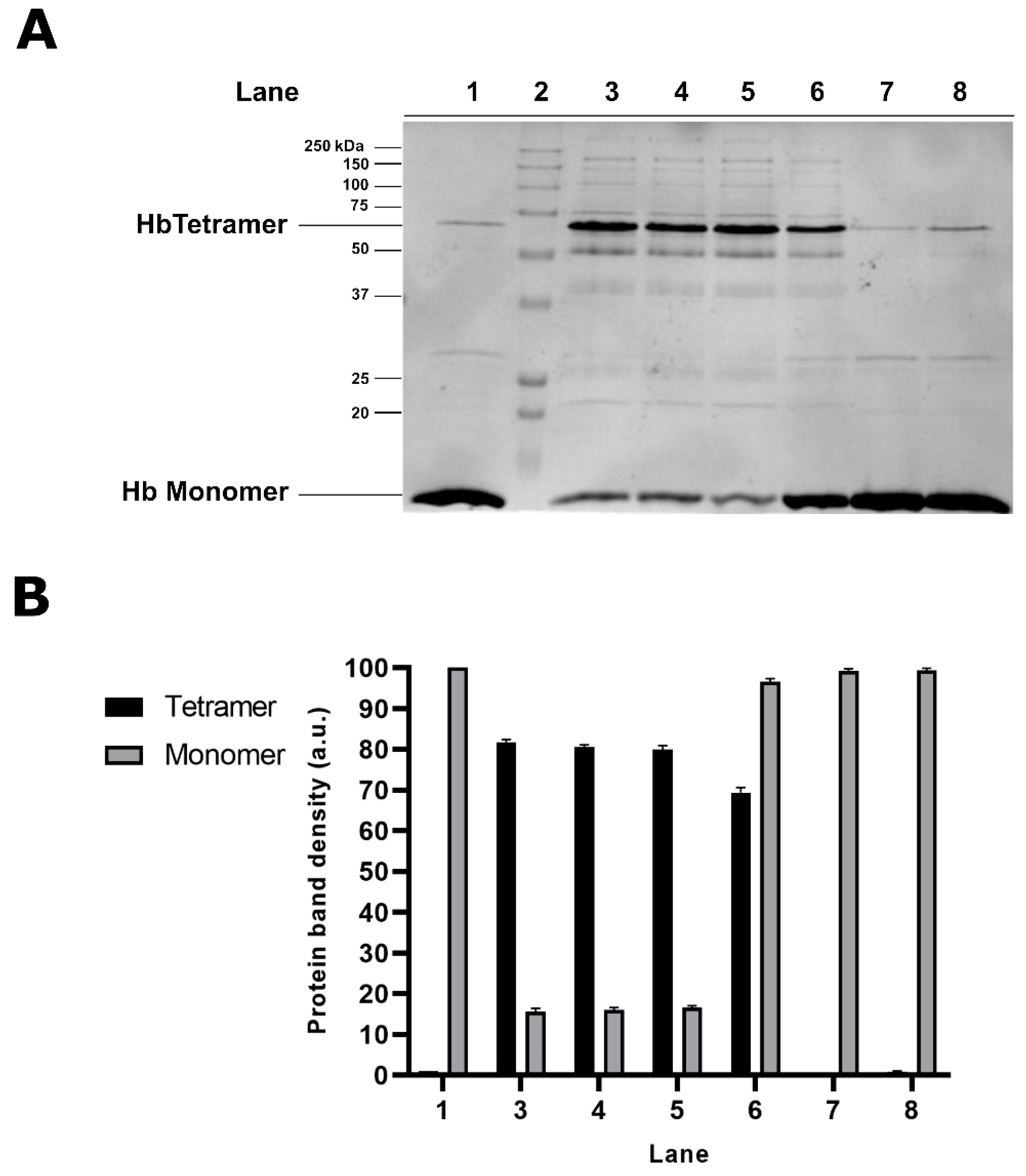
2.1. Electrophoretic Analyses of Cytosolic Proteins from Hg-Exposed Human RBC
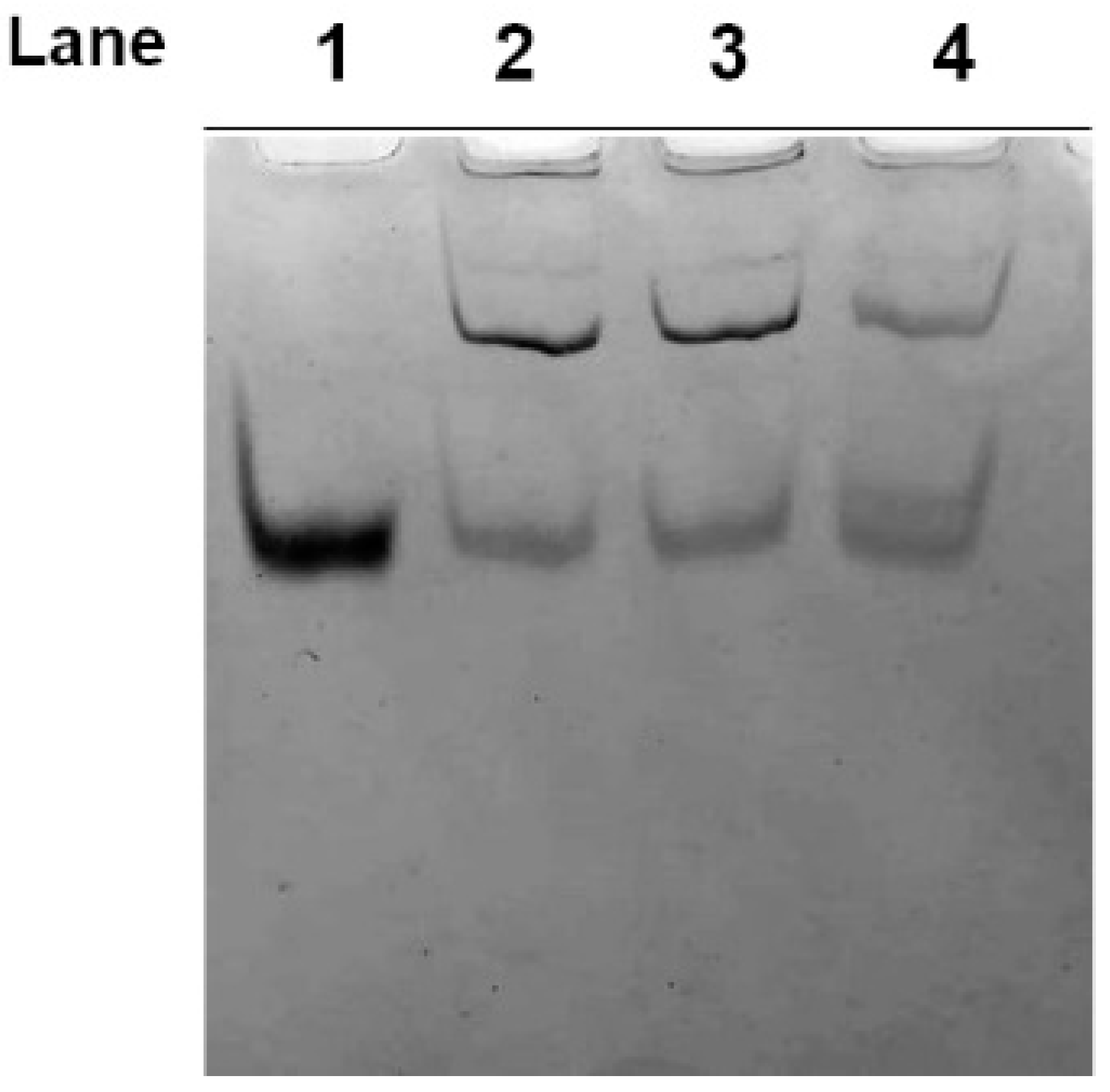
2.2. Effect of Dithiothreitol on Hg-Induced Protein Aggregate Formation in Human RBC
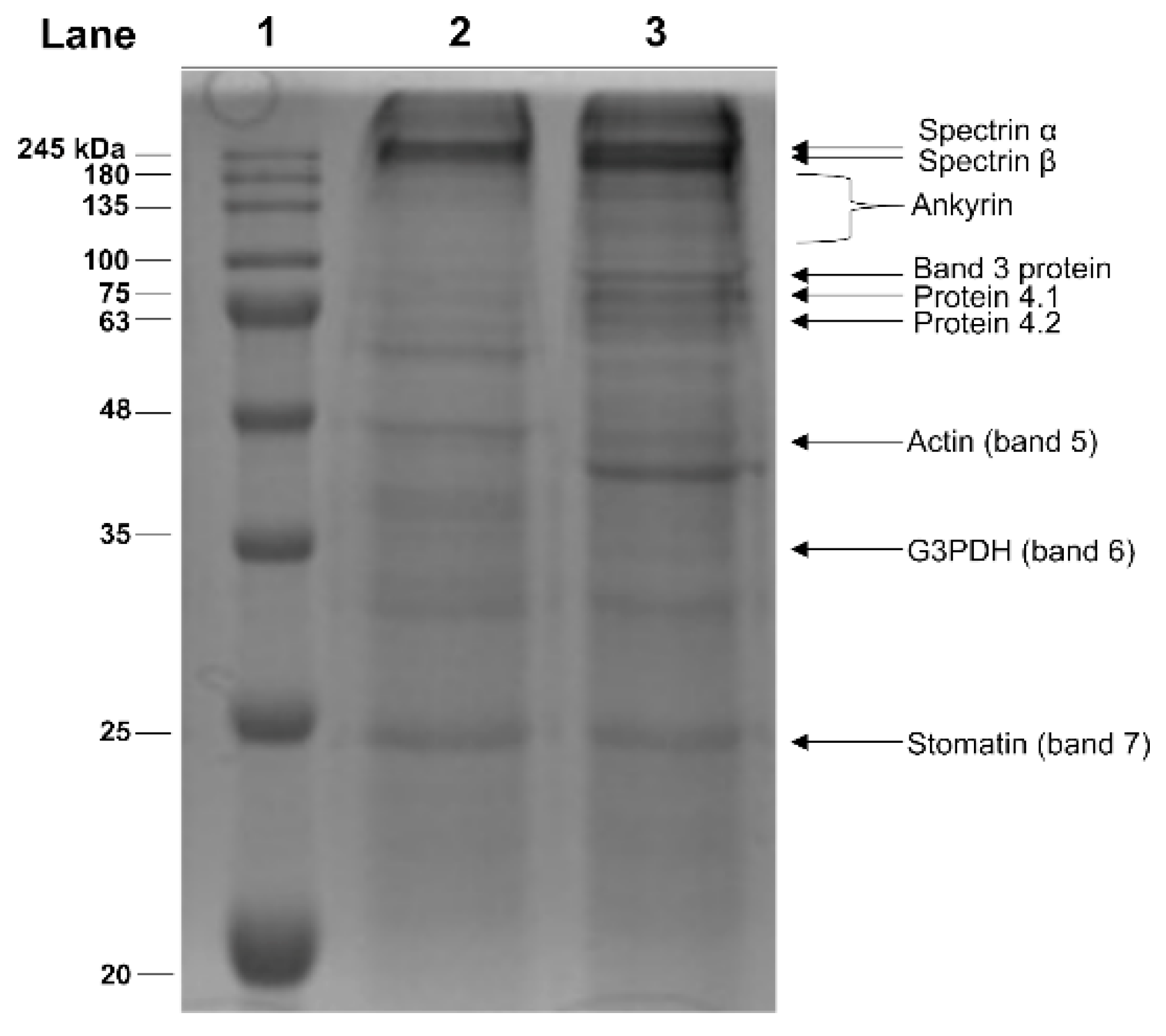
2.3. Electrophoretic Analyses of Membrane Proteins from Hg-Exposed Human RBC
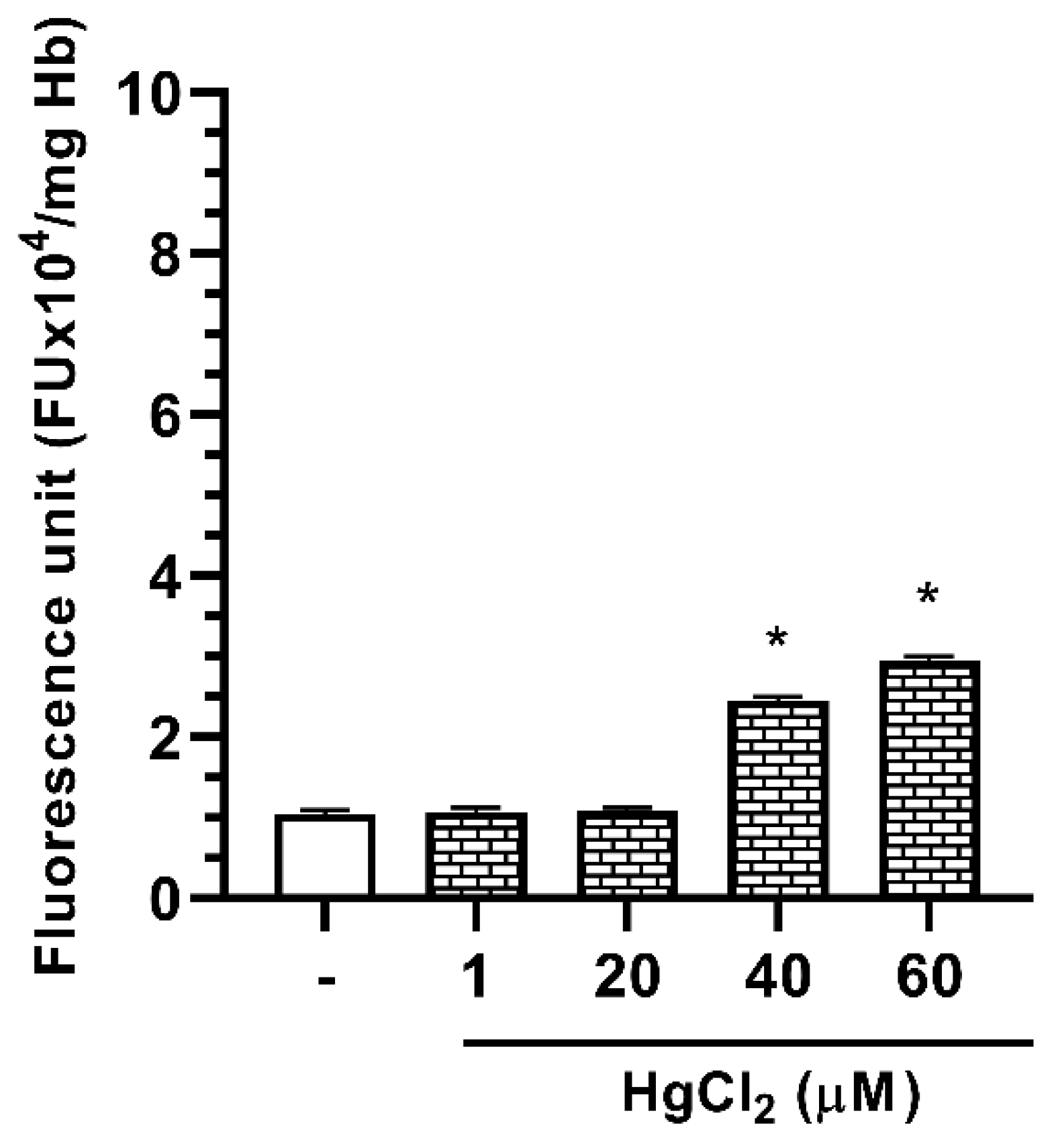
2.4. Effect of Hg on ROS Formation in Human RBC
3. Discussion
4. Materials and Methods
4.1. Preparation of RBC and HgCl2 Treatment
4.2. Dithiothreitol (DTT) Treatment
4.3. Preparation of Erythrocyte Membranes
4.4. Electrophoretic Analyses
4.5. Determination of Reactive Oxygen Species (ROS)
4.6. Protein Determination
4.7. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yousaf, B.; Amina; Liu, G.; Wang, R.; Imtiaz, M.; Rizwan, M.S.; Zia-Ur-Rehman, M.; Qadir, A.; Si, Y. The importance of evaluating metal exposure and predicting human health risks in urban-periurban environments influenced by emerging industry. Chemosphere 2016, 150, 79–89. [Google Scholar] [CrossRef]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotox. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The biological response chain to pollution: A case study from the “Italian Triangle of Death” assessed with the liverwort Lunularia cruciata. Environ. Sci. Pollut. Res. Int. 2017, 24, 26185–26193. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Paschoalini, A.L.; Savassi, L.A.; Arantes, F.P.; Rizzo, E.; Bazzoli, N. Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotox. Environ. Saf. 2019, 169, 539–550. [Google Scholar] [CrossRef]
- Spiegel, S.J. New mercury pollution threats: A global health caution. Lancet 2017, 390, 226–227. [Google Scholar] [CrossRef]
- Branco, V.; Caito, S.; Farina, M.; Teixeira da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Tecnol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Okpala, C.O.R.; Sardo, G.; Vitale, S.; Bono, G.; Arukwe, A. Hazardous properties and toxicological update of mercury: From fish food to human health safety perspective. Crit. Rev. Food Sci. Nutr. 2018, 58, 1986–2001. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K.; Silva, I.A.; Nyland, J.F. Mercury and autoimmunity: Implications for occupational and environmental health. Toxicol. Appl. Pharmacol. 2005, 207, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Popova, E.V.; Sinitskii, A.I.; Nemereshina, O.N.; Gatiatulina, E.R.; Nikonorov, A.A.; Skalny, A.V. Mercury and metabolic syndrome: A review of experimental and clinical observations. Biometals 2015, 28, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Pallan, S.; Gangji, A.S.; Lukic, D.; Clase, C.M. Mercury-associated nephrotic syndrome: A case report and systematic review of the literature. Am. J. Kidney Dis. 2013, 62, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Andrew, A.S.; Chen, C.Y.; Caller, T.A.; Tandan, R.; Henegan, P.L.; Jackson, B.P.; Hall, B.P.; Bradley, W.G.; Stommel, E.W. Toenail mercury levels are associated with amyotrophic lateral sclerosis (ALS) risk. Muscle Nerve 2018. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Houston, M.C. The Role of Mercury in Cardiovascular Disease. J. Cardiovasc. Dis. Diagn. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Hu, X.F.; Singh, K.; Chan, H.M. Mercury Exposure, Blood Pressure, and Hypertension: A Systematic Review and Dose-response Meta-analysis. Environ. Health Perspect. 2018, 126, 076002. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, R.; Prażanowski, M.; Michalska, M.; Krajewska, U.; Mielicki, W.P. Disorders in blood coagulation in humans occupationally exposed to mercuric vapors. J. Trace Elem. Exp. Med. 2002, 15, 21–29. [Google Scholar] [CrossRef]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-M.; Kim, S.; Noh, J.-Y.; Kim, K.; Jang, W.-H.; Bae, O.-N.; Chung, S.-M.; Chung, J.-H. Low-level mercury can enhance procoagulant activity of erythrocytes: A new contributing factor for mercury-related thrombotic disease. Environ. Health Perspect. 2010, 118, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef]
- Zalups, R.K.; Bridges, C.C. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol. Appl. Pharmacol. 2009, 235, 10–17. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Isab, A.A. A proton nuclear magnetic resonance study of the interaction of mercury with intact human erythrocytes. Biochim. Biophys. Acta 1982, 721, 374–384. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Ricchelli, F.; Drago, D.; Filippi, B.; Tognon, G.; Zatta, P. Aluminum-triggered structural modifications and aggregation of beta-amyloids. Cell. Mol. Life Sci. 2005, 62, 1724–1733. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef]
- Rooney, J.P.K. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 2007, 234, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ynalvez, R.; Gutierrez, J.; Gonzalez-Cantu, H. Mini-review: Toxicity of mercury as a consequence of enzyme alteration. Biometals 2016, 29, 781–788. [Google Scholar] [CrossRef]
- Ramírez-Bajo, M.J.; de Atauri, P.; Ortega, F.; Westerhoff, H.V.; Gelpí, J.L.; Centelles, J.J.; Cascante, M. Effects of Cadmium and Mercury on the Upper Part of Skeletal Muscle Glycolysis in Mice. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pirini, M.; Ventrella, V.; Pagliarani, A. Mercury and protein thiols: Stimulation of mitochondrial F1FO-ATPase and inhibition of respiration. Chem. Biol. Interact. 2016, 260, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tonazzi, A.; Giangregorio, N.; Console, L.; Scalise, M.; La Russa, D.; Notaristefano, C.; Brunelli, E.; Barca, D.; Indiveri, C. Mitochondrial carnitine/acylcarnitine transporter, a novel target of mercury toxicity. Chem. Res. Toxicol. 2015, 28, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef]
- Officioso, A.; Panzella, L.; Tortora, F.; Alfieri, M.L.; Napolitano, A.; Manna, C. Comparative Analysis of the Effects of Olive Oil Hydroxytyrosol and Its 5-S-Lipoyl Conjugate in Protecting Human Erythrocytes from Mercury Toxicity. Oxid. Med. Cell Longev. 2018, 2018, 9042192. [Google Scholar] [CrossRef]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol inhibits phosphatidylserine exposure and suicidal death induced by mercury in human erythrocytes: Possible involvement of the glutathione pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef]
- Eisele, K.; Lang, P.A.; Kempe, D.S.; Klarl, B.A.; Niemöller, O.; Wieder, T.; Huber, S.M.; Duranton, C.; Lang, F. Stimulation of erythrocyte phosphatidylserine exposure by mercury ions. Toxicol. Appl. Pharmacol. 2006, 210, 116–122. [Google Scholar] [CrossRef]
- Piscopo, M.; Ricciardiello, M.; Palumbo, G.; Troisi, J. Selectivity of metal bioaccumulation and its relationship with glutathione S-transferase levels in gonadal and gill tissues of Mytilus galloprovincialis exposed to Ni (II), Cu (II) and Cd (II). Rend. Fis. Acc. Lincei. 2016, 27, 737–748. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of arginine residues in Cu(II)-induced DNA breakage and Proteinase K resistance of H1 histones. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular effects of copper on the reproductive system of mytilus galloprovincialis. Mol. Reprod. Devel. 2019, 86, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. 2018, 25, 12957–12966. [Google Scholar] [CrossRef] [PubMed]
- Prchal, J.T.; Castleberry, R.P.; Parmley, R.T.; Crist, W.M.; Malluh, A. Hereditary Pyropoikilocytosis and Elliptocytosis: Clinical, Laboratory, and Ultrastructural Features in Infants and Children. Pediatr. Res. 1982, 16, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Myshkin, A.E.; Khromova, V.S. A new insight into mercurized hemoglobin aggregation mechanism. Biochim. Biophys. Acta 2005, 1749, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Akagi, H.; Malm, O.; Branches, F.J.P.; Kinjo, Y.; Kashima, Y.; Guimaraes, J.R.D.; Oliveira, R.B.; Haraguchi, K.; Pfeiffer, W.C.; Takizawa, Y.; et al. Human Exposure to Mercury Due to Goldmining in the Tapajos River Basin, Amazon, Brazil: Speciation of Mercury in Human Hair, Blood and Urine. Water Air Soil Pollut. 1995, 80, 85–94. [Google Scholar] [CrossRef]
- Forte, I.M.; Indovina, P.; Costa, A.; Iannuzzi, C.A.; Costanzo, L.; Marfella, A.; Montagnaro, S.; Botti, G.; Bucci, E.; Giordano, A. Blood screening for heavy metals and organic pollutants in cancer patients exposed to toxic waste in southern Italy: A pilot study. J. Cell Physiol. 2020, 235, 5213–5222. [Google Scholar] [CrossRef]
- McKelvey, W.; Gwynn, R.C.; Jeffery, N.; Kass, D.; Thorpe, L.E.; Garg, R.K.; Palmer, C.D.; Parsons, P.J. A Biomonitoring Study of Lead, Cadmium, and Mercury in the Blood of New York City Adults. Environ. Health Perspect. 2007, 115, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Lancaster, J.R.; Freeman, B.A.; Schechter, A.N. Nitric oxide’s reactions with hemoglobin: A view through the SNO-storm. Nat. Med. 2003, 9, 496–500. [Google Scholar] [CrossRef]
- Stratton, A.; Ericksen, M.; Harris, T.V.; Symmonds, N.; Silverstein, T.P. Mercury(II) binds to both of chymotrypsin’s histidines, causing inhibition followed by irreversible denaturation/aggregation. Protein Sci. 2017, 26, 292–305. [Google Scholar] [CrossRef]
- Steck, T.L. The organization of proteins in the human red blood cell membrane. J. Cell Biol. 1974, 62, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Marino, A. Natural Antioxidants Beneficial Effects on Anion Exchange through Band 3 Protein in Human Erythrocytes. Antioxidants 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Thevenin, B.J.; Willardson, B.M.; Low, P.S. The redox state of cysteines 201 and 317 of the erythrocyte anion exchanger is critical for ankyrin binding. J. Biol. Chem. 1989, 264, 15886–15892. [Google Scholar] [PubMed]
- Willardson, B.M.; Thevenin, B.J.; Harrison, M.L.; Kuster, W.M.; Benson, M.D.; Low, P.S. Localization of the ankyrin-binding site on erythrocyte membrane protein, band 3. J. Biol. Chem. 1989, 264, 15893–15899. [Google Scholar] [PubMed]
- Nigra, A.D.; Casale, C.H.; Santander, V.S. Human erythrocytes: Cytoskeleton and its origin. Cell. Mol. Life Sci. 2020, 77, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Leto, T.L.; Marchesi, V.T. A structural model of human erythrocyte protein 4.1. J. Biol. Chem. 1984, 259, 4603–4608. [Google Scholar]
- Satchwell, T.J.; Shoemark, D.K.; Sessions, R.B.; Toye, A.M. Protein 4.2: A complex linker. Blood Cells Mol. Dis. 2009, 42, 201–210. [Google Scholar] [CrossRef]
- Galletti, P.; Ki Paik, W.; Kim, S. Methyl acceptors for protein methylase II from human-erythrocyte membrane. Eur. J. Biochem. 1979, 97, 221–227. [Google Scholar] [CrossRef]
- Vassalli, Q.A.; Caccavale, F.; Avagnano, S.; Murolo, A.; Guerriero, G.; Fucci, L.; Ausió, J.; Piscopo, M. New insights into protamine-like component organization in Mytilus galloprovincialis sperm chromatin. DNA Cell Biol. 2015, 34, 162–169. [Google Scholar] [CrossRef]
- Fioretti, F.M.; Febbraio, F.; Carbone, A.; Branno, M.; Carratore, V.; Fucci, L.; Ausió, J.; Piscopo, M. A sperm nuclear basic protein from the sperm of the marine worm Chaetopterus variopedatus with sequence similarity to the arginine-rich C-termini of chordate protamine-likes. DNA Cell Biol. 2012, 31, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like proteins’ analysis as an emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. https://doi.org/10.3390/molecules25143278
Piscopo M, Notariale R, Tortora F, Lettieri G, Palumbo G, Manna C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules. 2020; 25(14):3278. https://doi.org/10.3390/molecules25143278
Chicago/Turabian StylePiscopo, Marina, Rosaria Notariale, Fabiana Tortora, Gennaro Lettieri, Giancarlo Palumbo, and Caterina Manna. 2020. "Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes" Molecules 25, no. 14: 3278. https://doi.org/10.3390/molecules25143278
APA StylePiscopo, M., Notariale, R., Tortora, F., Lettieri, G., Palumbo, G., & Manna, C. (2020). Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules, 25(14), 3278. https://doi.org/10.3390/molecules25143278







