Theoretical Investigation on Molecular Structure and Electronic Properties of BxLiy Cluster for Lithium-Ion Batteries with Quantum ESPRESSO Program
Abstract
1. Introduction
2. Computational and Mathematical Details
3. Structures and Stabilities of BxLiy (x = 1–3, y = 1–3) Clusters
3.1. The BxLiy (x = 1–3, y = 1–3) Clusters of Diatomic Group
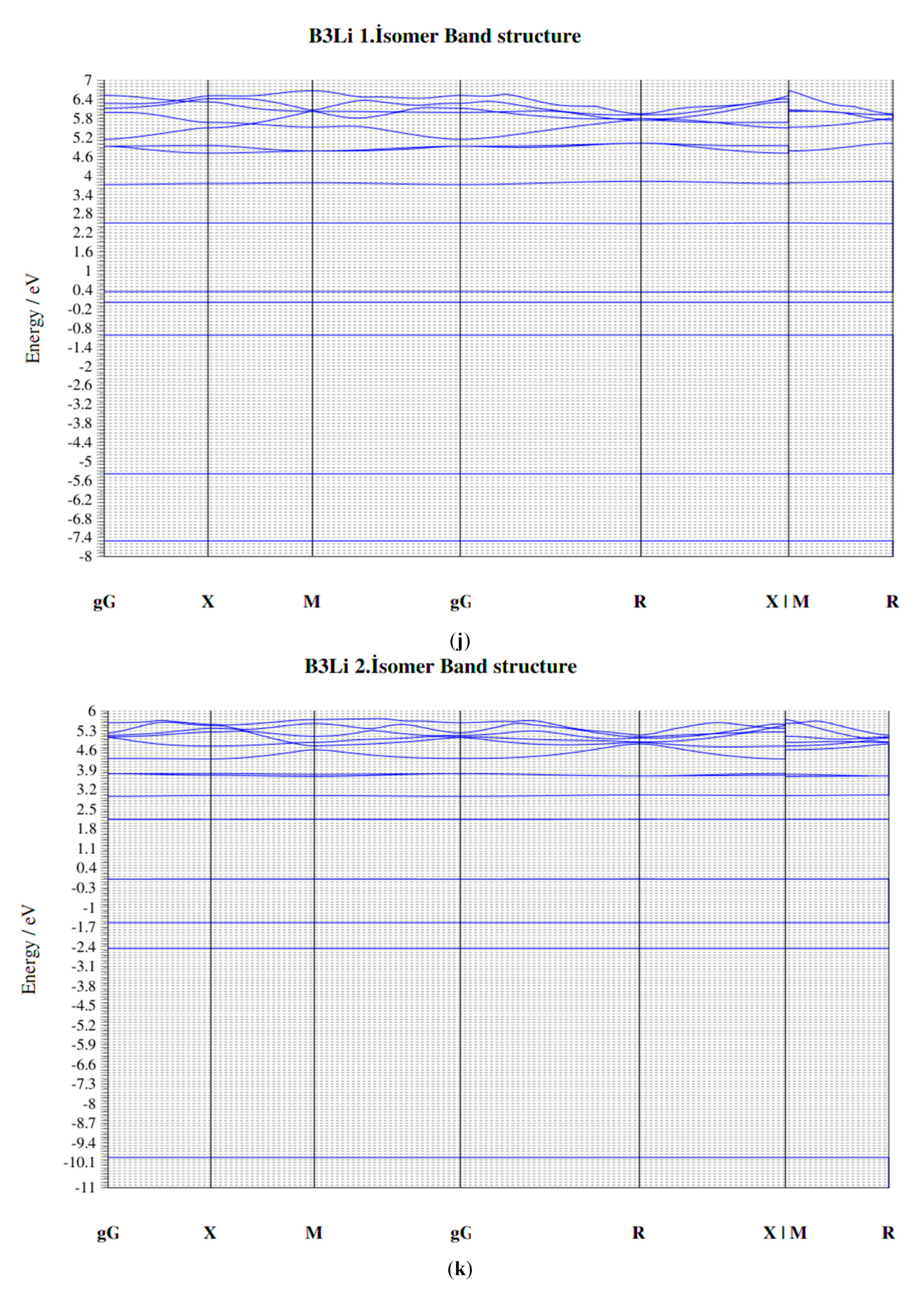
3.2. The BxLiy (x = 1–3, y = 1–3) Clusters of Triple Group
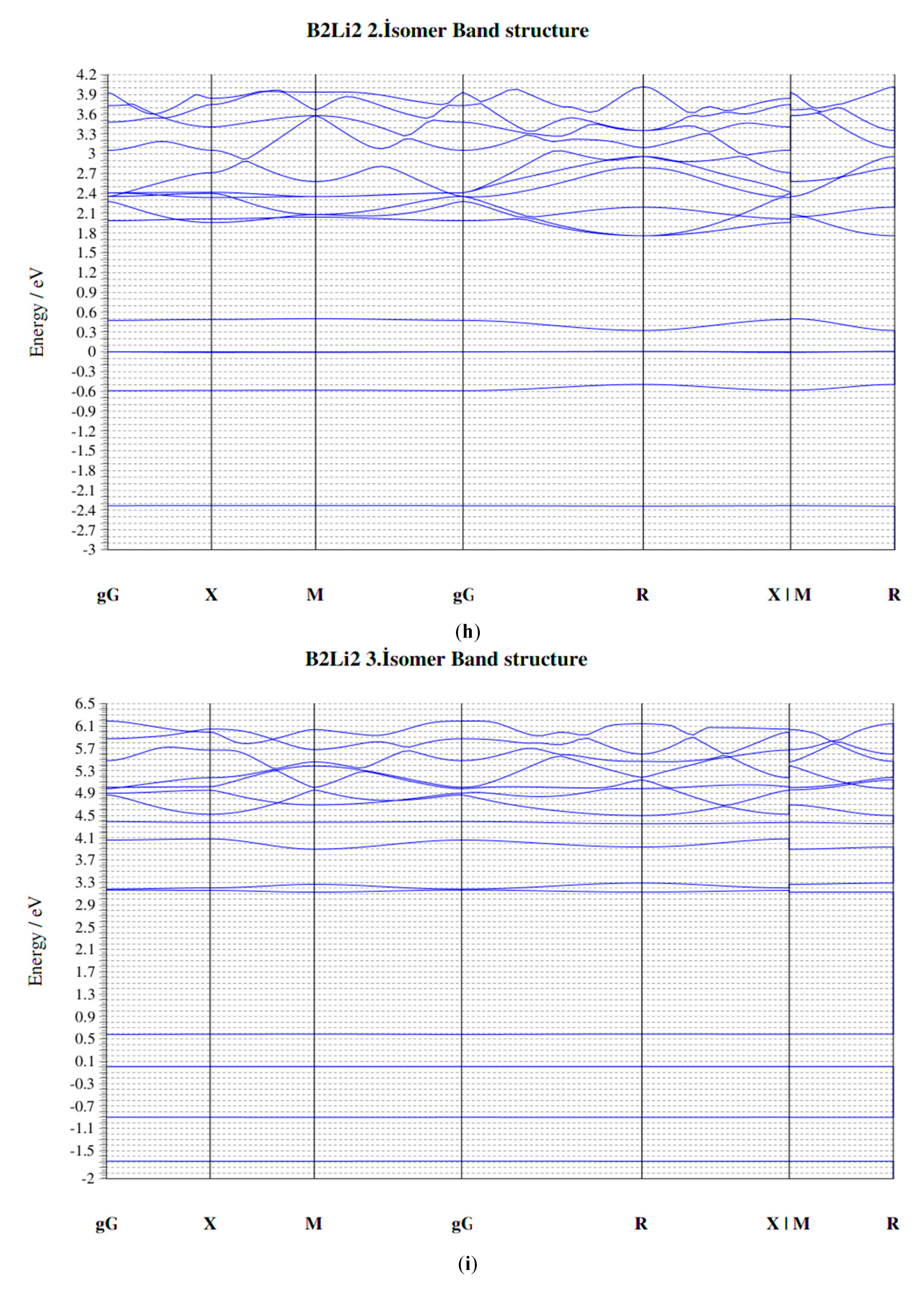
3.3. The BxLiy (x = 1–3, y = 1–3) Clusters of Quadrate Group
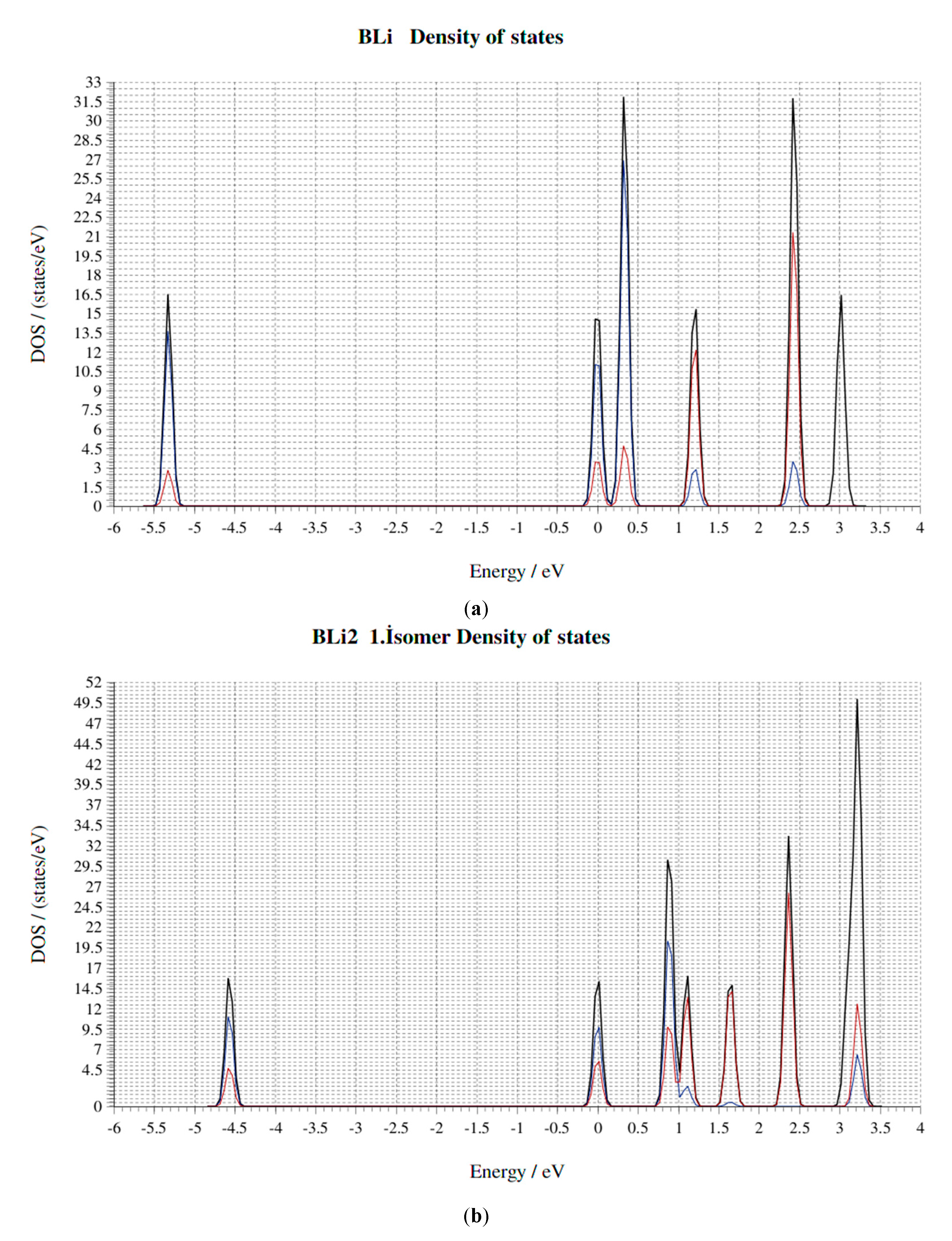
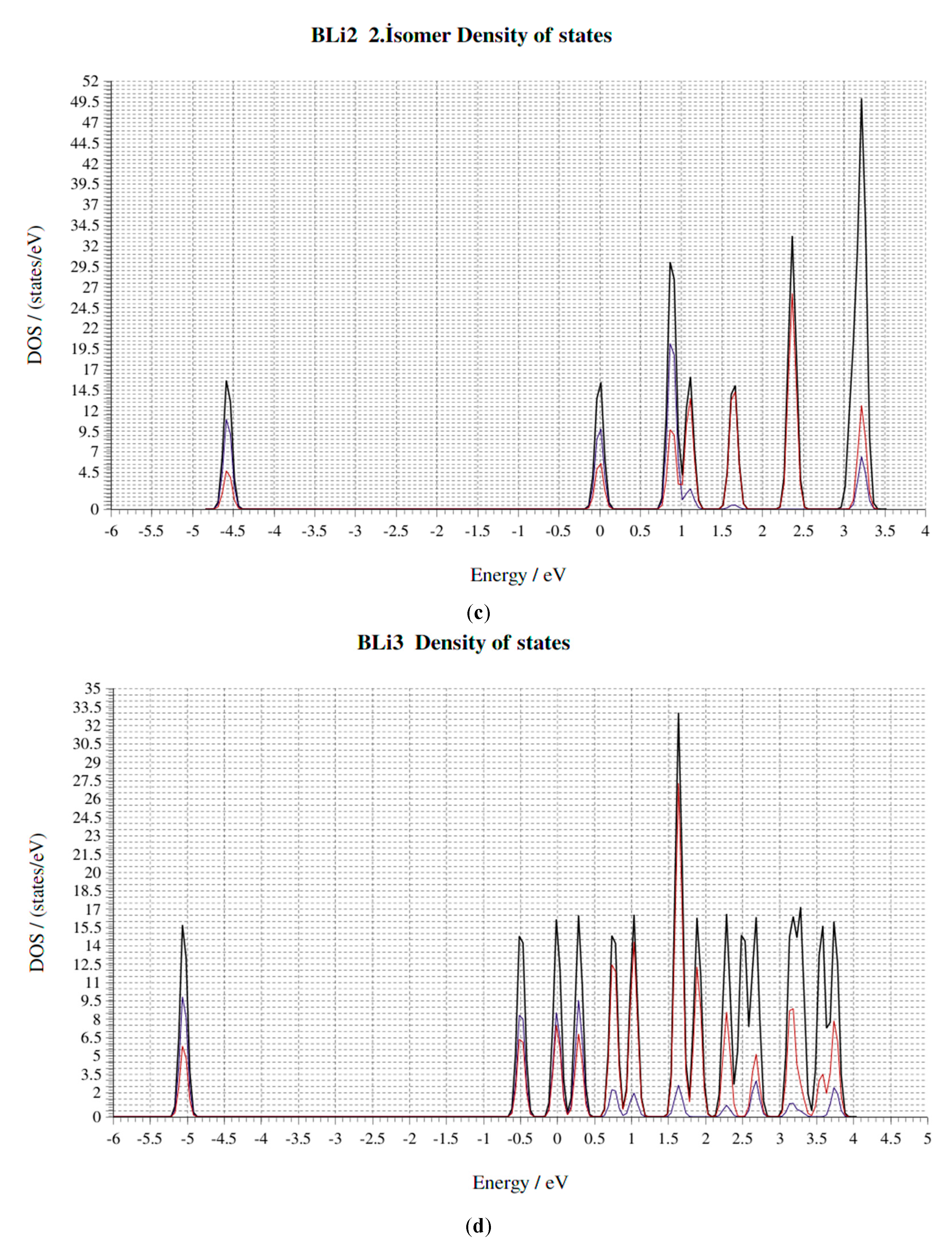
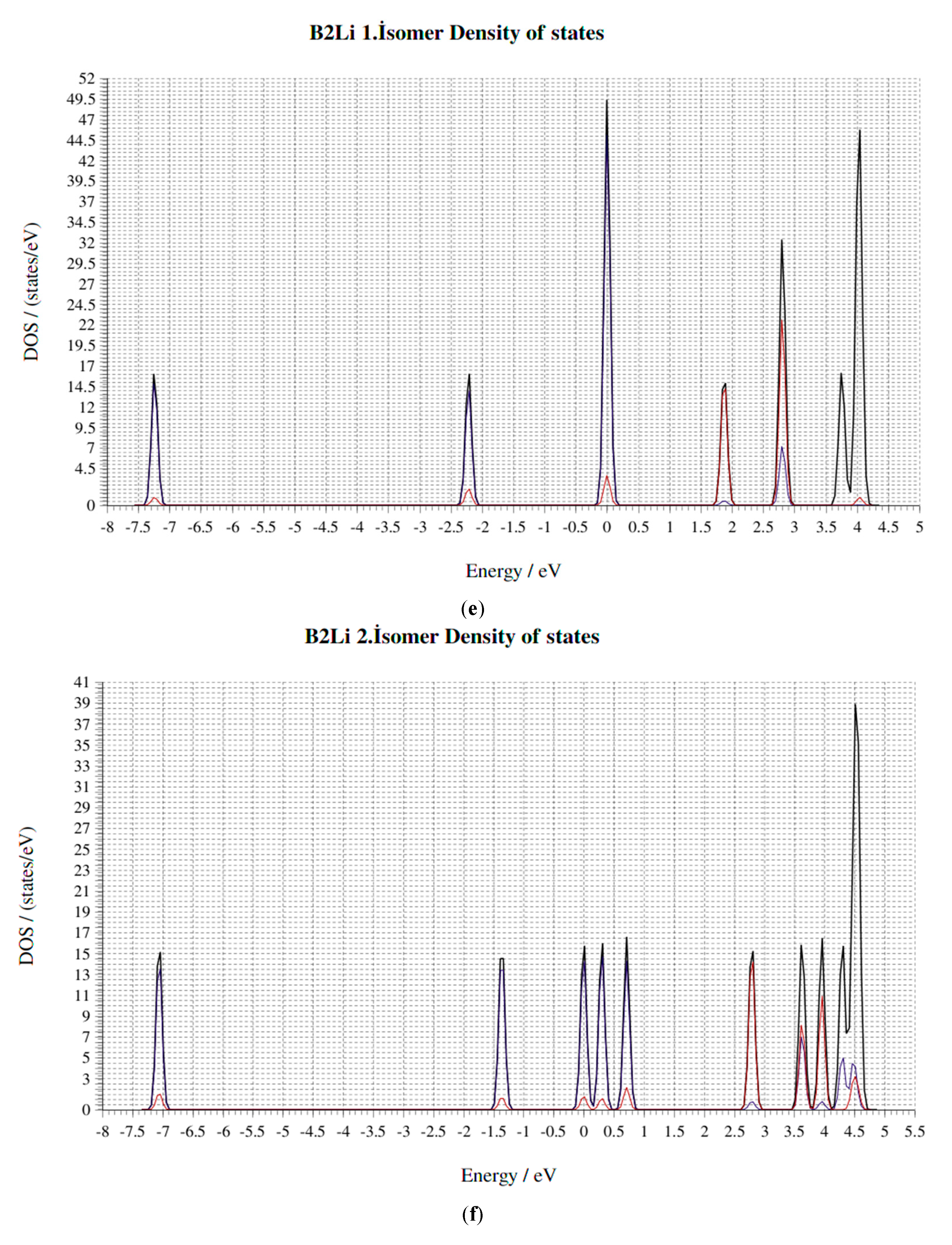
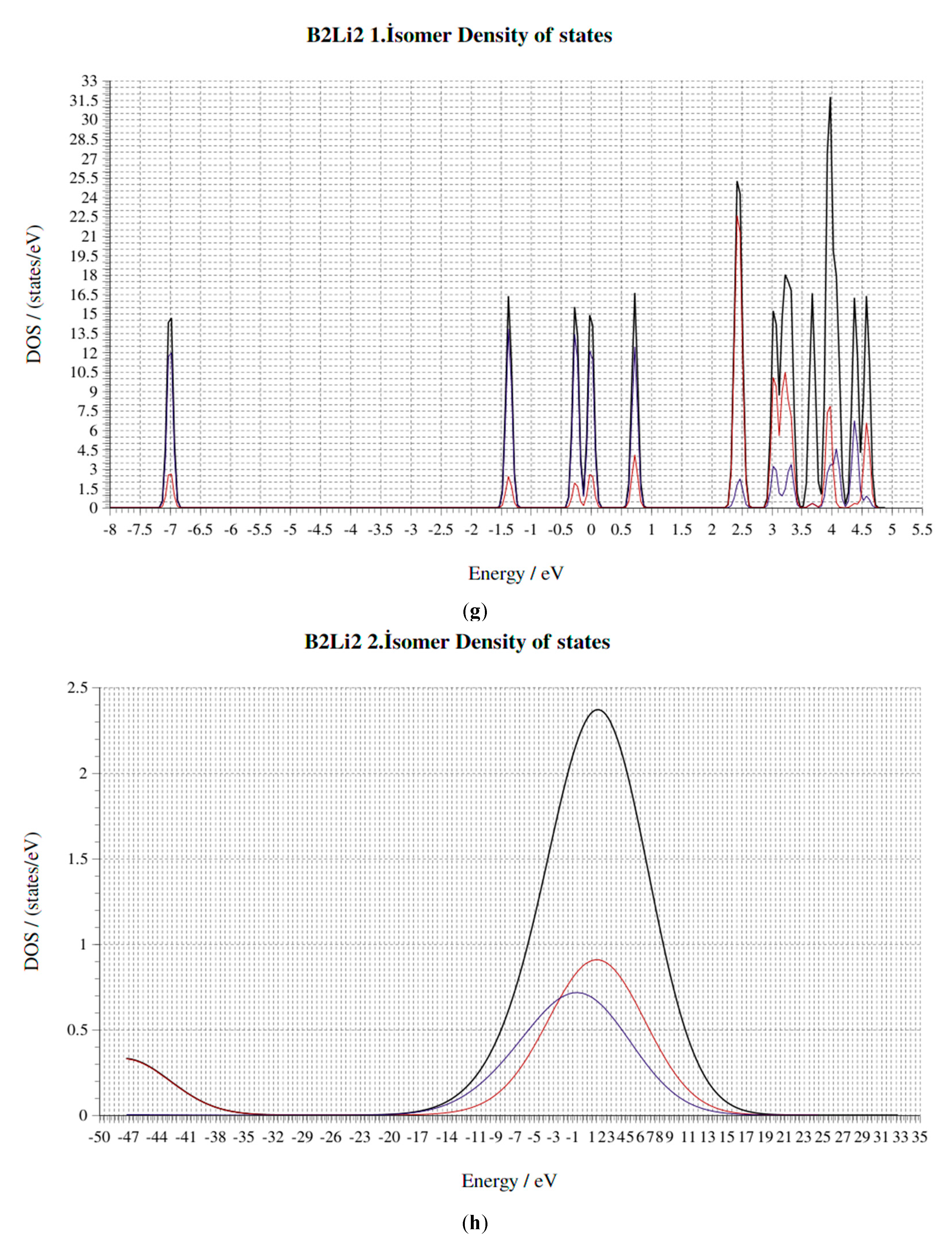
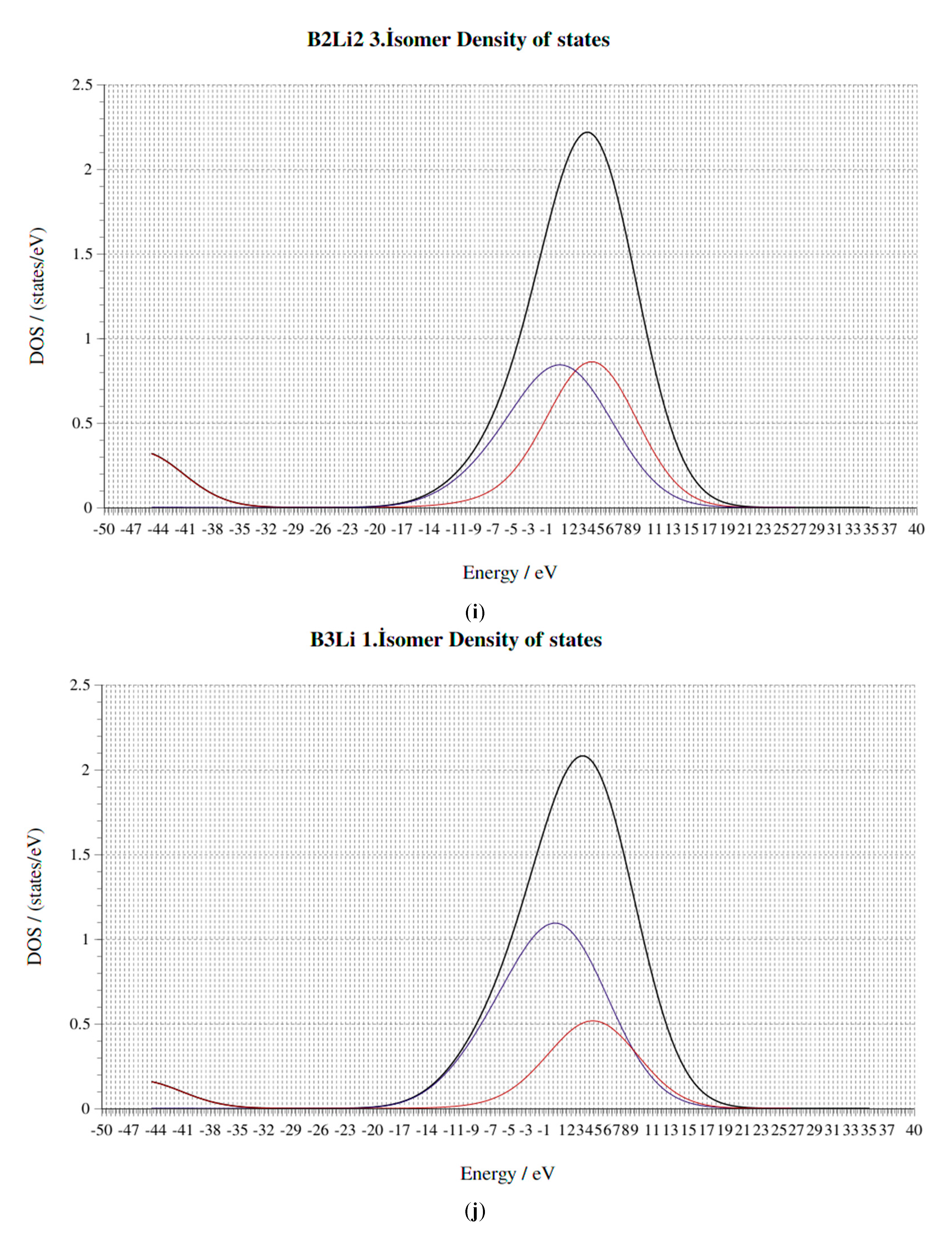
4. Electronic Properties of BxLiy (x = 1–3, y = 1–3) Cluster
4.1. The Second Difference in Energy (Δ2E) and Dissociation Energy (ΔE)
4.2. Binding Energy per Atoms
4.3. Total Energy (eV)
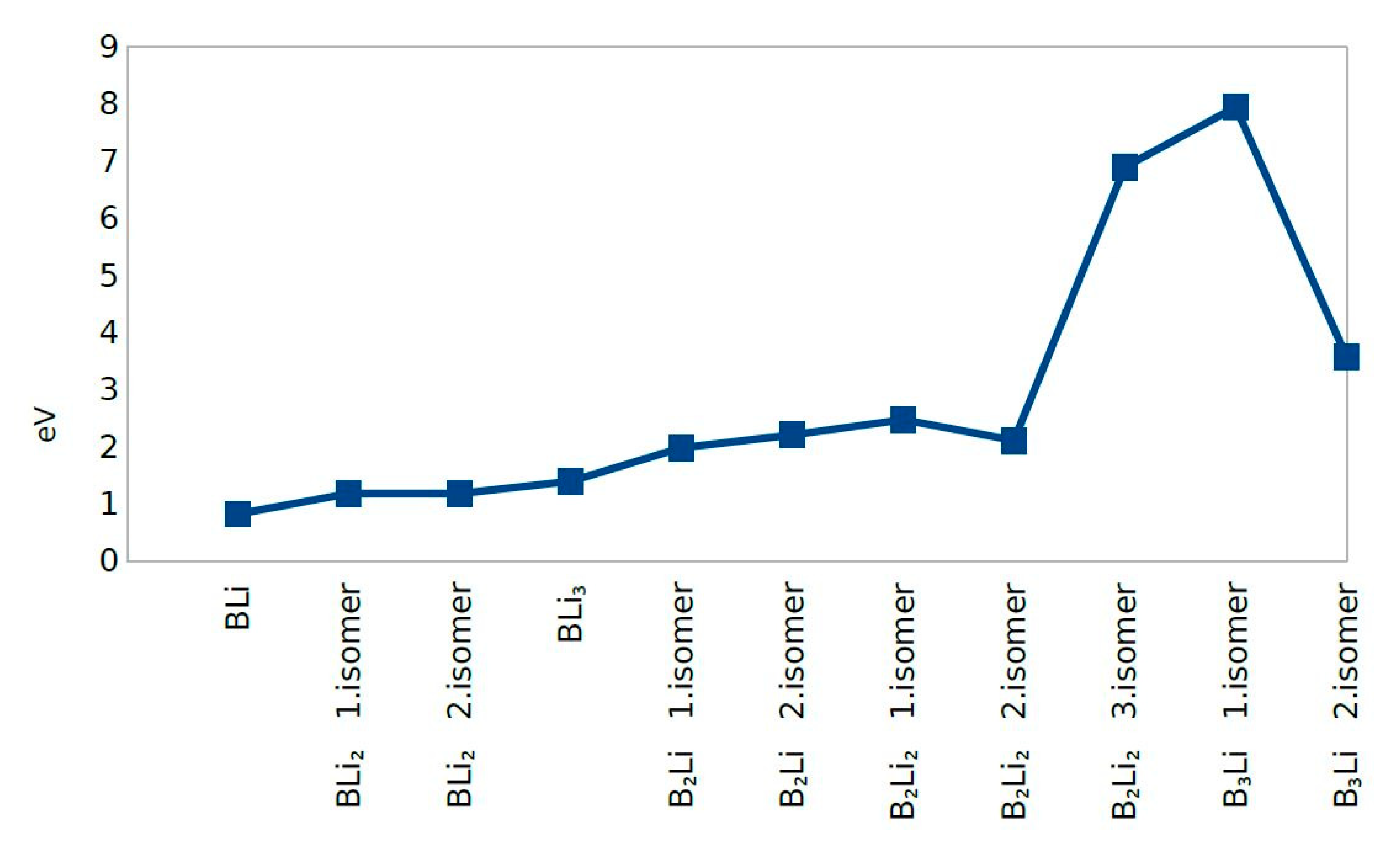
4.4. HOMO-LUMO Gap
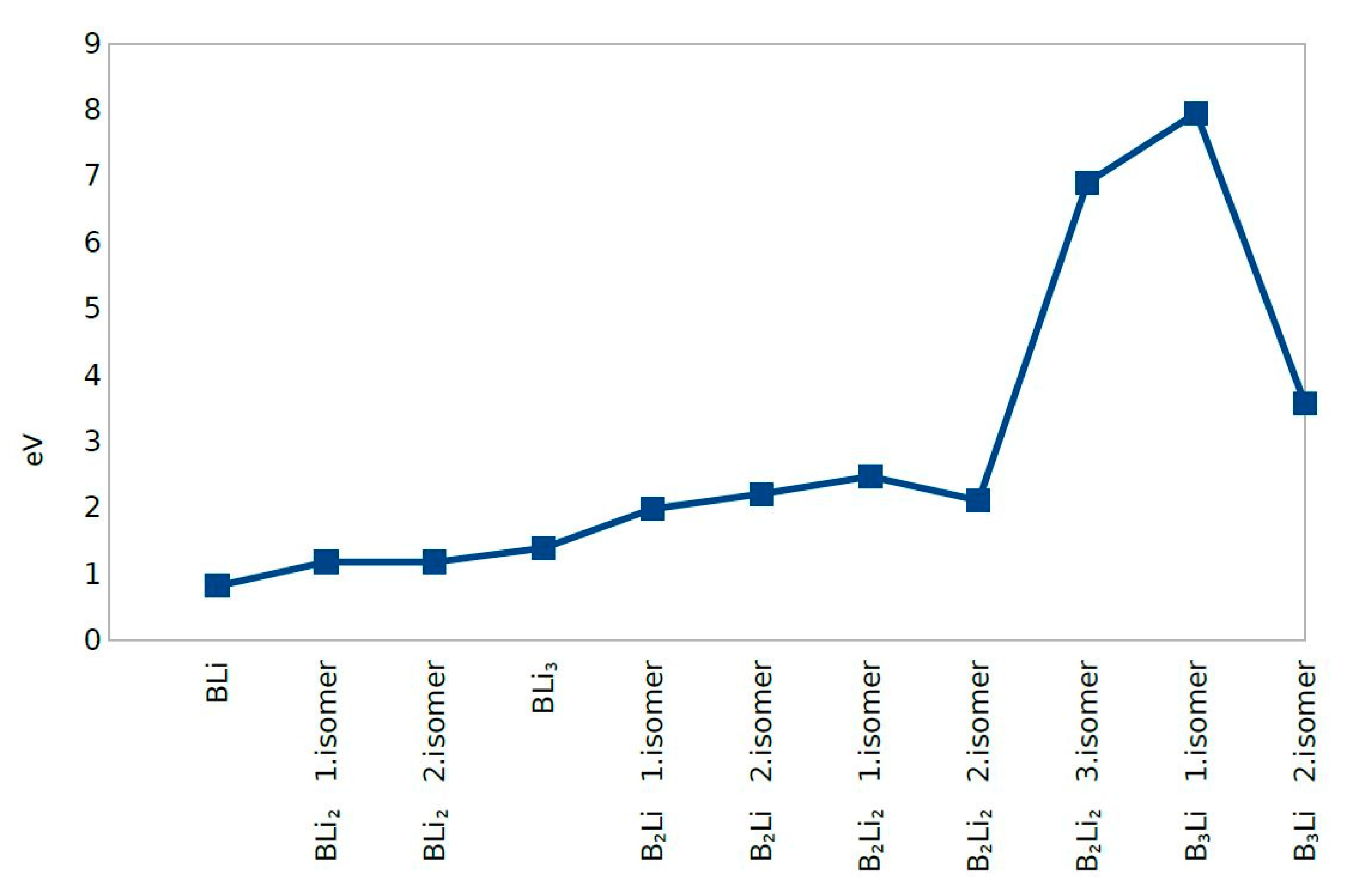
4.5. Fermi Energy (eV)
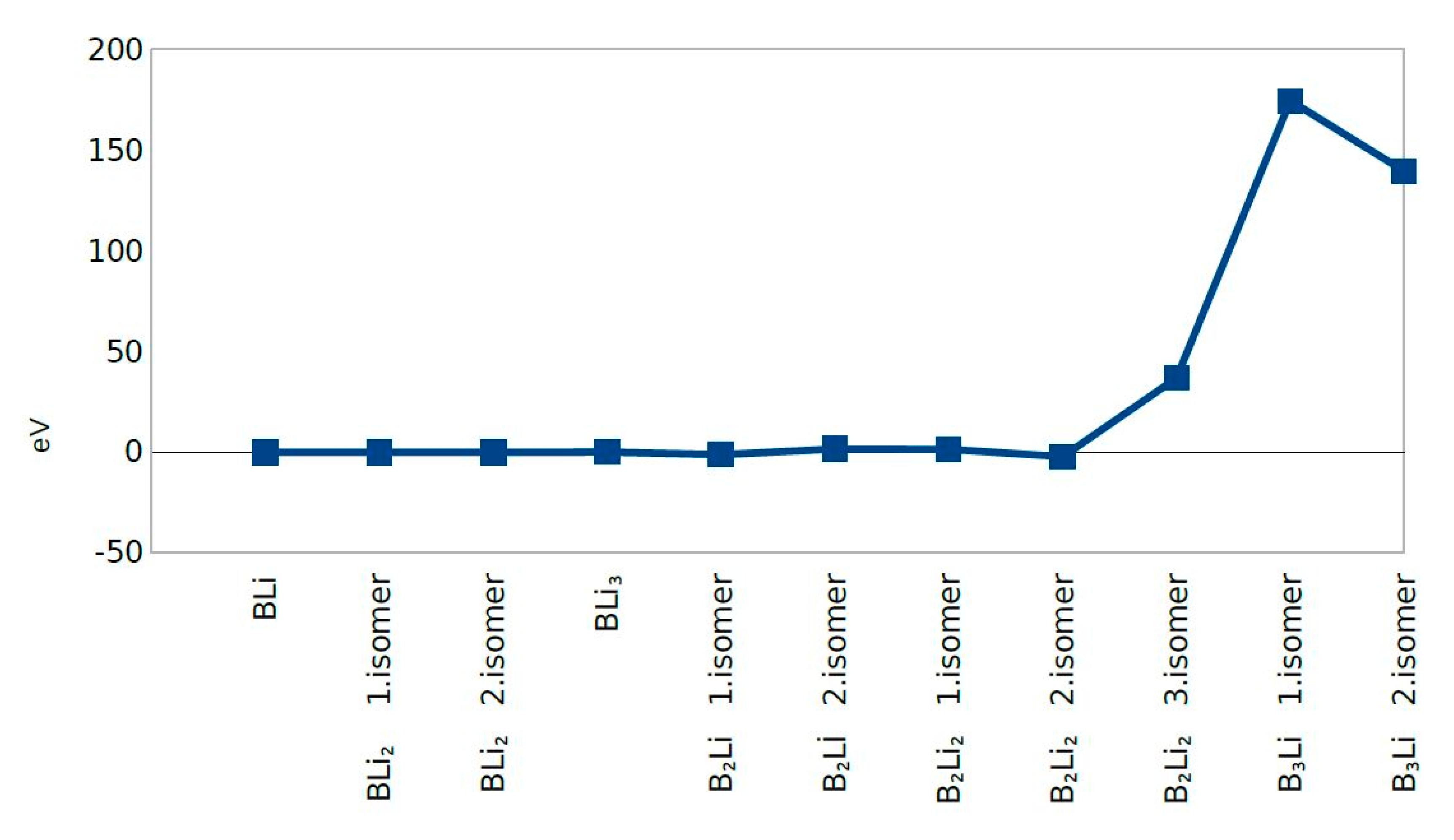
4.6. Force on Atom (eV/Å)
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balema, V.P. Mechanical processing in Hydrogen storage research and development. Mater. Matters 2007, 2, 16–18. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Metal borohydrides as hydrogen storage materials. Mater. Matters 2007, 2, 11–15. [Google Scholar]
- Demirbaş, A. Hydrogen and Boron as Recent Alternative Motor Fuels. Energy Sour. 2005, 27, 741–748. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Yong, C.K.; Wu, G.T.; Chen, P.; Shaw, W.; Karkambar, A.; Aurtrey, T.; Jones, M.O.; Johnson, S.R.; Edwards, P.P.; et al. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat. Mater. 2008, 7, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Mormillan, M.; Veziroğlu, T.; Mormillan, M.; Veziroğlu, T. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar]
- Ewald, R. Requirements for advanced mobile storage systems. Int. J. Hydrog. Energy 1998, 23, 803–814. [Google Scholar] [CrossRef]
- Karabacak, M.; Özçelik, S.; Güvenç, Z.B. Structures and energetics Of Pdn (n = 2–20) clusters using an embedded-atom model potential. Surf. Sci. 2003, 507, 636–642. [Google Scholar] [CrossRef]
- Böyükata, M.; Özdoğan, C.; Güvenç, Z.B. An investigation of hydrogen bonded neutral B4Hn (n = 1–11) and anionic B4H11(−1) clusters: Density functional study. J. Mol. Struct. THEOCHEM 2007, 805, 91–100. [Google Scholar] [CrossRef]
- Sudik, A.; Yang, J.; Halliday, D.; Wolverton, C. Hydrogen Storage Properties in (LiNH2)2−LiBH4−(MgH2)X Mixtures (X = 0.0−1.0). J. Phys. Chem. C 2008, 112, 4384–4390. [Google Scholar] [CrossRef]
- Böyükata, M.; Güvenç, Z.B. Density functional study of AlBn clusters for n = 1–14. J. Alloys Compd. 2011, 509, 4214–4234. [Google Scholar] [CrossRef]
- Bandaru, S.; Chakraborty, A.; Giri, S.; Chattaraj, P.K. Toward analyzing some neutral and cationic boron–lithium clusters (Bx Liy x = 2–6; y = 1, 2) as effective hydrogen storage materials: A conceptual density functional study. Int. J. Quantum Chem. 2012, 112, 695–702. [Google Scholar] [CrossRef]
- Li, Y.; Wu, D.; Li, Z.; Sun, C.C. Structural and electronic properties of boron-doped lithium clusters: Ab initio and DFT studies. J. Comput. Chem. 2007, 28, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.E.; Mitchell, M.A.; Sutula, R.A.; Holden, J.R. Crystal structure study of a new compound Li5B4. J. Less-Common Met. 1978, 61, 237–251. [Google Scholar] [CrossRef]
- Sutton, L.E. (Ed.) Tables of Interatomic Distances and Configuration in Molecules and Ions. In Interatomic Distances Supplement; The Chemical Society: London, UK, 1958. [Google Scholar]
- Hagenmueller, P.; Naslain, R. L’hexaboruce NaB6. Comptes Rendus de l’Académie des Sci. 1963, 257, 1294–1296. [Google Scholar]
- Lipp, A. Process for the Preparation of Alkali Borides. Fr. Patent 1461878, 9 December 1966. [Google Scholar]
- Kiessling, R. The Borides of Some Transition Elements. Acta Chem. Stand. 1950, 4, 209–212. [Google Scholar] [CrossRef]
- Rupp, L.W.; Hodges, D.J. Conduction-electron spin resonance in alkali hexaborides. J. Phys. Chem. Solids 1974, 35, 617–701. [Google Scholar] [CrossRef]
- Schmidt, P.H. Personal Communication; Be11 Telephone Laboratories: Murray Hill, NJ, USA, 1976. [Google Scholar]
- Secrist, D.R.; Childs, W.J. Lithium-Boron-Carbide Reaction Studies; USAEC Rep. TID-17149, Knolls Atomic Power Lab.: Schenectady, NY, USA, 1962. [Google Scholar]
- Secrist, D.R. Compound Formation in the Systems Lithium‐Carbon and Lithium-Boron. J. Am. Ceram. Sot. 1967, 50, 520–527. [Google Scholar] [CrossRef]
- Meden, A.; Mavri, J.; Bele, M.; Pejovik, S. Dissolution of Boron in Lithium Melt. J. Phys. Chem. 1995, 99, 4252–4259. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Lammertsma, K. Structure, Bonding, and Stability of Small Boron−Lithium Clusters. J. Phys. Chem A 1998, 102, 1608–1614. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Srinivas, G.N.; Hamilton, T.P.; Lammerstsma, K. Stability of Hyperlithiated Borides. J. Phys. Chem A 1999, 103, 710–715. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502–395521. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901–465931. [Google Scholar] [CrossRef] [PubMed]
- Zhurko, G.A. Chemcraft—Graphical Program for Visualization of Quantum Chemistry Computations. Available online: https://chemcraftprog.com (accessed on 18 January 2020).
- Burai. Available online: https://nisihara.wixsite.com/burai (accessed on 6 March 2020).
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundquist, B.I. Van der Waals Density Functional for General Geometrie. Phys. Rev. Lett. 2004, 92, 246401–246405. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Nagabalasubramanian, P.B.; Karabacak, M.; Periandy, S.; Govindarajan, M. Molecular structure, vibrational, electronic and thermal properties of 4-vinylcyclohexene by quantum chemical calculations. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2015, 145, 340–352. [Google Scholar] [CrossRef]
- Srinivas, G.N.; Hamilton, T.P.; Boatz, J.A.; Lammertsma, K. Theoretical Studies of B2Lin (n = 1−4). J. Phys. Chem. A 1999, 103, 9931–9937. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





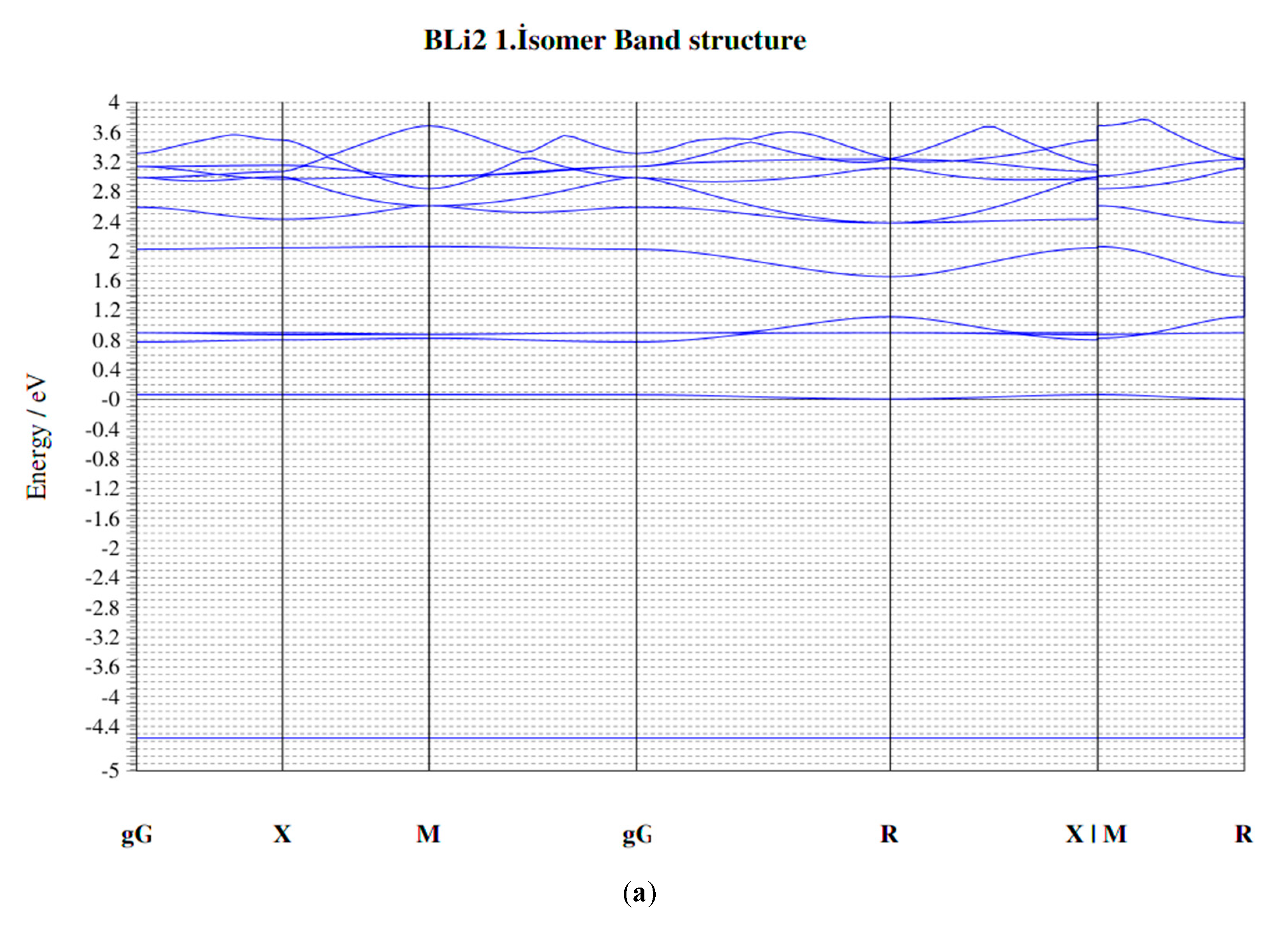
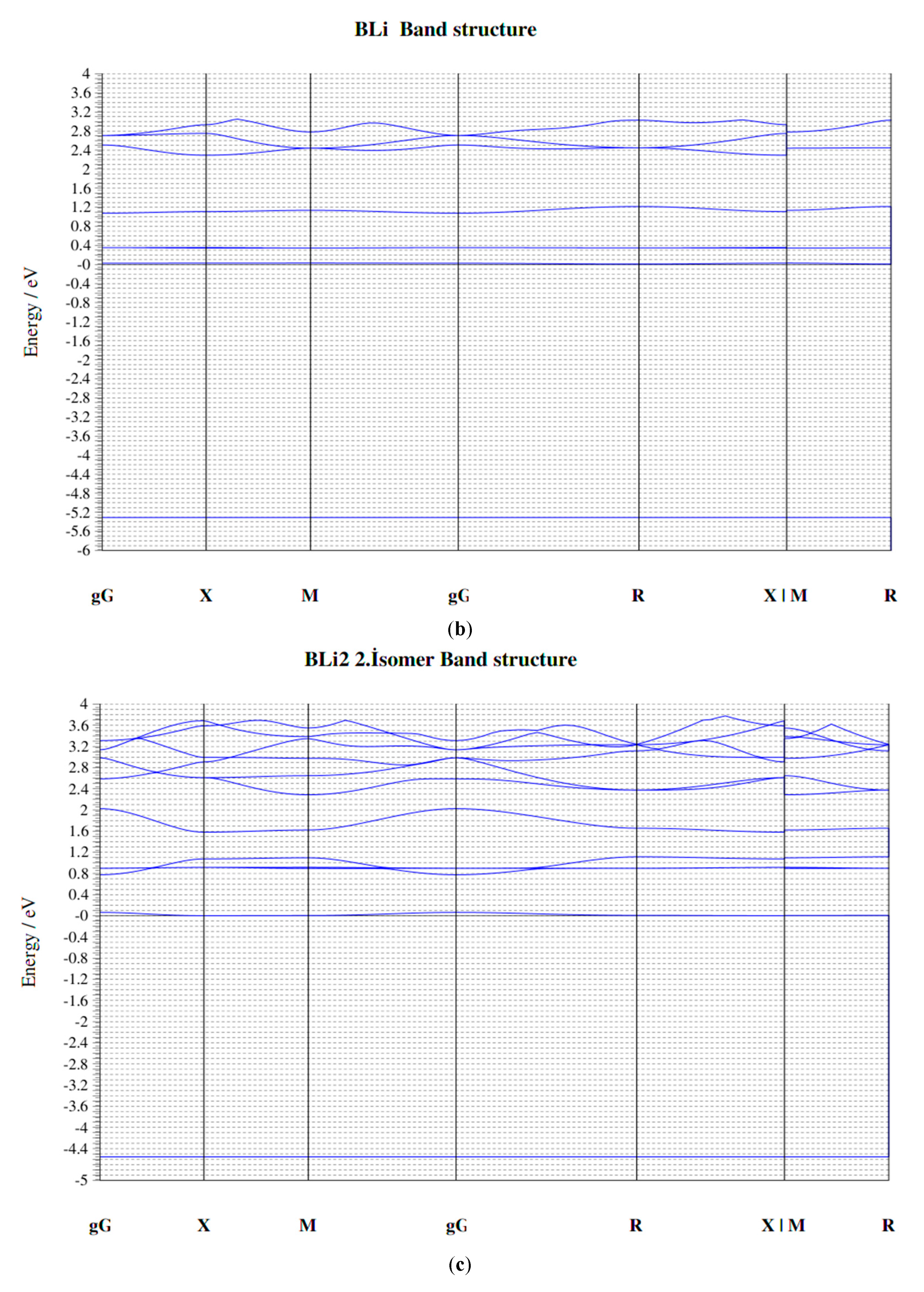
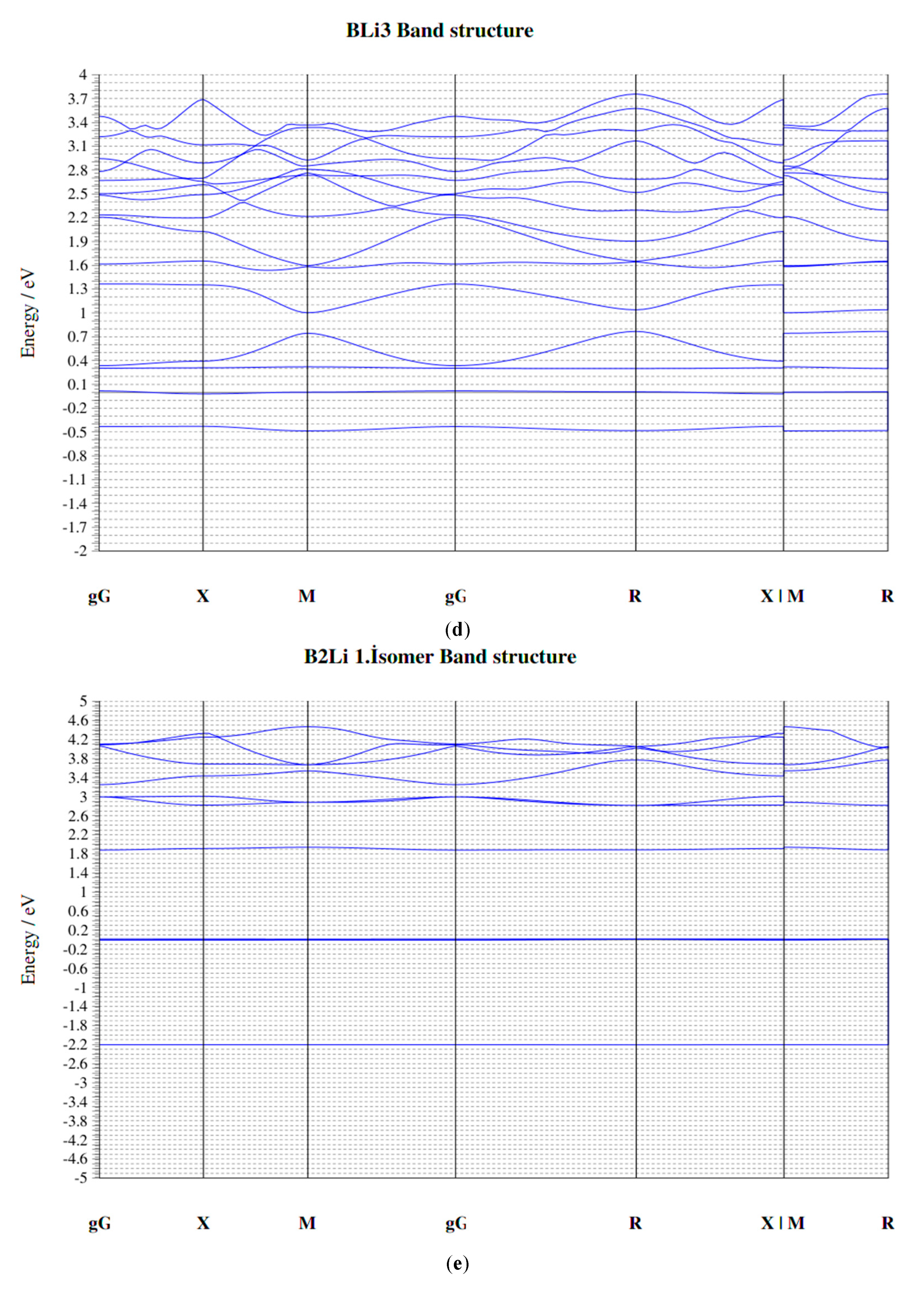
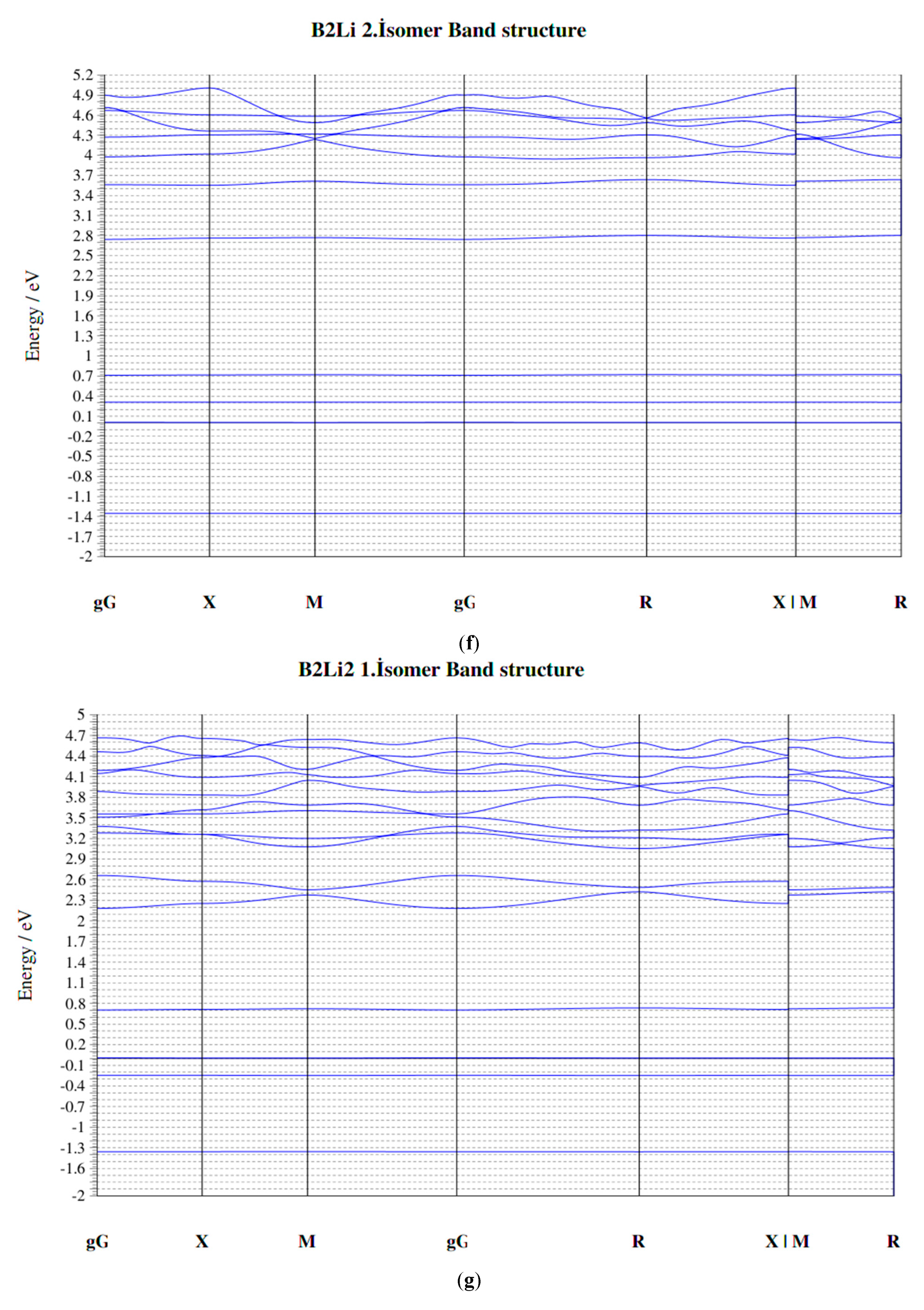
| Parameters | B–Li | B–B | Li–Li |
|---|---|---|---|
| Bond Lengths (Å) | |||
| BLi | 2.437 | - | - |
| BLi2 1. isomer | 2.207 | - | 4.414 |
| BLi2 2. isomer | 2.324 | - | 2.921 |
| BLi3 | 2.202 | - | 3.606 |
| B2Li 1. isomer | 2.113 | 1.617 | - |
| B2Li 2. isomer | 2.263 | 1.568 | - |
| B2Li2 1. isomer | 2.180 | 1.528 | 3.258 |
| B2Li2 2. isomer | 2.087 | 1.596 | 5.770 |
| B2Li2 3. isomer | 2.455 | 1.639 | 4.629 |
| B3Li 1. isomer | 2.305 | 1.618 | - |
| B3Li 2. isomer | 2.269 | 1.545 | - |
| Clusters/Isomer | Total Energy (eV) | Force on Atom (eV/Å) | Frequency Lowest/Highest (cm−1) | Point Group | Binding Energy Per Atom | HOMO-LUMO Gap |
|---|---|---|---|---|---|---|
| BLi | −277.37705 | 0.0217515 | 428.919/- | CV | 0.818640424 | 0.3392 |
| BLi2 1. isomer | −477.54732 | 0.0016969 | 76.22/601.68 | Dh | 1.178975414 | 0.8877 |
| BLi2 2. isomer | −477.54768 | 0.0329979 | 214.99/420.81 | C2V | 1.178987446 | 0.8901 |
| BLi3 | −677.85093 | 0.0350184 | 123.46/608.29 | C1 | 1.389984142 | 0.2944 |
| B2Li 1. isomer | −359.13939 | 0.0286678 | 164.70/1193.25 | CS | 1.978463931 | 0.0063 |
| B2Li 2. isomer | −359.81842 | 0.0086903 | 305.08/1112.38 | CS | 2.204807009 | 0.3025 |
| B2Li2 1. isomer | −561.36346 | 0.0230113 | 108.68/1170.00 | C1 | 2.470966369 | 0.7278 |
| B2Li2 2. isomer | −559.90784 | 0.0236314 | 76.05/1171.04 | Dh | 2.107060642 | 0.0000 |
| B2Li2 3. isomer | −579.07518 | 0.0407777 | 245.10/1148.02 | C2V | 6.898897329 | 0.5800 |
| B3Li 1. isomer | −462.46380 | 0.0324987 | 71.43/1526.35 | CV | 7.948900502 | 0.3191 |
| B3Li 2. isomer | −444.95986 | 0.0270737 | 244.13/1245.33 | C1 | 3.572915854 | 2.1249 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çipiloğlu, M.A.; Özkurt, A. Theoretical Investigation on Molecular Structure and Electronic Properties of BxLiy Cluster for Lithium-Ion Batteries with Quantum ESPRESSO Program. Molecules 2020, 25, 3266. https://doi.org/10.3390/molecules25143266
Çipiloğlu MA, Özkurt A. Theoretical Investigation on Molecular Structure and Electronic Properties of BxLiy Cluster for Lithium-Ion Batteries with Quantum ESPRESSO Program. Molecules. 2020; 25(14):3266. https://doi.org/10.3390/molecules25143266
Chicago/Turabian StyleÇipiloğlu, Mustafa Ali, and Ali Özkurt. 2020. "Theoretical Investigation on Molecular Structure and Electronic Properties of BxLiy Cluster for Lithium-Ion Batteries with Quantum ESPRESSO Program" Molecules 25, no. 14: 3266. https://doi.org/10.3390/molecules25143266
APA StyleÇipiloğlu, M. A., & Özkurt, A. (2020). Theoretical Investigation on Molecular Structure and Electronic Properties of BxLiy Cluster for Lithium-Ion Batteries with Quantum ESPRESSO Program. Molecules, 25(14), 3266. https://doi.org/10.3390/molecules25143266





