Abstract
Di(hetero)aryl ketones are important motifs present in natural products, pharmaceuticals or agrochemicals. In recent years, Pd(II)-catalyzed acylation of (hetero)arenes in the presence of an oxidant has emerged as a catalytic alternative to classical acylation methods, reducing the production of toxic metal waste. Different directing groups and acyl sources are being studied for this purpose, although further development is required to face mainly selectivity problems in order to be applied in the synthesis of more complex molecules. Selected recent developments and applications are covered in this review.
1. Introduction
Transition-metal direct C-H functionalization has emerged as an efficient, atom-economical and environmentally friendly synthetic tool for the preparation of complex multifunctional molecules, which is a good alternative to traditional cross-coupling chemistry. However, this transformation represents a significant challenge in chemical synthesis, largely due to the difficulties associated with the chemoselective activation of relatively inert C-H bonds. Palladium (II) catalysis has been widely used in C-H activation and functionalization of C(sp2)-H bonds of arenes, heteroarenes or even simple alkenes [1,2]. In this context, Pd(II)-catalyzed acylation of arenes in the presence of an oxidant has emerged as an interesting tool for the synthesis of di(hetero)aryl ketones, important motifs present in natural products, pharmaceuticals or agrochemicals. This procedure constitutes a catalytic alternative to classical acylation methods, reducing the production of toxic metal waste, although it still requires the use of a stoichiometric amount of an oxidant and, in some cases, additives such as acids or metal salts.
A schematic general mechanism proposal is depicted in Scheme 1 that implies three distinct fundamental steps. First, palladation of the arene or heteroarene 1 occurs to afford an arylpalladium(II) intermediate I, via C-H activation. The regioselectivity of this step is generally controlled by the use of a directing group (DG) [3] that provides the ortho-arylpalladium(II) intermediate I. On the other hand, the oxidant promotes de formation of an acyl radical II that, upon reaction with I, forms a Pd(IV) intermediate III after oxidation. Reductive elimination would afford the ketone 3, recovering the Pd(II) catalyst. The first example of Pd(II)-catalyzed acylation reaction with aldehydes was reported in 2009 by Cheng using 2-pyridine as directing group [4]. Since then, significant advances have been made in the field, by the assistance of a wide variety of directing groups for the metalation (DG in 1) and by the practical use of different precursors 2 for the acyl radical. The scope of the reaction is wide regarding the both the (hetero)aromatic ring 1 and the acyl radical equivalent 2. The use of the directing group on 1 allows the reaction to proceed with both electron-donating and electron-withdrawing substituents on the aromatic ring, although better reactivity is generally obtained with electron-rich aromatic rings. Regarding the acyl radical equivalents 2, in most cases aoryl groups are (ArCO) are introduced. Higher reactivities are generally observed when the aromatic ring of the acyl radical equivalent 2 is substituted with electron donating groups, what would be in accordance with a more nucleophilic radical intermediate II. The use aliphatic acyl equivalents, although possible, generally provides much lower yields. The use of peroxides, such as t-butylhydroperoxide (TBHP), as oxidants for the formation of the acyl radical is the most general, although more recently their generation via photoredox catalysis [5] has also been studied for these reactions.
Scheme 1.
Schematic mechanistic proposal for Pd(II)-catalyzed acylation via C-H activation.
Although most reactions take place according to Scheme 1, the actual mechanism is still not clear. For instance, the exact nature of species III formed after radical addition is not known in most cases. Density Functional Theory (DFT) calculations [6] support that the oxidative addition of an acyl radical, obtained by TBHP hydrogen atom abstraction from an aldehyde, to an arylpalladium (II) species would generate a Pd(IV) intermediate through a very exergonic process. Reductive elimination would lead to the acylated compound. An alternative aldehyde insertion mechanism was found to be unfavorable. Kinetic isotopic effect experiments have also been used to try to establish the rate-determining step. However, the results obtained for the KH/KD ratio in some cases support that the C-H bond cleavage is involved in the rate-determining step [6] but not in other cases [7]. Alternatively, binding of the substrate with palladium and the formation of an aroyl palladium π complex has been proposed [7]. Consequently, further studies are required for mechanistic understanding and further development is necessary for this type of reactions in order to be applied in the synthesis of more complex molecules. A review appeared in 2015 covering the major achievements in this field [8] and the topic has also been included in a broader review [9]. Therefore, the present article will not attempt to provide exhaustive coverage of the literature but it is intended to focus on significant recent advances in this type of Pd(II) catalyzed reactions in arenes and heteroarenes and their applications.
2. Acylation of Arenes
As indicated, the first example of Pd(II)-catalyzed acylation reaction was reported using 2-pyridine as directing group, aldehydes as the acyl precursor, although an aldehyde insertion mechanism was proposed in a Pd(II)/Pd(0)/Pd(II) cycle, using air as the oxidant [4] (Scheme 2a). Later, the use of TBHP as an oxidant was reported and a Pd(II)/Pd(IV)/Pd(II) cycle was proposed [10]. Homogeneous conditions are generally used for these reactions, using standard commercial Pd(II) pre-catalysts, such as Pd(OAc)2, Pd(TFA)2, PdCl2(CH3CN)2, sometimes in the presence of additives. However, it has been shown that a recyclable heterogeneous palladium complex under on-water conditions, using toluenes as the acyl source (Scheme 2b), can replace homogeneous catalysts. Polymer supported furan-2-ylmethanamine complex is stable and could be reused for 5 cycles without loss of efficiency [11]. α-Oxoacids have been also used as the acyl source in decarboxylative acylation reactions in the presence of silver salts [12]. Generally these reactions require the use of high temperatures (80–140 °C) but more recently it has been shown that aliphatic and aromatic aldehydes 5, α-oxoacids 8 and α-oxoaldehydes 9 can be reacted at room temperature in CH3CN, in presence of Pd(OAc)2 as catalyst and K2S2O8 (for 8 and 9) or TBHP (for aldehydes 5) as oxidants. The formation of an acyl radical intermediate is proposed in all cases. Generally, good yields of 6 are obtained, although aldehydes required longer reaction times (36 h) (Scheme 2c) [13]. Interestingly, when 2-pyrimidine was used as directing group instead, mixtures of mono- and diacylated products were obtained.

Scheme 2.
Acylation of arenes using a pyridine-directing group.
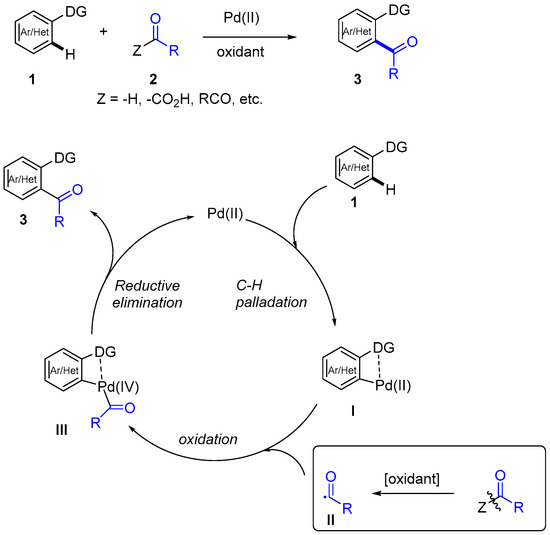
If the pyridine-directing group is linked to the aromatic ring through a heteroatom [7], it can be easily removed, so further transformations can be performed on the acylated compounds, significantly increasing the synthetic applicability of these transformations. Along these lines, the effect of the substitution on the pyridine ring was studied in the acylation of N-aryl-2-pyridinylamines 10 (Scheme 3) [14].
Scheme 3.
Pyridine as removable directing group. Effect of the substitution.
The introduction of an electron donating group in C-4 of the pyridine ring (R3 = OMe) promotes the reactivity of the Pd(II)-catalyzed C-H activation step. On the other hand, the amino nitrogen has to be protected (R2 = Boc, Ac, Bn, Moc, Piv) and Boc was selected as the most effective group. The reaction was extended to a series of aldehydes obtaining 11 in moderate to good yields, although in most cases significant amounts of the diacylated products were also obtained. Both protecting groups could be efficiently removed to obtain amine 12, which could also be transformed into an acridanone [14]. Besides the use of simple 2-pyridinyl, other related directing groups based on nitrogen coordination have been developed. As shown in Scheme 4, β-carboline was used as directing group for the selective acylation of ketones 13 with α-oxoacids 8 using K2S2O8 as oxidant. Diketones 14 were obtained in good yields, which could be derivatized to phthalizines 15. In this case, the pyridine nitrogen would facilitate the metalation by the formation of a six-membered palladacycle [15].

Scheme 4.
β-Carboline as directing group.
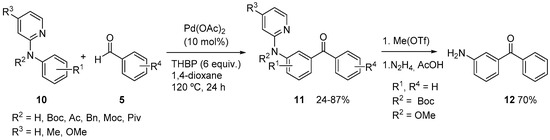
A very interesting application has been developed for the late stage functionalization of peptides, as shown in Scheme 5. Using a picolinamide directing group it was possible to carry out the selective acylation of phenylalanine-containing peptides 16 (from dipeptides to pentapeptides). The selective palladation would be favored by the bidentate directing group through the formation of a palladacycle intermediate such as IV that would later react with the acyl radical to generate a Pd(IV) intermediate. Aromatic, heteroaromatic and aliphatic aldehydes 5 could be used, using dicumyl peroxide (DCP) as oxidant in the presence of silver carbonate (TBHP gave lower yields). Under these conditions, generally excellent yields of the selectively monoacylated peptides 17 were obtained, minimizing the formation of diacylated compounds and with complete retention of stereochemistry [16].
Scheme 5.
Late stage functionalization of peptides using a picolinamide directing group.
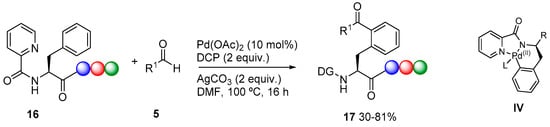
Besides pyridine, other nitrogen heterocycles have been used as directing groups, such as triazoles The acylation of 2-aryl-1,2,3-triazoles has been accomplished with aldehydes [17]. More recently, when 1,4-diaryl-1,2,3-triazoles 18 were used, the acylation using aldehydes 5 [18] or oxoacids 8 [19] took place selectively on the aromatic ring on C-4 of the triazole (Scheme 6). In the first case (Scheme 6a), the yields of 19 were improved in the presence of a ligand such as X-Phos. The reaction using oxoacids 8 in the presence of silver oxide (Scheme 6b) does not seem to involve the formation of an acyl radical intermediate, as no change in reactivity was found when the reactions were carried out in the presence of radical scavengers such as 2,2,6,6-tetramethylpiperidin-1-yl-oxydanyl (TEMPO). A mechanism (Scheme 6) is proposed in which coordination to the more electron rich N-3 atom of the triazole would favor the ortho-palladation. The oxoacid 8 would generate an acylsilver intermediate, which would transmetalate to generate an acylpalladium(II) intermediate. Reductive elimination produces 19 and the resulting palladium(0) species is reoxidized to Pd(II), closing the catalytic cycle. In addition, the higher directing ability of nitrogen over oxygen atom has been applied for the completely regioselective acylation of 3,5-diarylisoxazoles 20 with aldehydes, which are acylated at the ortho-position of the C-3 aromatic ring to obtain 21 [20] (Scheme 6c).
Scheme 6.
1,2,3-triazole and oxazole as directing groups.
The use of tertiary amides as directing groups in ortho-metalation reactions is widely recognized and employed [21,22]. Diethyl amides have been used in rhodium-catalyzed aroylation reactions with aldehydes [23]. However, as tertiary amides may undergo insertion of palladium into the N-C(O) bond [24], they have been used as aroyl sources in cross-coupling reactions. Besides, the weak coordination of the amide and the electron deficient aryl ring makes them difficult substrates for palladium catalyzed C-H activation. Despite this, two examples of decarboxylative acylation of benzamides 22 with α-oxocarboxylic acids 8 were described almost simultaneously (Scheme 7) [25,26]. In both cases, dialkyl or cyclic amides could be used as directing groups. Diethyl amides (R2 = Et) were selected as the best candidates in the first case (Scheme 7a, [25]) using (NH4)2S2O8 as oxidant, while dimethyl amides (R2 = Me) were selected for extension in the second case (Scheme 7b, [26]). The mode of activation of the amide is not clear. Control experiments suggest that the initial palladation is assisted by initial coordination to the amide nitrogen, although DFT calculations showed that an O-coordinated intermediate would be more favorable [25]. Besides, intermolecular KIE experiments (KH/KD = 2.5) suggest that the C-H cleavage might be the rate determining step. In this case, O-coordination to the amide is proposed [26].

Scheme 7.
Tertiary amides as directing groups. Acylation of benzamides.
Secondary amides, with a free NH group, have also been successfully used as directing groups in the acylation reaction with aldehydes [27] or α-oxoacids [26], leading to the formation of hydroxyisoindolones through acylation and subsequent cyclization. More recently, toluene derivatives 7 have been used as acyl source for the acylation of N-methoxybenzamides 24 (Scheme 8a) [28].

Scheme 8.
Secondary amides as directing groups. Formation of isoindolinones.
In this case, the mechanistic proposal differs from Scheme 1, although a coupling with an acyl radical is also involved. In agreement with previous reports [27], it is proposed that the secondary amide would be activated by TBHP, generating an amide nitrogen radical V, which forms intermediate VI after electrophilic palladation. TBHP also oxidizes the toluene 7 to the acyl radical, forming a high-valent Pd(IV) intermediate VII prior to a fast C-H activation. Hydroxyindolinones 25 would be obtained through reductive elimination and subsequent cyclization, as depicted in Scheme 8a. Amino acid-derived amides have also been used as bidentate directing groups (Scheme 8b) [29]. The mechanistic proposal is analogous to the one depicted in Scheme 8a, although in this case, C-H activation is assisted by prior coordination of Pd(II) to both the nitrogen atom and the carboxylic oxygen atom forming a palladacycle intermediate. The directing group on benzamides 26 is incorporated in the oxazoloisoindolinones 27 through a cascade reaction, with complete diastereoselectivity.
Besides benzamides, anilides have also been successfully acylated with aldehydes [30,31,32]. Also, an amide-directed metalation was applied in the selective C-7 acylation of indolines with aldehydes [33]. More recently, acylation of acetanilides 28 has been achieved with inexpensive benzylic alcohols 29 under aqueous conditions at 40 °C in the presence of a catalytic amount of TFA, obtaining very good yields of ketones 30, with wide functional group tolerance (Scheme 9a) [34]. A bimetallic palladium 6-membered cyclopalladated complex could be obtained and used as catalyst for this reaction. Recently, carbamates have also been used as directing groups for the acylation with α-oxoacids 8 (Scheme 9b) obtaining 32 in moderate to good yields [35]. Carbamate directing group could be easily removed obtaining amine 33 that could be derivatized to various heterocyclic systems, such as quinazoline or phenylquinoline.
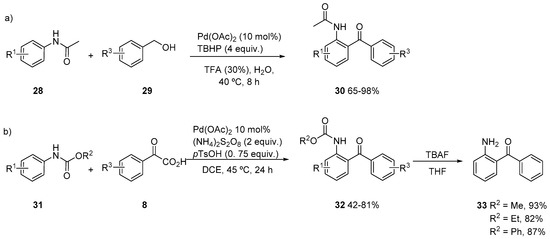
Scheme 9.
Acylation of acetanilides and N-aryl carbamates.
More recently, sulfonamides an N-sulfoximine amides have also been developed as directing groups (Scheme 10). Sulfonamides 34 are acylated efficiently with aldehydes 5 in good yields in only 15 min [36]. Aliphatic aldehydes can also be used, although with lower yields. Only a change in the solvent and an extension of the reaction time led to the direct formation of cyclic sulfonyl ketimines 36 in generally good yields (Scheme 10a). In the case of the N-sulfoximine benzamides 37, the reaction could be efficiently performed with α-oxocarboxylic acids 8 at room temperature. The reactivity was similar when electron withdrawing and electron donating groups were introduced in the sulfoximine unit. (Scheme 10b). The directing group could be easily hydrolyzed to obtain the corresponding carboxylic acid 39 or directly transformed in other heterocyclic systems, such as phthalazinone 40 [37].

Scheme 10.
Acylation of sulfonamides and N-sulfoximine amides.
Besides these examples, other nitrogen containing directing groups have been also used. For instance, azobenzenes 41 have been selectively ortho-acylated with aldehydes [38], also using aqueous conditions [39] and with α-oxoacids 8 [40], even at room temperature [41]. The acylated compounds 42 could be very efficiently transformed into indazoles 43 (Scheme 11a). More recently, α-hydroxyl ketones 44 have been identified as acylating agents (Scheme 11b). In most examples, symmetrically substituted azoarenes are used, leading to the selective formation of monoacylated compounds, although excellent selectivities could also be obtained when non-symmetrically substituted azoarenes 41 were used, depending on the substitution pattern. This acylation reaction could be applied to the synthesis of a liver(X) receptor agonist 43a. It is proposed that the hydroxyketone is oxidized to the corresponding diketone, generating the acyl radical. Both acyl fragments are transferred, so symmetrically substituted 44 have to be used to obtain selective reactions [42].

Scheme 11.
Acylation of azoarenes.
Nitrosoamines are also effective directing groups (Scheme 12). Thus, nitrosoanilines 45 could be acylated with oxoacids 8 at room temperature, using 5 mol % of the palladium catalyst. Ketones 46 are obtained in good yields, with wide functional group tolerance. The nitroso group could be removed or transformed, as exemplified in Scheme 12 for the two step synthesis of indole 47 [43]. Related examples have also been described [44,45]. The mechanism in these cases is still not clear but the formation of an intermediate acyl radical from the ketoacid 8 is not proposed. Instead, palladation of nitrosoanilines 45 lead to an intermediate IX, which would react with ketoacid 8 to obtain a Pd(II)carboxylate such as X. Decarboxylation would lead to an acyl-palladium(IV) intermediate XI after oxidation.

Scheme 12.
Nitrosoamine as directing group.
To finish this section, an important contribution is depicted in Scheme 13. Recently, the combination of visible light photoredox catalysis with transition metal catalysis has attracted much attention, as it can open opportunities for new reactivity [46]. In this context, it has been shown that the acylation reaction of acetanilides 28 [47] (Scheme 13a) and azoarenes 41 [48] (Scheme 13b) can be carried out at room temperature using a combination of a photoredox catalyst with a palladium(II) catalyst. In both cases, the reaction would work on a Pd(II)/Pd(IV) catalytic cycle, analogous to the one depicted in Scheme 1. This cycle would be coupled with the corresponding photocatalytic cycle, through which the acyl radical is generated from the oxoacid 8. The presence of the acyl radical has been supported by TEMPO trapping experiments and the presence of oxygen (air) is required. In both cases, several photoredox catalysts were screened and Eosin Y and the acridinium salt perchlorate PC-A (Fukuzumi salt) were selected as the best candidates. In both cases, yields of the ketones 30 and 42 are competitive with the ones obtained in the presence of an oxidant (Scheme 8 and Scheme 10), with the advantage of using milder conditions in the absence of an excess of oxidant. The proposed mechanism is illustrated in Scheme 13a for the reaction of acetanilides 28. Palladium catalytic cycle would start by a C-H activation to form palladacycle XII, which reacts with the acyl radical to afford a Pd(III) intermediate XIII. A one electron oxidation via superoxide radical anion generates a Pd(IV) intermediate XIV. Reductive elimination leads to 30 and regenerates the Pd(II) catalyst. The photocatalytic cycle starts with the visible irradiation of Eosin Y to take it to the excited state (Eosin Y*). One electron oxidation of the ketoacid 8 generates the acyl radical, after loss of CO2 and Eosin Y radical anion (Eosin Y−·). Electron transfer by molecular oxygen regenerates Eosin Y and produces superoxide radical anion (detected by Electron Spin Resonance (ESR)), who acts as an oxidant in the palladium cycle.

Scheme 13.
Photoredox/palladium catalyzed acylation of acetanilides and azoarenes.
3. Acylation of Heteroarenes
The Pd(II)-catalyzed oxidative acylation of heteroarenes has been less developed than the acylation of arenes covered in the previous section. In some cases, it is possible to take advantage of the electronic properties of the heteroaromatic ring (mainly electron rich), so the C-H palladation step can be performed without the need of a directing group. However, directing groups are frequently used to overcome the electronic bias and direct the metalation to other positions in the ring. Among them, the most frequently used are nitrogen-based directing groups (2-pyridine, 2-pyrimidine). The acylation of indole derivatives has received much attention. It has been shown that the Pd(II)-catalyzed acylation of N-alkylindoles with aldehydes in the presence of TBHP occurs selectively at the C-3 position, through an acyl radical insertion into the initially generated 3-indoly-Pd(II) intermediate [49]. However, C-2 selective acylation of indoles has been described using 2-pyrimidine or 2-pyridine as a directing group on nitrogen and α-oxocarboxylic acids [50] and aldehydes [51]. More recently, using 2-pyrimidine as directing group, it has been shown that the acylation of indoles 48 can be achieved with both aliphatic and aromatic aldehydes 5 at the C-2 position (Scheme 14a) [52]. The directing group can be efficiently removed leading to 2-acylindoles 51. Besides, a second metalation at C-7 can also take place, so 2,7-diacylated indoles 50 can be obtained in good yields, using a larger excess of aldehyde. If the reaction is carried out sequentially with different aldehydes 5, non-symmetrically substituted diacylindoles 50 could also be obtained (Scheme 14 b) [52]. In a similar way, diketones 52 (Scheme 14c) [53] and toluene derivatives 7 (Scheme 14d) [54] have been used as acyl surrogates. In all cases, the oxidative formation of an acyl radical from 5, 7 or 52 is proposed, so the mechanistic proposal would be in accordance with the general mechanism shown in Scheme 1. In all cases, high temperatures and long reaction times are required but it has been shown that the use of an acid additive (pivalic acid, Scheme 14d) increases the reaction rate, presumably by generating more electrophilic palladium species and, consequently, facilitating the C-H activation event. However, the acid may also have a detrimental effect by protonation of the substrate. Both with toluenes 7 and aromatic aldehydes 5, the introduction of electron donating groups on the aromatic ring leads to increased yields of 49, which would be in accordance with a more nucleophilic acyl radical. However, the opposite trend was observed with diketones 52. Interestingly, when other directing groups, such as sulfonylpyridyl, Boc or N,N-dimethylcarbamoyl, were tested, no reaction took place [54], which is in contrast with related rhodium-catalyzed acylation reactions [55].
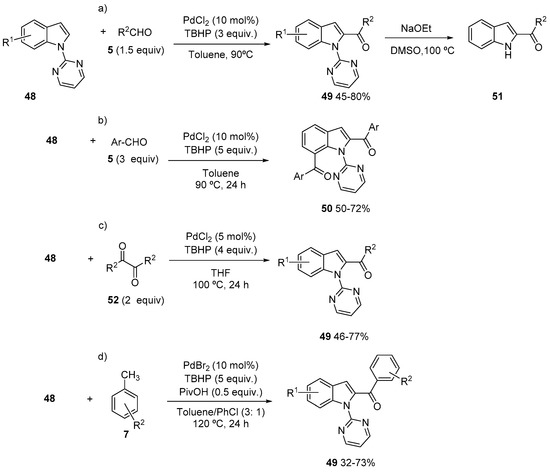
Scheme 14.
Pyrimidine-directed C-2 acylation of indoles.
Dual visible light photoredox/palladium catalysis has been also developed for the acylation of indole, using pyrimidine as directing group and aldehydes as the acyl source. Thus, indoles 48 could be efficiently acylated at room temperature in excellent yields with a wide variety of aromatic, heteroaromatic and aliphatic aldehydes 5 (Scheme 15a) [56]. The reaction was carried out both in batch and into a continuous-flow micro reactors, under blue LED light, using fac-[Ir(ppy)3] as photoredox catalyst and TBHP as the oxidant. In general, much shorter reaction times (2 h vs. 20 h), decreased catalyst loading and higher yields were obtained when the reaction was carried out in a continuous-flow reactor (Scheme 15a). Almost simultaneously, a dual catalytic system that uses a ruthenium photoredox catalyst was reported, also at room temperature with consistently good yields (Scheme 15b) [57]. As in the previously discussed examples (Scheme 13), the Pd(II)/Pd(IV) cycle for the C-H activation and acylation would be combined with the photoredox cycle but in these cases, the presence of an oxidant (TBHP) is also necessary. A mechanistic proposal is depicted in Scheme 15. In the presence of light, Ir3+ or Ru2+ are excited to Ir3+* or Ru2+*. Single electron transfer to TBHP produces the t-butoxy radical that, in turn, abstracts a hydrogen from the aldehyde 5 to generate the acyl radical. The addition of the acyl radical to palladium (II) intermediate XV generates a Pd(III) intermediate XVI, which is oxidized by Ru3+ or Ir4+ closing the photocatalytic cycle and generating Pd(IV) intermediate XVII. Reductive elimination produces 49 and regenerates de Pd(II) catalyst.

Scheme 15.
Photoredox/palladium catalyzed C-2 acylation of indoles.
Other directing groups have also been used for C-2 acylation. As shown in Scheme 16, the amine directed palladation of indoles 53 was possible through the formation of a six-membered ring palladacycle. Kinetic isotopic effect (KIE) experiments suggested that the cleavage of the C-H would be rate determining. The acylation takes place efficiently with a variety of aromatic α-oxoacids 8, obtaining the indolo[1,2-a]quinazolines 54 after condensation. The reaction takes also place in the presence of TEMPO and other radical scavengers, suggesting that an acyl radical is not involved. The authors propose an alternate Pd(II)/Pd(0) mechanism, in which the oxidant is required for the reoxidation of the palladium catalyst [58].

Scheme 16.
Amine directed acylation/cyclization. Access to indolo[1,2-a]quinazolines.
C-4 position of indoles 55 can also be selectively acylated using a ketone carbonyl group in C-3 as directing group (Scheme 17) [59]. The metalation takes place with complete regioselectivity at the C-4 position, through the formation of a six-membered palladacycle, which would be more favorable than C-2 metalation that would imply a more strained five-membered palladacycle. The choice of the protecting group on the nitrogen atom is also critical for reactivity.

Scheme 17.
Ketone directed selective C-4 acylation of indoles.
In contrast with the examples discussed for indoles, only a few examples of acylation of pyrroles have been described. When 2-pyrimidine was used as directing group, mixtures of C2-acylated pyrrole and the corresponding 2,5-diacylated product were obtained in modest yields [53]. Diacylated pyrroles have also been obtained using 2-pyridine as directing group on pyrrole, under conditions optimized for indole systems [51]. However, we have recently shown that the use of 2-pyrimidine as directing group, and the use of an acidic additive (PivOH), allows the C-2 acylation of pyrrole 57 with aldehydes 5 in moderate to good yields (Scheme 18) [60]. However, in most of the cases, a minor amount of the corresponding 2,5-diacylated pyrrole was also obtained. We reasoned that a change in the directing group, introducing a substituent at C-3, could result in a steric interaction that may prevent the adoption of the required conformation to assist the second palladation. In fact, when 3-methyl-2-pyridinyl was used as directing group (58), monoacylated pyrroles 60 were obtained with complete selectivity. Both directing groups could be easily removed, so selected acylated pyrroles have been used as intermediates in the synthesis of Celastramycin analogues, such as 62 and in an improved synthesis of Tolmetin 61.
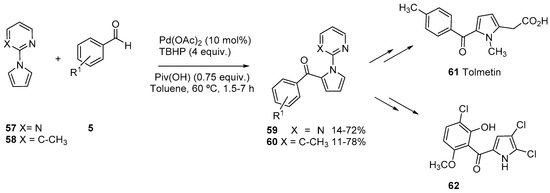
Scheme 18.
Pyrimidine and 6-methylpyridine-directed acylation of pyrroles.
2-Pyridine has been used for the acylation of carbazoles 63 (Scheme 19) [61] with various aromatic and aliphatic aldehydes 5. When only one equivalent of the aldehyde 5 was used, a mixture of mono- and diacylated carbazoles were obtained in low yields. However, the use of 4 equivalents of 5 led to the selective formation of diacylated carbazoles 64 in generally high yields in the case of aromatic aldehydes. Lower yields were obtained with aliphatic aldehydes. Interestingly, when 3,6-dihalogenated carbazoles 63 (X = Br, I) were used, monoacylated carbazoles 65 were selectively obtained.

Scheme 19.
Pyridine-directed acylation of carbazoles.
2-Pyridine has also been introduced at the C-2 position of benzothiophenes 66 and benzofurans 67 to obtain the C-acylated derivatives 68 and 69 using aromatic aldehydes (Scheme 20a) [62] or α-oxoacids 8 (Scheme 20b) [63] as the acyl sources. In the latter case, a Pd(0) pre-catalyst is employed that would be oxidized in situ.

Scheme 20.
Pyridine-directed acylation of benzofurans and benzothiophenes.
The examples of heteroarene acylation shown so far involve electron rich heteroaromatic rings that, in principle, could be more easily metalated with an electrophilic Pd(II) catalyst. However, selective C-8 palladium catalyzed acylation of quinolines has also been accomplished (Scheme 21) [64] using an N-oxide as the directing group. The formation of an N-oxide chelated palladacycle would favor the regioselective acylation of quinoline N-oxides 70 with a variety of aromatic α-oxoacids 8. Besides, quinolines with electron donating groups gave better yields of acylquinolines 71 than those with electron withdrawing groups. Intermolecular KIE experiments indicate that the C-H bond cleavage would be rate determining. Besides, the reaction of quinoline N-oxide with stoichiometric PdCl2 gave a chloride-bridged palladacycle dimer XVIII. This complex XVIII was reacted with an oxoacid 8 in the presence of oxidant, which suggests the formation of a five membered palladacycle in the catalytic cycle.

Scheme 21.
N-oxide directed C-8 acylation of quinolines.
4. Conclusions and Outlook
As has been shown through selected examples, the oxidative Pd(II)-catalyzed acylation is an effective alternative to the classical acylation methods. This methodology has been successfully applied to the acylation of arenes and heteroarenes, using different acyl sources. In most cases, a directing group is necessary to favor the initial palladation. Although highly selective reactions have been accomplished under relatively mild conditions, further development is required, also regarding the mechanistic aspects, to face selectivity problems in order to be applied in the synthesis of more complex molecules. Reactivity problems are also apparent as, in many cases, long reaction times and relatively high temperatures are required. Besides, the use aliphatic acyl equivalents, if possible, generally provides much lower yields. The use of other techniques, such as microwave (MW) irradiation or the combination of this metal catalyzed reaction with photoredox catalysis may indeed open new opportunities for development. In addition, the demand of more sustainable processes would foster the translation of homogeneous catalytic systems into heterogeneous catalysts that may be recovered and reused. Although there are interesting examples in related Pd(II)-catalyzed alkenylation reactions using metal-organic framewrks (MOFs) [65], the use of heterogeneous systems for acylation reactions is still underdeveloped. On the other hand, the use of other more abundant and less toxic 3d transition metals on [66], such as nickel [67] or cobalt [68] for these acylation reactions would be of a great importance.
Author Contributions
Writing—original draft preparation, C.S.; writing—review and editing, N.S. and E.L.; supervision, N.S. and E.L.; funding acquisition, N.S. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Ministerio de Economía y Competitividad (CTQ2016-74881-P), Ministerio de Ciencia e Innovación (PID2019-104148GB-I00) and Gobierno Vasco (IT1045-16).
Acknowledgments
Technical and human support provided by Servicios Generales de Investigación SGIker (UPV/EHU, MINECO, GV/EJ, ERDF and ESF) is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lei, A.; Shi, W.; Liu, W.; Zhang, H.; He, C. Oxidative Cross-Coupling Reactions; Wiley: Weinheim, Germany, 2017. [Google Scholar]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild Metal-catalyzed C–H Activation: Examples and Concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalization Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, S.; Wang, W.; Luo, F.; Cheng, J. Palladium-Catalyzed Acylation of sp2 C-H bond: Direct Access to Ketones from Aldehydes. Org. Lett. 2009, 11, 3120–3123. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Lei, Z.; Ngai, M.Y. Acyl Radical Chemistry via Visible-Light Photoredox Catalysis. Synthesis 2019, 51, 303–333. [Google Scholar] [CrossRef]
- Duan, P.; Yang, Y.; Ben, R.; Yan, Y.; Dai, l.; Hong, M.; Wu, Y.D.; Wang, D.; Zhang, X.; Zhao, J. Palladium-catalyzed Benzo[d]isoxazole Synthesis by C-H Activation/[4+1] Annulation. Chem. Sci. 2014, 5, 1574–1578. [Google Scholar] [CrossRef]
- Chu, J.H.; Chen, S.T.; Chiang, M.F.; Wu, M.J. Palladium Catalyzed Direct Ortho Aroylation of 2-Phenoxypyridines with Aldehydes and Catalytic Mechanistic Investigation. Organometallics 2015, 34, 953–966. [Google Scholar] [CrossRef]
- Wu, X.-F. Acylation of (Hetero)Arenes through C-H Activation with Aroyl Surrogates. Chem. Eur. J. 2015, 21, 12252–12265. [Google Scholar] [CrossRef]
- Hummel, J.R.; Boerth, J.A.; Ellman, J.A. Transition-Metal-Catalyzed C−H Bond Addition to Carbonyls, Imines and Related Polarized π Bonds. Chem. Rev. 2017, 117, 9163–9227. [Google Scholar] [CrossRef]
- Baslé, O.; Bidange, J.; Shuai, Q.; Li, C.J. Palladium-Catalyzed Oxidative sp2 CH Bond Acylation with Aldehydes. Adv. Synth. Catal. 2010, 352, 1145–1149. [Google Scholar] [CrossRef]
- Perumgani, C.P.; Parvathaneni, S.P.; Keesara, S.; Mandapati, M.R. Recyclable Pd(II) Complex Catalyzed Oxidative sp2 C-H Bond Acylation of 2-Aryl Pyridines with Toluene Derivatives. J. Organomet. Chem. 2016, 822, 189–195. [Google Scholar] [CrossRef]
- Li, M.; Ge, H. Decarboxylative Acylation of Arenes with α-Oxocarboxylic Acids via Palladium-Catalyzed C-H Activation. Org. Lett. 2010, 12, 3464–3467. [Google Scholar] [CrossRef] [PubMed]
- Hossian, A.; Manna, M.K.; Manna, K.; Jana, R. Palladium-catalyzed Decarboxylative, Decarbonylative and Dehydrogenative C(sp2)–H Acylation at Room Temperature. Org. Biomol. Chem. 2017, 15, 6592–6603. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Chiang, M.F.; Li, C.W.; Su, Z.H.; Lo, S.C.; Wu, M.J. Palladium Catalyzed Late Stage ortho-C-H Bond Aroylation of anilines using 4-Methoxy-2-pyridinyl as a Removable Directing Group. Organometallics 2019, 38, 2105–2119. [Google Scholar] [CrossRef]
- Kolle, S.; Batra, S. β-Carboline-directed Decarboxylative Acylation of Ortho-C(sp2)–H of the Aryl Ring of Aryl(β-carbolin-1-yl)methanones with α-Ketoacids under Palladium Catalysis. RSC Adv. 2016, 6, 50658–50665. [Google Scholar] [CrossRef]
- San Segundo, M.; Correa, A. Pd-catalyzed Site-selective C(sp2)–H Radical Acylation of Phenylalanine Containing Peptides with Aldehydes. Chem. Sci. 2019, 10, 8872–8879. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Q.; Yu, X.; Kuang, C. Palladium-Catalyzed Acylation of 2-Aryl-1,2,3-triazoles with Aldehydes. Adv. Synth. Catal. 2014, 356, 961–966. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Z.; Liu, Y.; Xie, K.; Jiang, Y. Palladium-Catalyzed Acylation of Arenes by 1,2,3-Triazole-Directed C–H Activation. Eur. J. Org. Chem. 2016, 5971–5979. [Google Scholar] [CrossRef]
- Ma, X.; Huang, H.; Yang, J.; Feng, X.; Xie, K. Palladium-Catalyzed Decarboxylative N-3-ortho-C–H Acylation of 1,4-Disubstituted 1,2,3-Triazoles with α-Oxocarboxylic Acids. Synthesis 2018, 50, 2567–2576. [Google Scholar] [CrossRef]
- Banerjee, A.; Bera, A.; Santra, S.K.; Guin, S.; Patel, B.K. Palladium-catalysed Regioselective Aroylation and Acetoxylation of 3,5-Diarylisoxazole via Ortho C–H Functionalisations. RSC Adv. 2014, 4, 8558–8566. [Google Scholar] [CrossRef]
- Snieckus, V. Directed Ortho Metalation. Tertiary Amide and O-carbamate Directors in Synthetic Strategies for Polysubstituted Aromatics. Chem. Rev. 1990, 90, 879–933. [Google Scholar] [CrossRef]
- Whisler, M.C.; MacNeil, S.; Snieckus, V.; Beak, P. Beyond Thermodynamic Acidity: A Perspective on the Complex-Induced Proximity Effect (CIPE) in Deprotonation Reactions. Angew. Chem. Int. Ed. 2004, 43, 2206–2225. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, E.; Kim, A.; Lee, Y.; Chi, K.W.; Kwak, J.H.; Jung, Y.H.; Kim, I.S. Rhodium-Catalyzed Oxidative ortho-Acylation of Benzamides with Aldehydes: Direct Functionalization of the sp2 C–H Bond. Org. Lett. 2011, 13, 4390–4393. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar] [CrossRef]
- Laha, J.K.; Patel, K.V.; Sharma, S. Palladium-Catalyzed Decarboxylative Ortho-Acylation of Tertiary Benzamides with Arylglyoxylic Acids. ACS Omega. 2017, 2, 3806–3815. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Yao, J.-P.; Li, Z.-Y.; Li, Q.-L.; Lin, H.-S.; Wang, G.-W. Palladium-Catalyzed Decarboxylative Ortho-Acylation of Benzamides with α-Oxocarboxylic Acids. J. Org. Chem. 2017, 82, 12715–12725. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, N.; Huang, J.; Lu, S.; Zhu, Y.; Yu, X.; Zhao, K. Efficient Synthesis of Hydroxyl Isoindolones by a Pd-Mediated C-H Activation/Annulation Reaction. Chem. Eur. J. 2013, 19, 11184–11188. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, L.; Xu, B.; Zhao, L.; Zhou, J.; Zhang, H. Palladium-Catalyzed C-H Bond Ortho Acylation/Annulation with Toluene Derivatives. Asian J. Org. Chem. 2016, 5, 62–65. [Google Scholar] [CrossRef]
- Jing, K.; Wang, X.-N.; Wang, G.-W. Diastereoselective Synthesis of Oxazoloisoindolinones via Cascade Pd-Catalyzed Ortho-Acylation of N-Benzoyl α-Amino Acid Derivatives and Subsequent Double Intramolecular Cyclizations. J. Org. Chem. 2019, 84, 161–172. [Google Scholar] [CrossRef]
- Chan, C.W.; Zhou, Z.; Yu, W.Y. Palladium(II)-Catalyzed Direct Ortho-C−H Acylation of Anilides by Oxidative Cross-Coupling with Aldehydes using Tert-Butyl Hydroperoxide as Oxidant. Adv. Synth. Catal. 2011, 353, 2999–3006. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Mao, F.; Li, X.; Kwong, F.Y. Palladium-Catalyzed Oxidative C−H Bond Coupling of Steered Acetanilides and Aldehydes: A Facile Access to ortho-Acylacetanilides. Org. Lett. 2011, 13, 3258–3261. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Li, P.; Zhou, W. Palladium(II)-Catalyzed Direct Ortho-C−H Acylation of Anilides by Oxidative Cross-Coupling with Aldehydes using Tert-Butyl Hydroperoxide as Oxidant. Chem. Eur. J. 2011, 17, 10208–10212. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Sharma, S.; Mishra, N.K.; Han, S.; Park, J.; Oh, H.; Ha, J.; Yoo, H.; Jung, H.-Y.; Kim, I.S. Direct and Site-Selective Palladium-Catalyzed C-7 Acylation of Indolines with Aldehydes. Adv. Synth. Catal. 2015, 357, 594–600. [Google Scholar] [CrossRef]
- Luo, F.; Yang, J.; Li, Z.; Xiang, H.; Zhou, X. Palladium-Catalyzed C–H Bond Acylation of Acetanilides with Benzylic Alcohols under Aqueous Conditions. Eur. J. Org. Chem. 2015, 2463–2469. [Google Scholar] [CrossRef]
- Li, Q.-L.; Li, Z.-Y.; Wang, G.-W. Palladium-Catalyzed Decarboxylative Ortho-Acylation of Anilines with Carbamate as a Removable Directing Group. ACS. Omega. 2018, 3, 4187–4198. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Panda, N. Palladium-Catalyzed Ortho-Benzoylation of Sulfonamides through C−H Activation: Expedient Synthesis of Cyclic N-Sulfonyl Ketimines. Adv. Synth. Catal. 2020, 362, 561–571. [Google Scholar] [CrossRef]
- Das, P.; Biswas, P.; Guin, J. Palladium-Catalyzed Decarboxylative Ortho-C(sp2)−H Aroylation of N-Sulfoximine Benzamides at Room Temperature. Chem. Asian J. 2020, 15, 920–925. [Google Scholar] [CrossRef]
- Li, H.; Li, P.; Wang, L. Direct Access to Acylated Azobenzenes via Pd-Catalyzed C–H Functionalization and Further Transformation into an Indazole Backbone. Org. Lett. 2013, 15, 620–623. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Huang, H.; Deng, G.J. Palladium-Catalyzed Oxidative Direct Ortho-C–H Acylation of Arenes with Aldehydes under Aqueous Conditions. Eur. J. Org. Chem. 2015, 2015, 7919–7925. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, D.-D.; Wang, G.-W. Palladium-Catalyzed Decarboxylative Ortho Acylation of Azobenzenes with α-Oxocarboxylic Acids. J. Org. Chem. 2013, 78, 10414–10420. [Google Scholar] [CrossRef]
- Li, H.; Li, P.; Tan, H.; Wang., L.A. Highly Efficient Palladium-Catalyzed Decarboxylative ortho-Acylation of Azobenzenes with α-Oxocarboxylic Acids: Direct Access to Acylated Azo Compounds. Chem. Eur. J. 2013, 19, 14432–14436. [Google Scholar] [CrossRef]
- Majhi, B.; Ahammed, S.; Kundu, D.; Ranu, B.C. Palladium-Catalyzed Oxidative C−C Bond Cleavage of α-Hydroxyketones: Application to C−H Acylation of Azoarenes and Synthesis of a Liver(X) Receptor Agonist. Asian J. Org. Chem. 2015, 4, 154–163. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, L.; Chen, Y.; Zhou, Q.; Huang, J.W.; Miao, H.; Luo, H.B. Palladium-Catalyzed Decarboxylative Acylation of N-Nitrosoanilines with α-Oxocarboxylic Acids. J. Org. Chem. 2016, 81, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Guo, P.; Sun, W.; Li, Y.-M.; Sun, M.; Hua, C. Palladium-catalyzed Ortho-acylation of N-Nitrosoanilines with α-Oxocarboxylic Acids: A Convenient Method to Synthesize N-Nitroso Ketones and Indazoles. Tetrahedron Lett. 2016, 57, 2511–2514. [Google Scholar] [CrossRef]
- Yao, J.P.; Wang, G.W. Palladium-catalyzed Decarboxylative Ortho-acylation of N-Nitrosoanilines with α-Oxocarboxylic Acids. Tetrahedron Lett. 2016, 57, 1687–1690. [Google Scholar] [CrossRef]
- De Abreu, M.; Belmont, P.; Brachet, E. Synergistic Photoredox/Transition-Metal Catalysis for Carbon–Carbon Bond Formation Reactions. Eur. J. Org. Chem. 2020, 2020, 1327–1378. [Google Scholar] [CrossRef]
- Zhou, C.; Li, P.; Zhu, X.; Wang, L. Merging Photoredox with Palladium Catalysis: Decarboxylative Ortho-Acylation of Acetanilides with α-Oxocarboxylic Acids under Mild Reaction Conditions. Org. Lett. 2015, 17, 6198–6201. [Google Scholar] [CrossRef]
- Xu, N.; Li, P.; Xie, Z.; Wang, L. Merging Visible-Light Photocatalysis and Palladium Catalysis for C-H Acylation of Azo- and Azoxybenzenes with α-Keto Acids. Chem. Eur. J. 2016, 22, 2236–2242. [Google Scholar] [CrossRef]
- Kianmehr, E.; Kamezi, S.; Foroumadi, A. Palladium-catalyzed Oxidative C−H bond coupling of Indoles and Benzaldehydes: A New Approach to the Synthesis of 3-Benzoylindoles. Tetrahedron 2014, 70, 349–354. [Google Scholar] [CrossRef]
- Pan, C.; Jin, H.; Liu, X.; Cheng, Y.; Zhu, C. Palladium-catalyzed Decarboxylative C2-Acylation of Indoles with α-Oxocarboxylic Acids. Chem. Commun. 2013, 49, 2933–2935. [Google Scholar] [CrossRef]
- Yan, X.-B.; Shen, Y.-W.; Chen, D.-Q.; Gao, P.; Li, Y.-X.; Song, X.-R.; Liu, X.-Y.; Liang, Y.-M. Palladium-catalyzed C2-Acylation of Indoles with Aryl and Alkyl Aldehydes. Tetrahedron 2014, 70, 7490–7495. [Google Scholar] [CrossRef]
- Kumar, G.; Sekar, G. Pd-catalyzed Direct C2-Acylation and C2,C7-Diacylation of Indoles: Pyrimidine as an Easily Removable C–H Directing Group. RSC Adv. 2015, 5, 28292–28298. [Google Scholar] [CrossRef]
- Li, C.; Shu, S.; Wu, X.; Liu, H. Palladium-Catalyzed C2-Acylation of Indoles with α-Diketones Assisted by the Removable N-(2-Pyrimidyl) Group. Eur. J. Org. Chem. 2015, 2015, 3743–3750. [Google Scholar] [CrossRef]
- Zhao, Y.; Sharma, U.K.; Schröder, F.; Sharma, N.; Song, G.; Van der Eycken, E.V. Direct C-2 Acylation of Indoles with Toluene Derivatives via Pd(II)-catalysed C–H Activation. RSC Adv 2017, 7, 32559–32563. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Y.; Li, Y. Rhodium-catalyzed Oxidative C2-Acylation of Indoles with Aryl and Alkyl Aldehydes. Chem. Commun. 2012, 48, 5163–5165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.K.; Gemoets, H.P.L.; Schröder, F.; Nöel, T.; Van der Eycken, E.V. Merger of Visible-Light Photoredox Catalysis and C−H Activation for the Room-Temperature C-2 Acylation of Indoles in Batch and Flow. ACS Catal. 2017, 7, 3818–38232. [Google Scholar] [CrossRef]
- Manna, K.M.; Bairy, G.; Jana, R. Dual Visible-light Photoredox and Palladium(II) Catalysis for Dehydrogenative C2-Acylation of Indoles at Room Temperature. Org. Biomol. Chem. 2017, 15, 5899–5903. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, S.; Zhang, J.; Yu, J.; Zhang, Z.; Ji, F. Palladium-Catalyzed Primary Amine-Directed Decarboxylative Annulation of α-Oxocarboxylic Acids: Access to Indolo [1,2-a] Quinazolines. Adv. Synth. Catal. 2019, 361, 1798–1802. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Fan, J.; Xu, Q.; Xie, M. Selective C–H Acylation of Indoles with α-Oxocarboxylic Acids at the C4 Position by Palladium Catalysis. Chem. Commun. 2019, 55, 8102–8105. [Google Scholar] [CrossRef]
- Santiago, C.; Rubio, I.; Sotomayor, N.; Lete, E. Selective Pd(II)-catalyzed Acylation of Pyrrole with Aldehydes. Application to the Synthesis of Celastramycin analogues and Tolmetin. Eur. J. Org. Chem. 2020, (in press). [CrossRef]
- Maiti, S.; Burgula, L.; Chakraborti, G.; Dash, J. Palladium Catalyzed Pyridine Group Directed Regioselective Oxidative C-H Acylation of Carbazoles using Aldehydes as the Acyl Source. Eur. J. Org. Chem. 2017, 332–340. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, H.; Xie, C.; Han, J.; Li, G.; Pan, Y. Palladium-Catalyzed C3 Acylation of Benzofurans and Benzothiophenes with Aromatic Aldehydes by Cross-Dehydrogenative Coupling Reactions. Asian J. Org. Chem. 2013, 2, 1044–1047. [Google Scholar] [CrossRef]
- Gong, W.-J.; Liu, D.-X.; Li, F.-L.; Gao, J.; Li, H.-X.; Lang, J.-P. Palladium-catalyzed Decarboxylative C3-Acylation of Benzofurans and Benzothiophenes with α-Oxocarboxylic Acids via Direct sp2 C-H Bond Activation. Tetrahedron 2015, 71, 1269–1275. [Google Scholar] [CrossRef]
- Chen, X.; Cui, X.; Wu, Y. C8-Selective Acylation of Quinoline N-Oxides with α-Oxocarboxylic Acids via Palladium-Catalyzed Regioselective C−H Bond Activation. Org. Lett. 2016, 18, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Cirujano, F.G.; Leo, P.; Vercammen, J.; Smolders, S.; Orcajo, G.; De Vos, D.E. MOFs Extend the Lifetime of Pd(II) Catalyst for Room Temperature Alkenylation of Enamine-Like Arenes. Adv. Synth. Catal. 2018, 360, 3872–3876. [Google Scholar] [CrossRef]
- Gandeepan, P.; Muüller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C−H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, C.; Wang, P.; Zhang, Y.; Ge, H. Nickel-Catalyzed Decarboxylative Acylation of Heteroarenes by sp2 C−H Functionalization. Chem. Eur. J. 2014, 20, 1–5. [Google Scholar] [CrossRef]
- Yang, K.; Chen, X.; Wang, Y.; Li, W.; Kadi, A.A.; Fun, H.-K.; Sun, H.; Zang, Y.; Li, G.; Lu, H. Cobalt-Catalyzed Decarboxylative 2-Benzoylation of Oxazoles and Thiazoles with α-Oxocarboxylic Acids. J. Org. Chem. 2015, 80, 11065–11072. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).