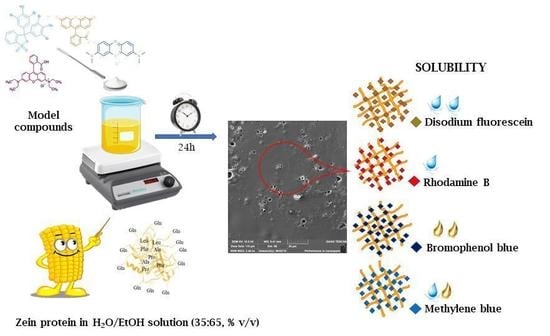
Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zein Gels
2.3. Rheological Measurements of Zein Gels
2.4. In Vitro Release of Model Compounds from Zein Gels
2.5. Statistical Analysis
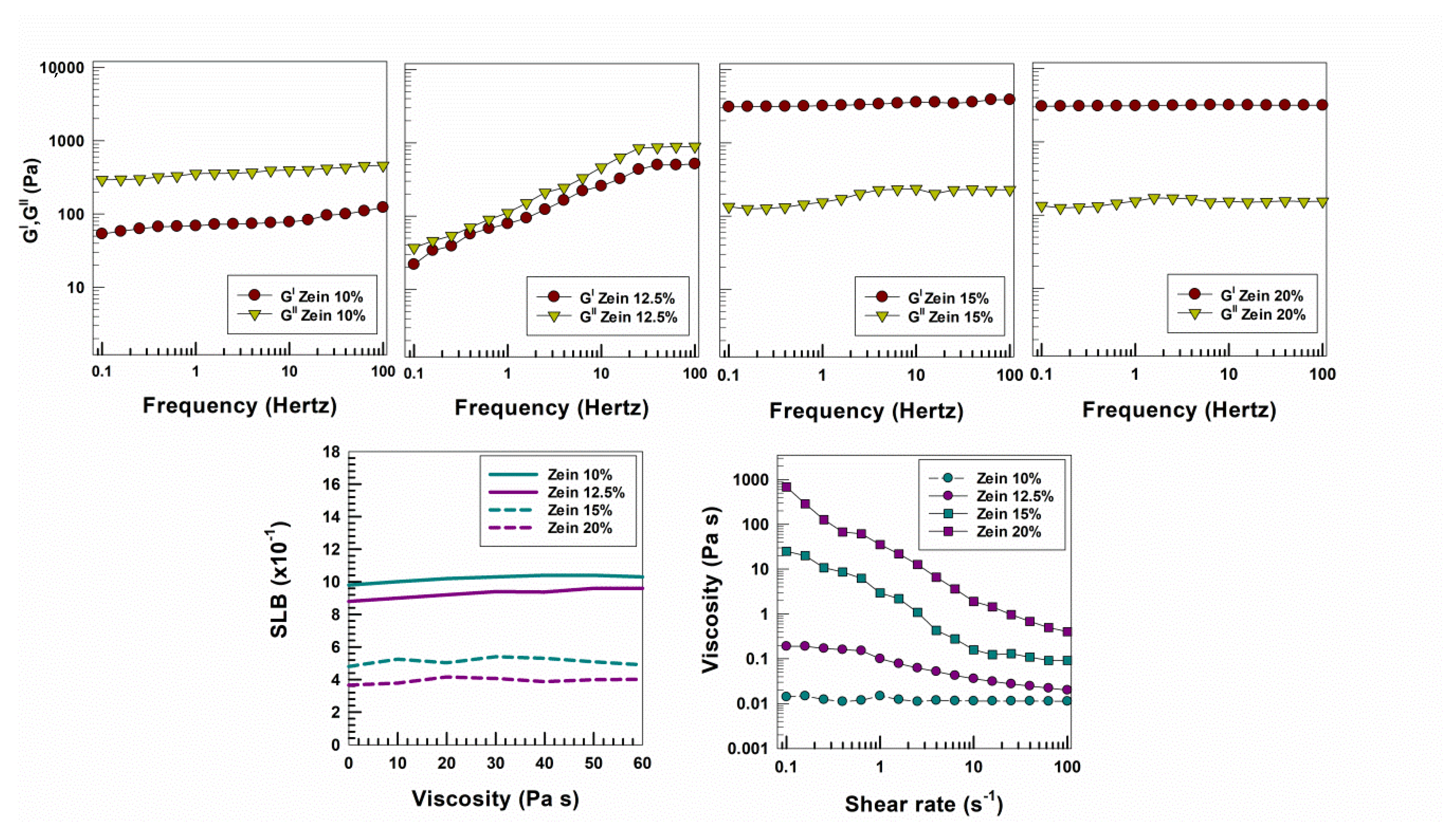
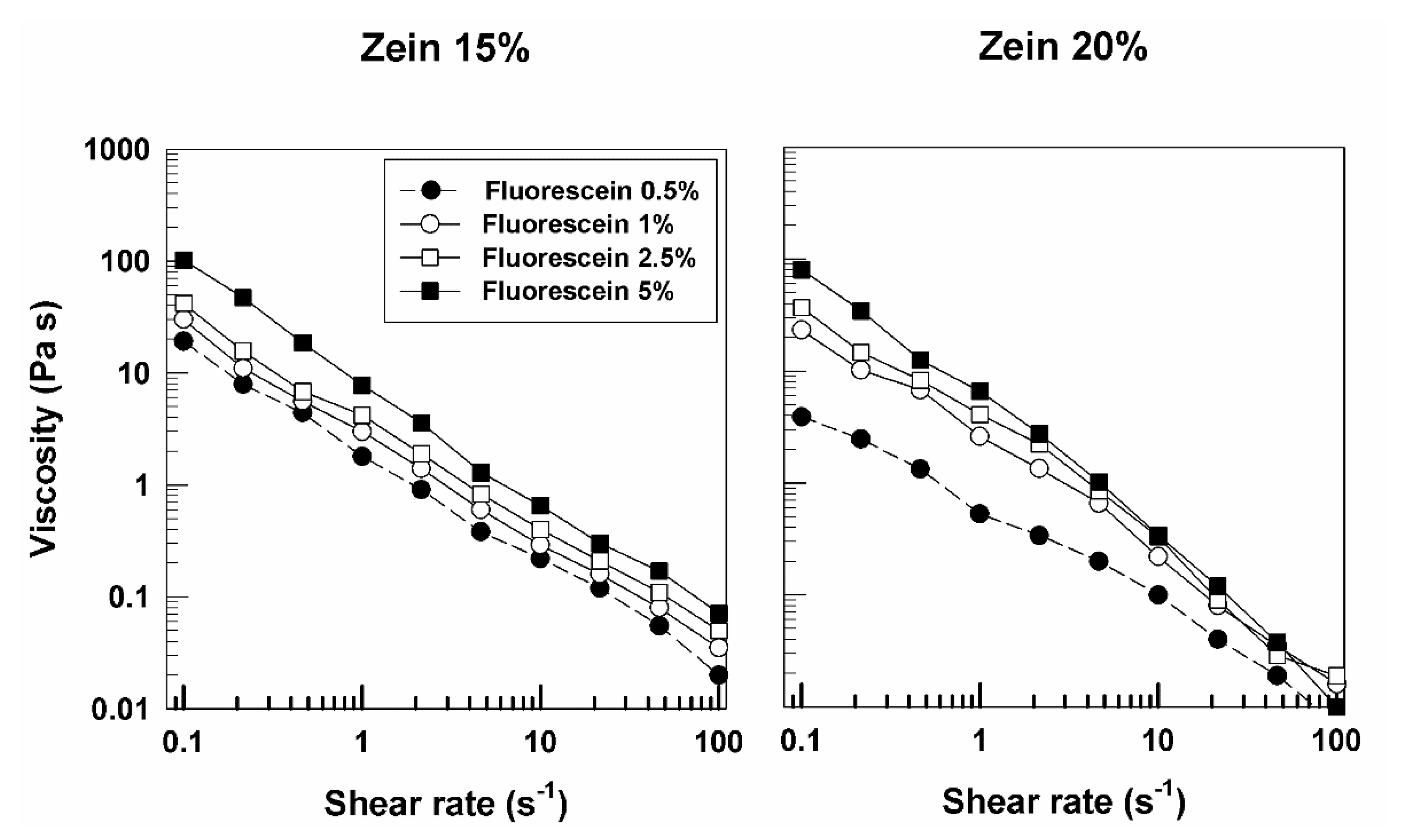
3. Results and Discussion
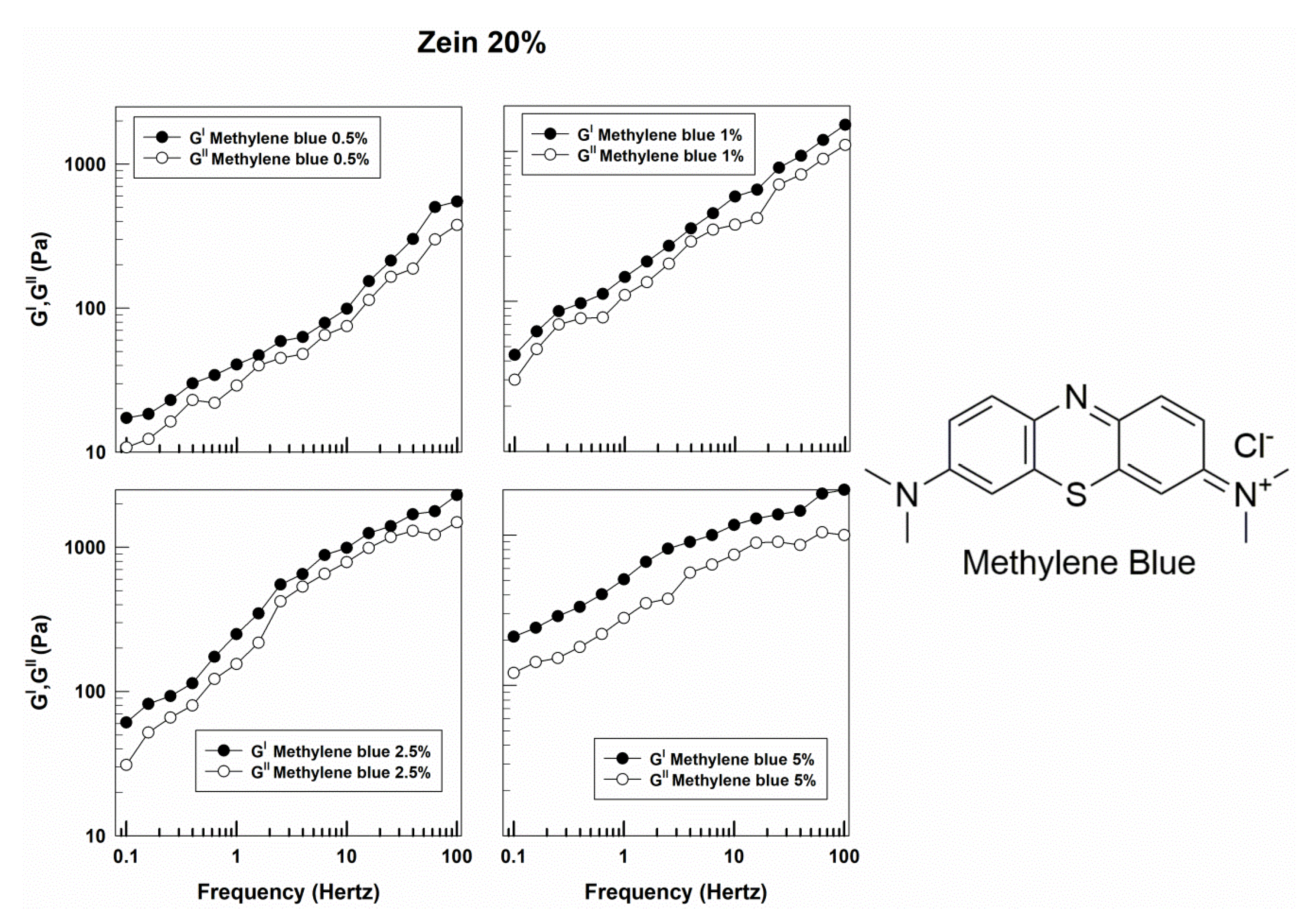
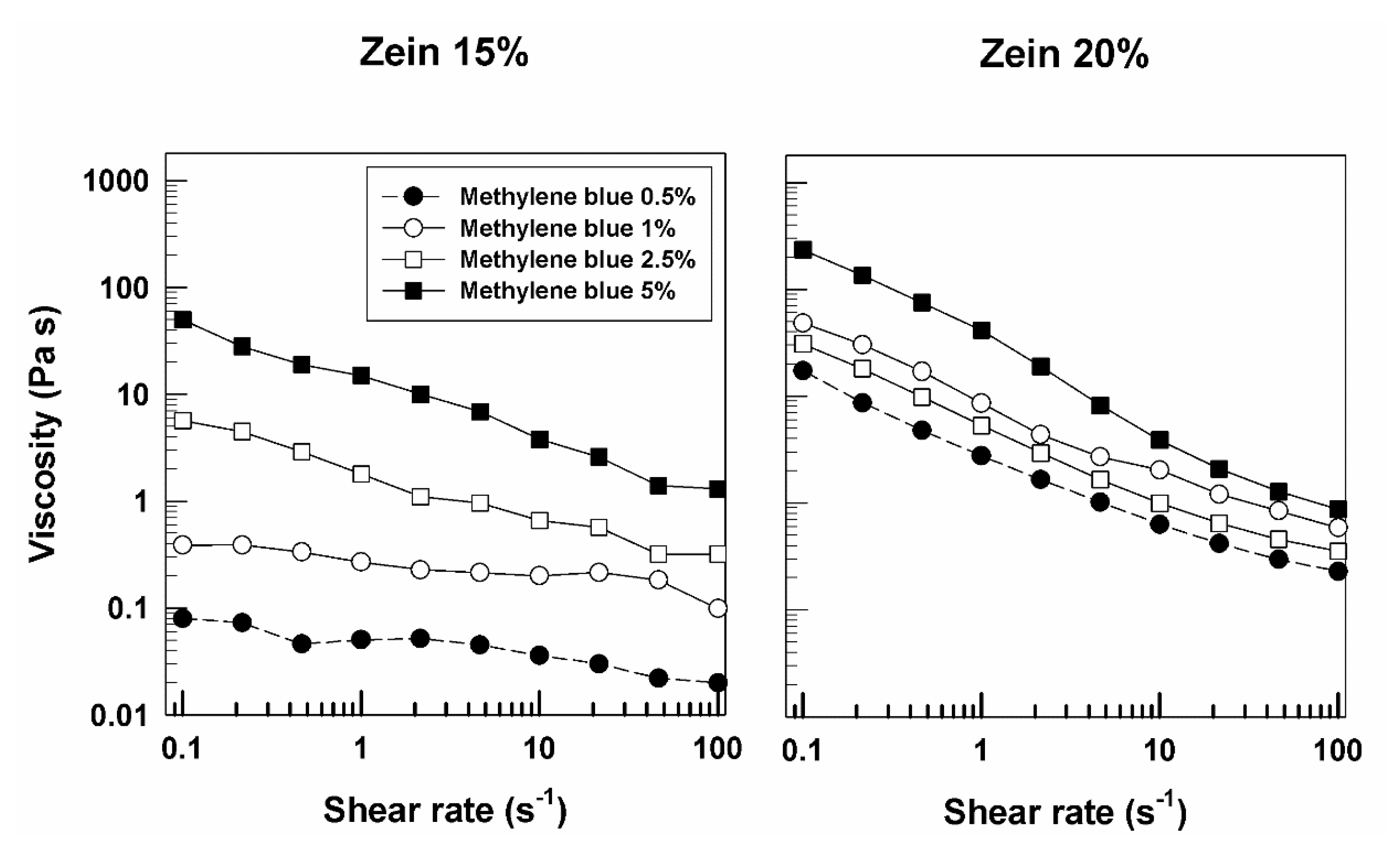
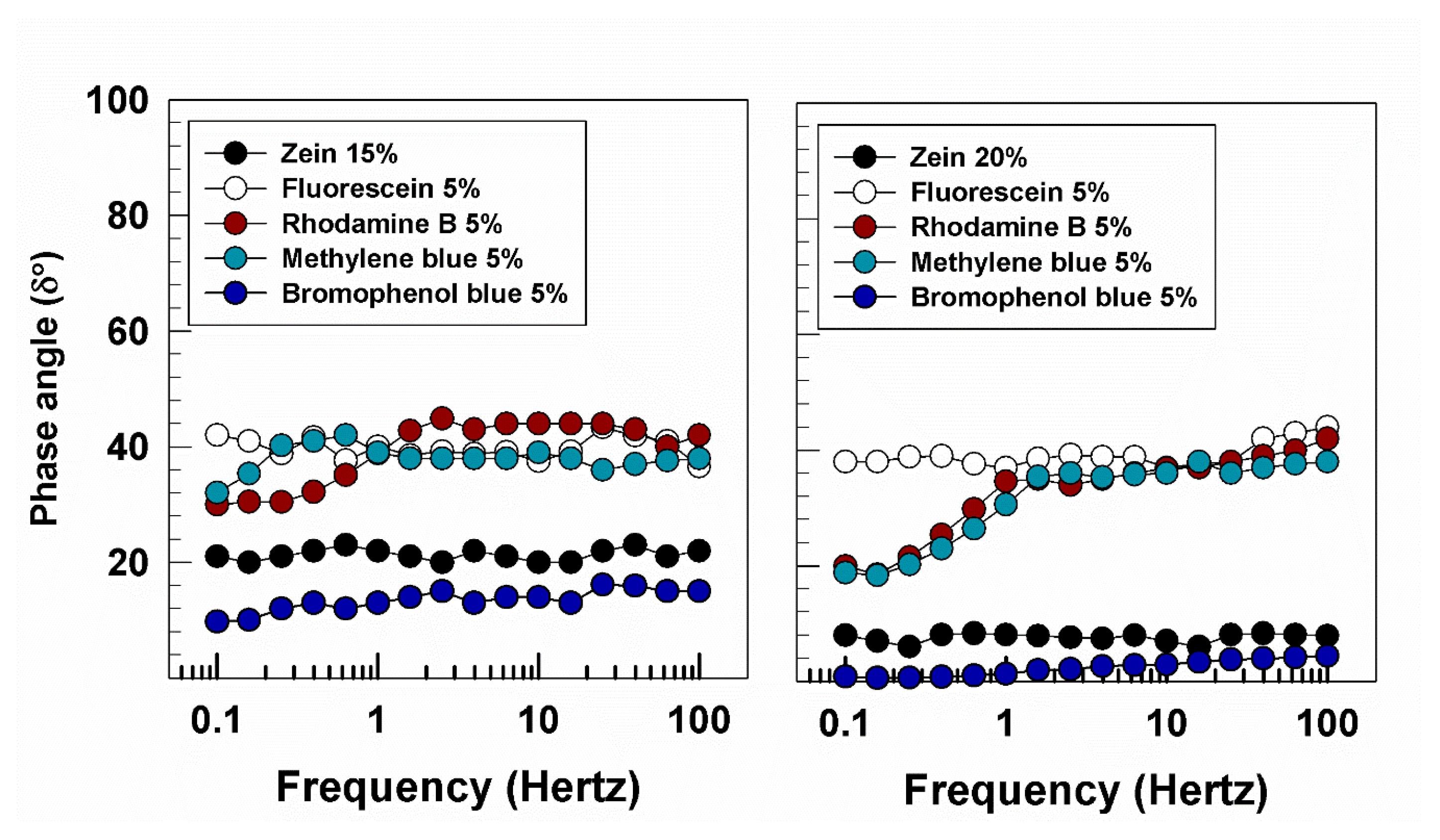
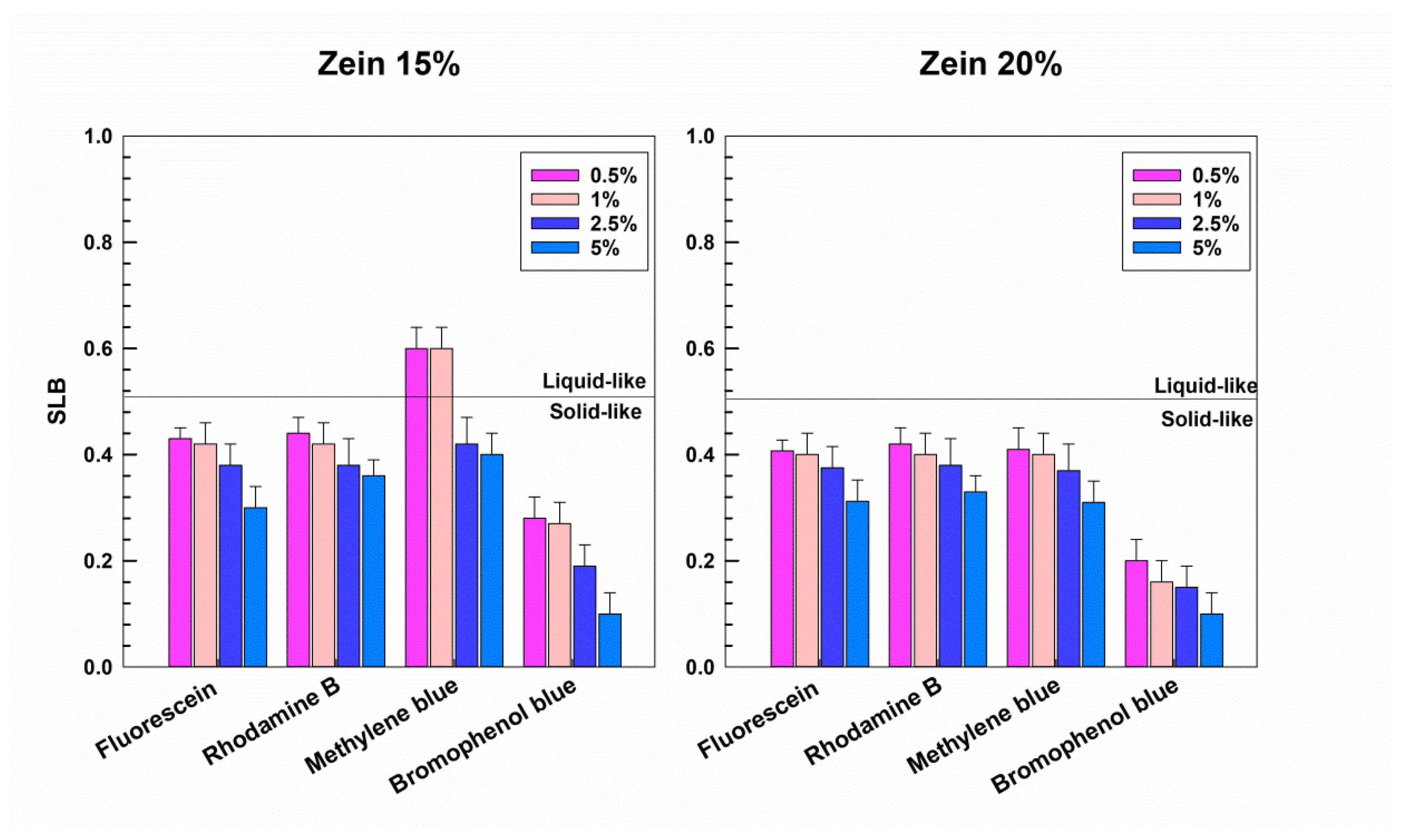
3.1. Rheological Features of Zein Gels
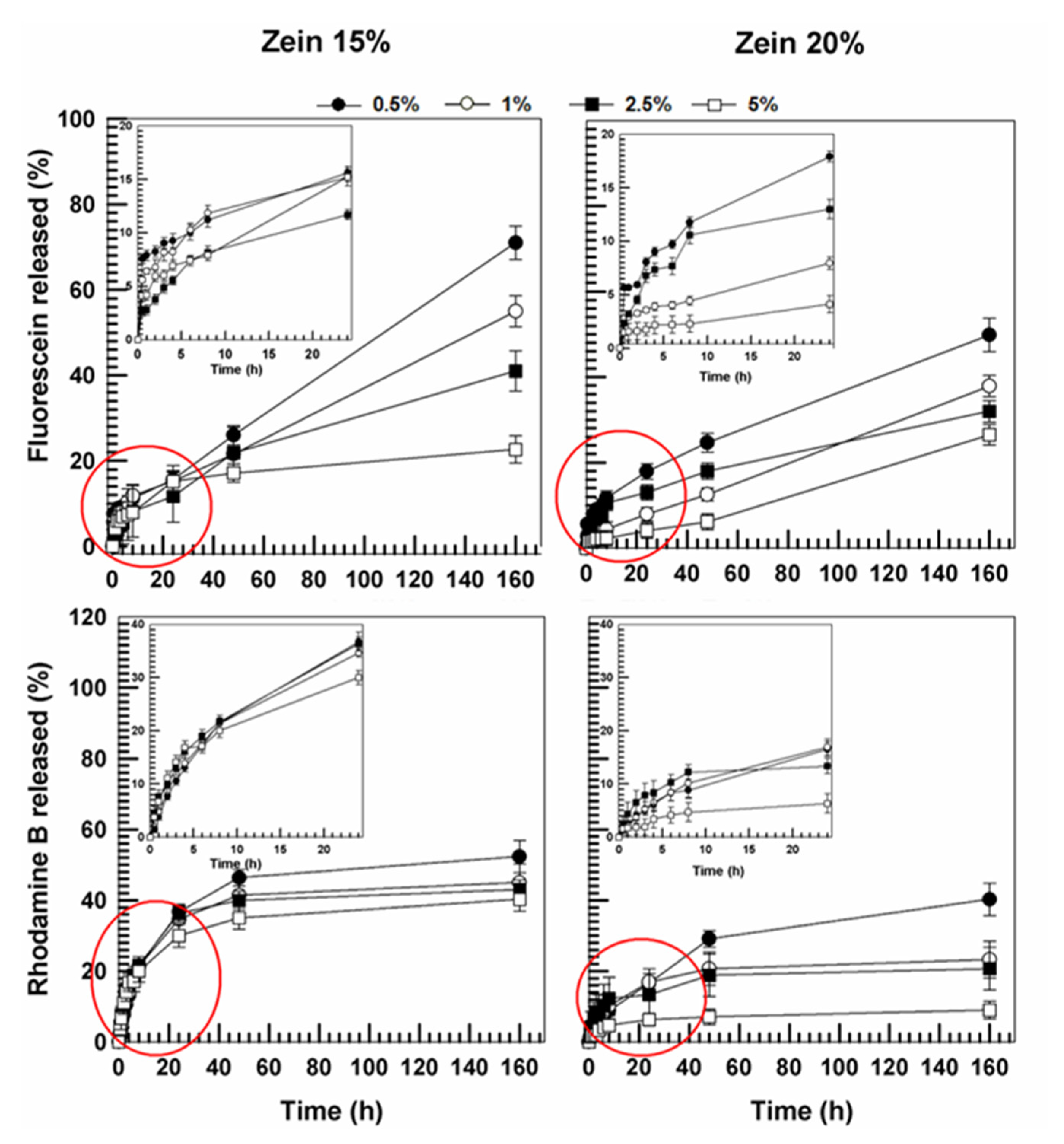
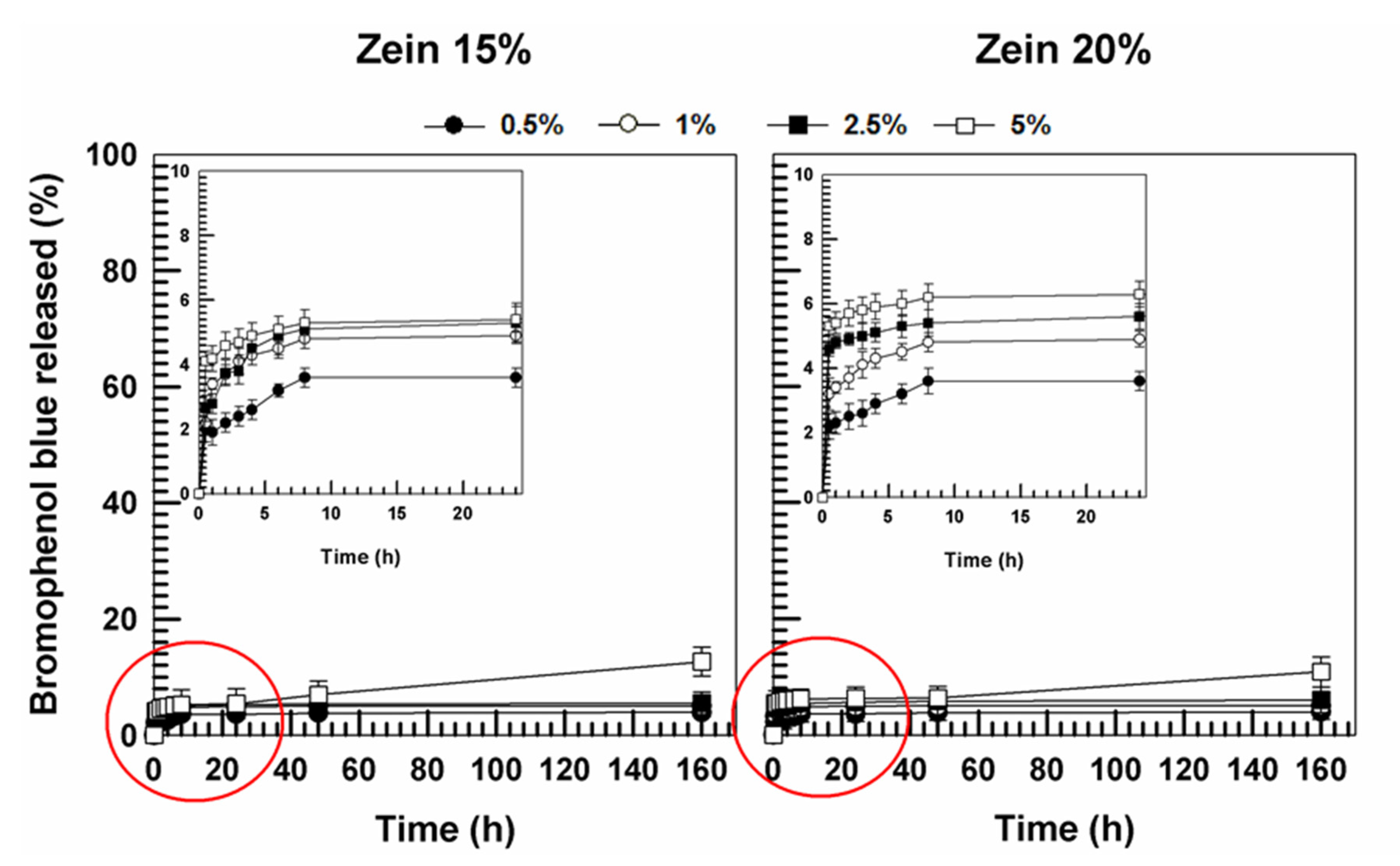
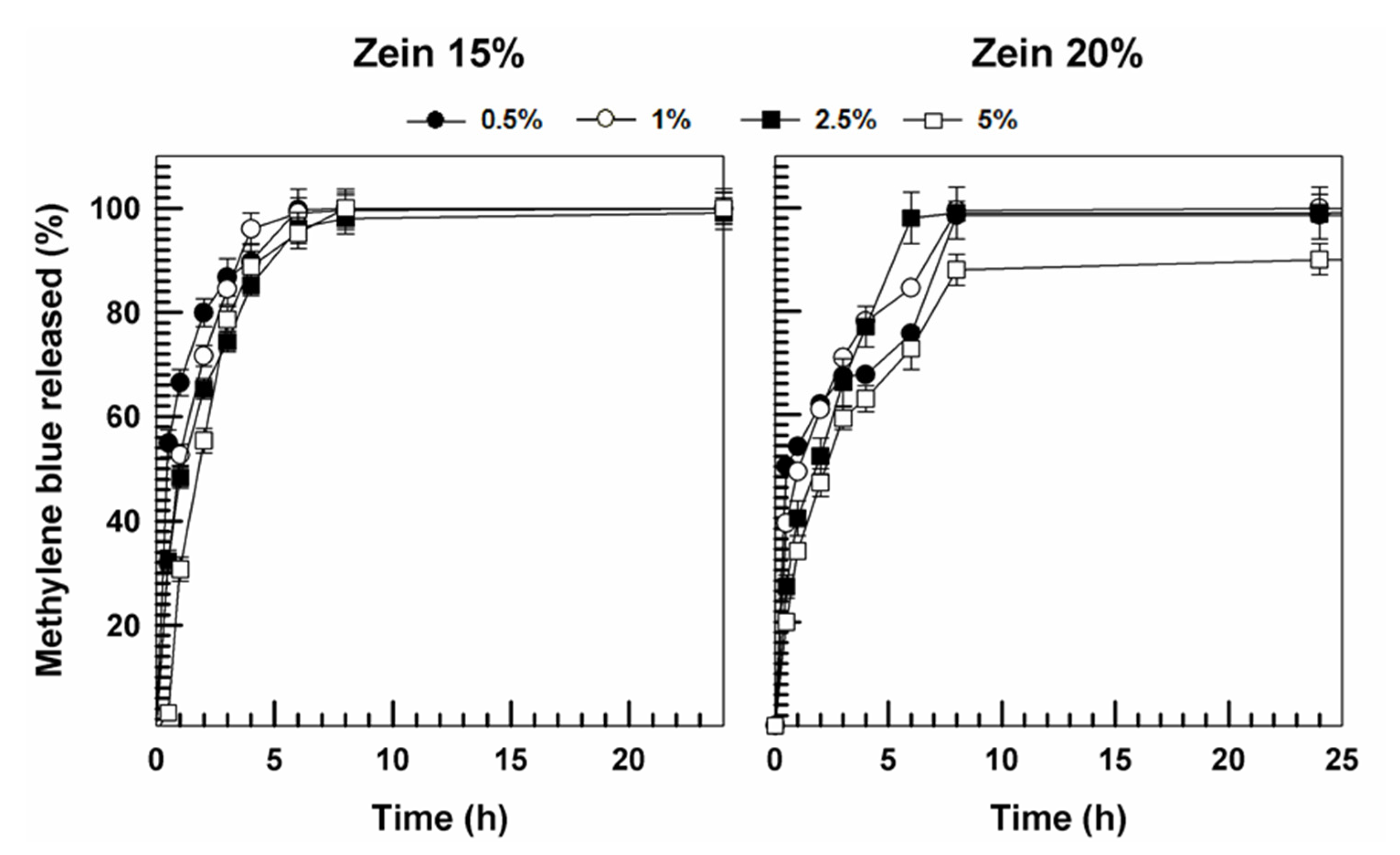
3.2. Evaluation of Release Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 16. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Lamsal, B.P. REVIEW: Zein Extraction from Corn, Corn Products, and Coproducts and Modifications for Various Applications: A Review. Cereal Chem. 2011, 88, 159–173. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A history of processing and use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Che, X.; Zhang, H.; Shi, N.; Li, C.; Chen, Y.; Kong, W. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Control. Release 2015, 206, 206–219. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, X.; Ren, S.; Wang, J.; Zhang, H. Physicochemical characterization of a zein prepared using a novel aqueous extraction technology and tensile properties of the zein film. Ind. Crops Prod. 2019, 130, 57–62. [Google Scholar] [CrossRef]
- Sousa, F.F.O.; de Luzardo-Álvarez, A.; Blanco-Méndez, J.; Otero-Espinar, F.J.; Martín-Pastor, M.; Macho, I.S. Use of 1H NMR STD, WaterLOGSY, and Langmuir monolayer techniques for characterization of drug–zein protein complexes. Eur. J. Pharm. Biopharm. 2013, 85, 790–798. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: the industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Bisharat, L.; Berardi, A.; Perinelli, D.R.; Bonacucina, G.; Casettari, L.; Cespi, M.; AlKhatib, H.S.; Palmieri, G.F. Aggregation of zein in aqueous ethanol dispersions: Effect on cast film properties. Int. J. Biol. Macromol. 2018, 106, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Duan, W.; Lee, B.-J.; Tran, T.T.D. The use of zein in the controlled release of poorly water-soluble drugs. Int. J. Pharm. 2019, 566, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Wang, Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem. 2011, 124, 210. [Google Scholar] [CrossRef]
- Zhong, Q.; Ikeda, S. Viscoelastic properties of concentrated aqueous ethanol suspensions of α-zein. Food Hydrocoll. 2012, 28, 46–52. [Google Scholar] [CrossRef]
- Nonthanum, P.; Lee, Y.; Padua, G.W. Effect of γ-Zein on the Rheological Behavior of Concentrated Zein Solutions. J. Agric. Food Chem. 2012, 60, 1742–1747. [Google Scholar] [CrossRef]
- Chen, X.-W.; Fu, S.-Y.; Hou, J.-J.; Guo, J.; Wang, J.-M.; Yang, X.-Q. Zein based oil-in-glycerol emulgels enriched with β-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef]
- Qi, H.; Cao, J.; Xin, Y.; Mao, X.; Xie, D.; Luo, J.; Chu, B. Dual responsive zein hydrogel membrane with selective protein adsorption and sustained release property. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 70, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, R.; Mao, L.; Gao, Y. Ethanol-induced composite hydrogel based on propylene glycol alginate and zein: Formation, characterization and application. Food Chem. 2018, 255, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Glusac, J.; Davidesko-Vardi, I.; Isaschar-Ovdat, S.; Kukavica, B.; Fishman, A. Tyrosinase-crosslinked pea protein emulsions: Impact of zein incorporation. Food Res. Int. 2019, 116, 370–378. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Theivendran, P.; Baskararaj, S.; Sankaranarayanan, B.; Palanisamy, P.; Saravanan, G.; Arunachalam, S.; Sankaranarayanan, M.; Natarajan, J.; Somasundaram, B. Modeling a pH-sensitive Zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery. 3 Biotech 2019, 9, 185. [Google Scholar] [CrossRef]
- Gagliardi, A.; Froiio, F.; Salvatici, M.C.; Paolino, D.; Fresta, M.; Cosco, D. Characterization and refinement of zein-based gels. Food Hydrocoll. 2020, 101, 105555. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, M.; Fu, B.M. Temporal effects of vascular endothelial growth factor and 3, 5-cyclic monophosphate on blood–brain barrier solute permeability in vivo. J. Neurosci. Res. 2014, 92, 1678–1689. [Google Scholar] [CrossRef]
- Nishida, K.; Sato, N.; Sasaki, H.; Nakamura, J. Absorption of organic anions as model drugs following application to rat liver surface in-vivo. J. Pharm. Pharmacol. 1994, 46, 867–870. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Han, Y.; Henderson, S.C.; Majeska, R.J.; Weinbaum, S.; Schaffler, M.B. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc. Natl. Acad. Sci. USA 2005, 102, 11911–11916. [Google Scholar] [CrossRef]
- Cumming, H.; Rücker, C. Octanol–water partition coefficient measurement by a simple 1H NMR method. ACS Omega 2017, 2, 6244–6249. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Methylene blue-a therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Brownell, J.; Patel, P.; Tsoukas, M.M. The role of photodynamic therapy in acne: An evidence-based review. Am. J. Clin. Dermatol. 2017, 18, 311–321. [Google Scholar] [CrossRef]
- Müller, O.; Lu, G.; Jahn, A.; Mockenhaupt, F.P. How worthwhile is methylene blue as a treatment of malaria. Expert Rev. Anti.-Infect. Ther. 2019, 17, 471. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Ceniti, C.; Froiio, F.; Gagliardi, A.; Britti, D.; Paolino, D.; Costanzo, N. Observations on passive microrheology for monitoring the fermentation process in yoghurt. Int. Dairy J. 2020, 102, 104604. [Google Scholar] [CrossRef]
- Critello, C.D.; Fiorillo, A.S.; Cristiano, M.C.; de Franciscis, S.; Serra, R. Effects of sulodexide on stability of sclerosing fiams. Phlebology 2019, 34, 191–200. [Google Scholar] [CrossRef]
- Di Francesco, M.; Primavera, R.; Fiorito, S.; Cristiano, M.C.; Taddeo, V.A.; Epifano, F.; Di Marzio, L.; Genovese, S.; Celia, C. Acronychiabaueri Analogue Derivative-Loaded Ultradeformable Vesicles: Physicochemical Characterization and Potential Applications. Planta Med. 2017, 83, 482–491. [Google Scholar] [CrossRef]
- Hamed, R.; Al Baraghthi, T.; Sunoqrot, S. Correlation between the viscoelastic properties of the gel layer of swollen HPMC matrix tablets and their in vitro drug release. Pharm. Dev. Technol. 2018, 23, 838–848. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Vijayalakshmi, E.; Korrapati, P.S. Selective interactions of zein microspheres with different class of drugs: an in vitro and in silico analysis. AAPS PharmSciTech 2014, 15, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.L.; Wong, A.I.C.; Xu, Y.; Yuliarti, O. Zein as a water insoluble excipient for spray dry encapsulation of hydrophilic bioactives. J. Food Eng. 2020, 283, 110054. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |
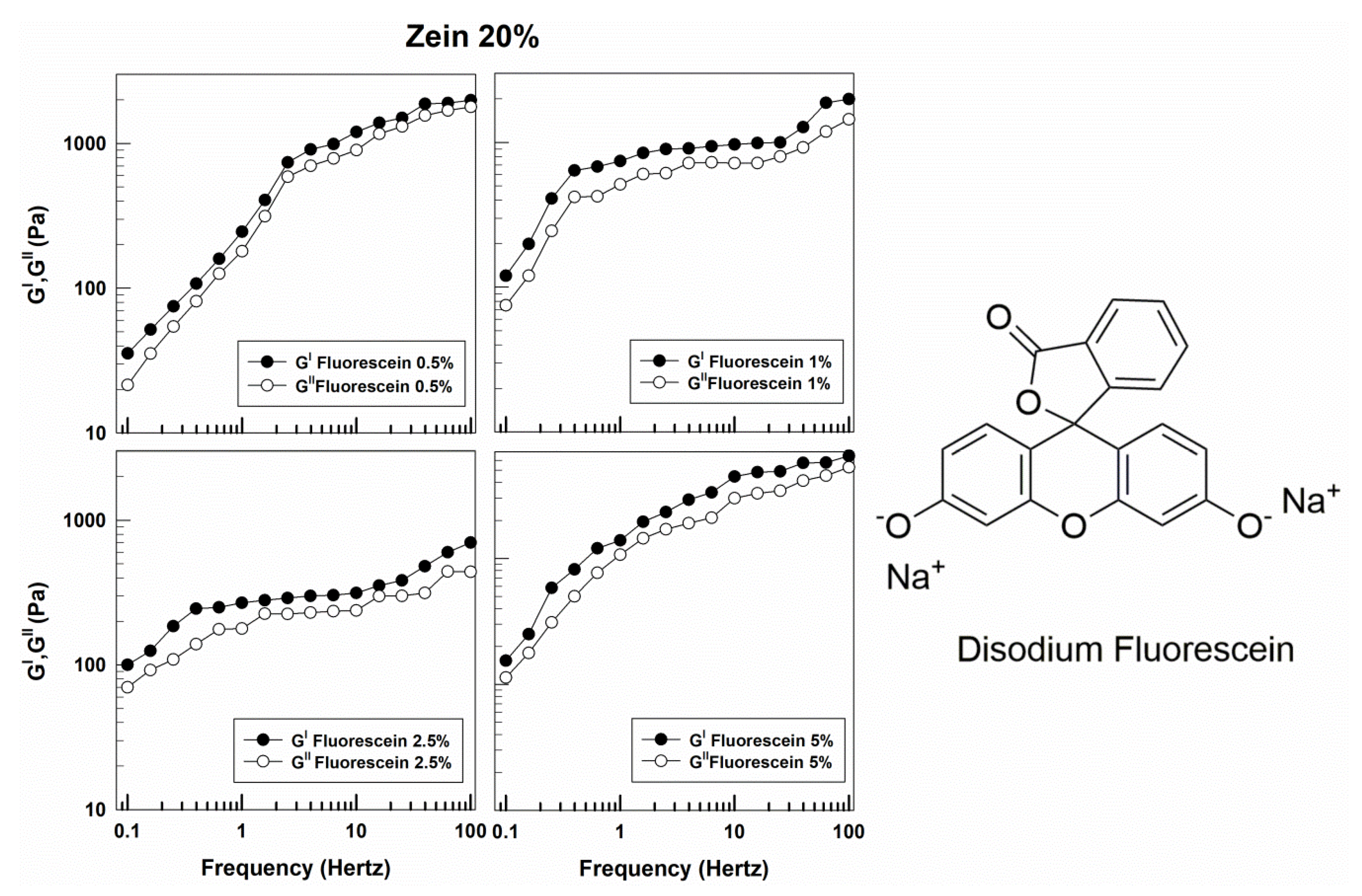
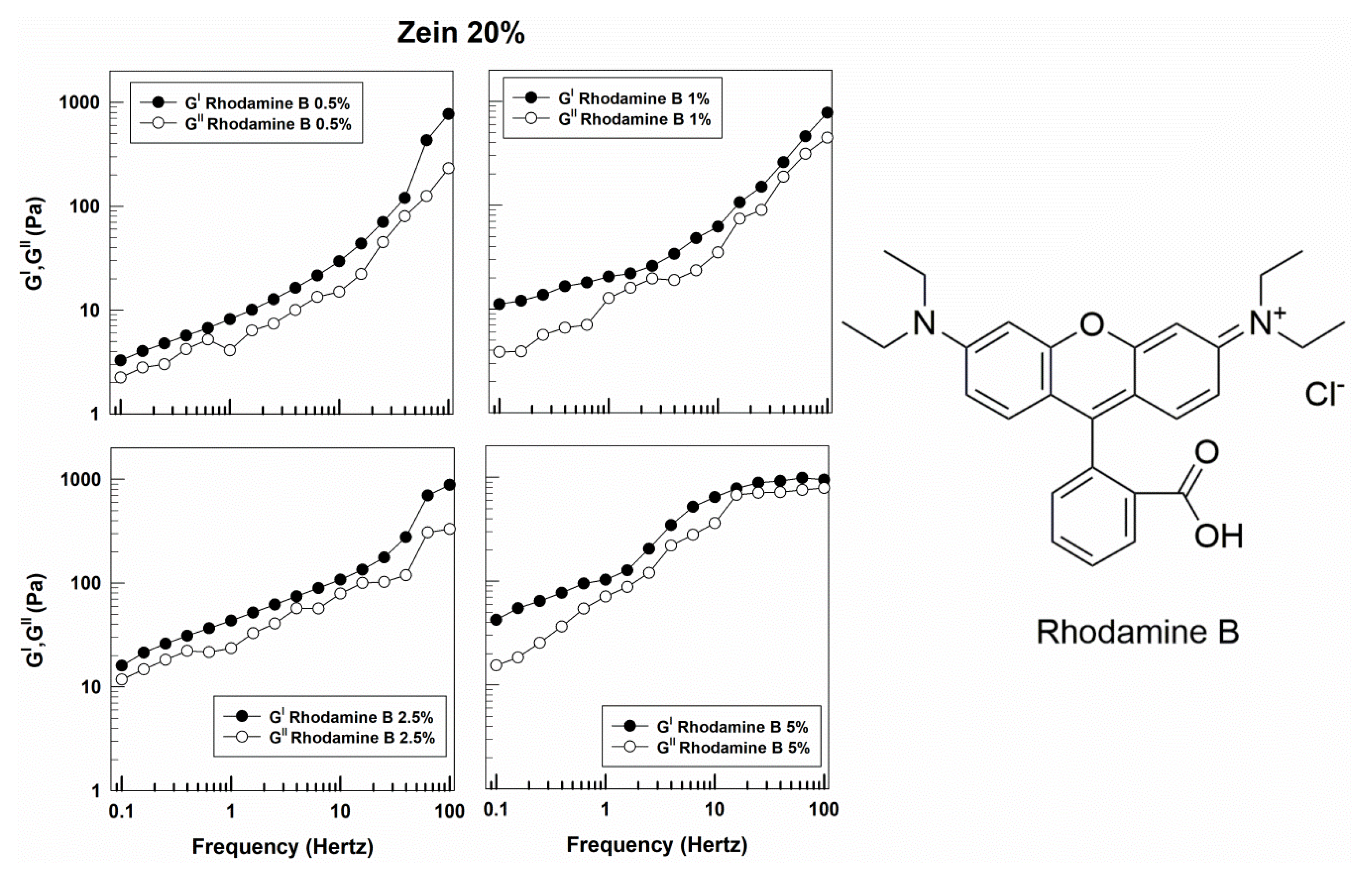
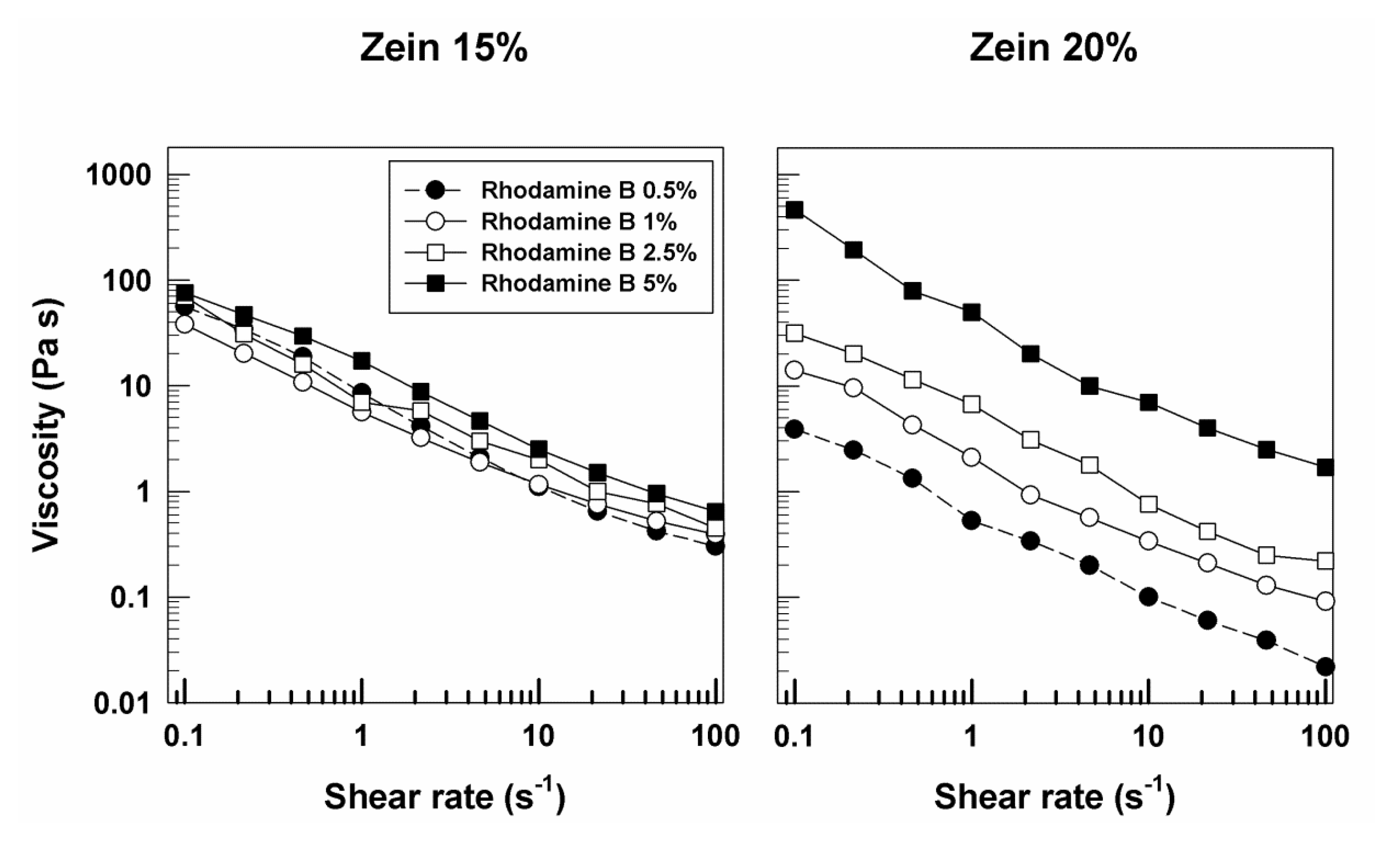
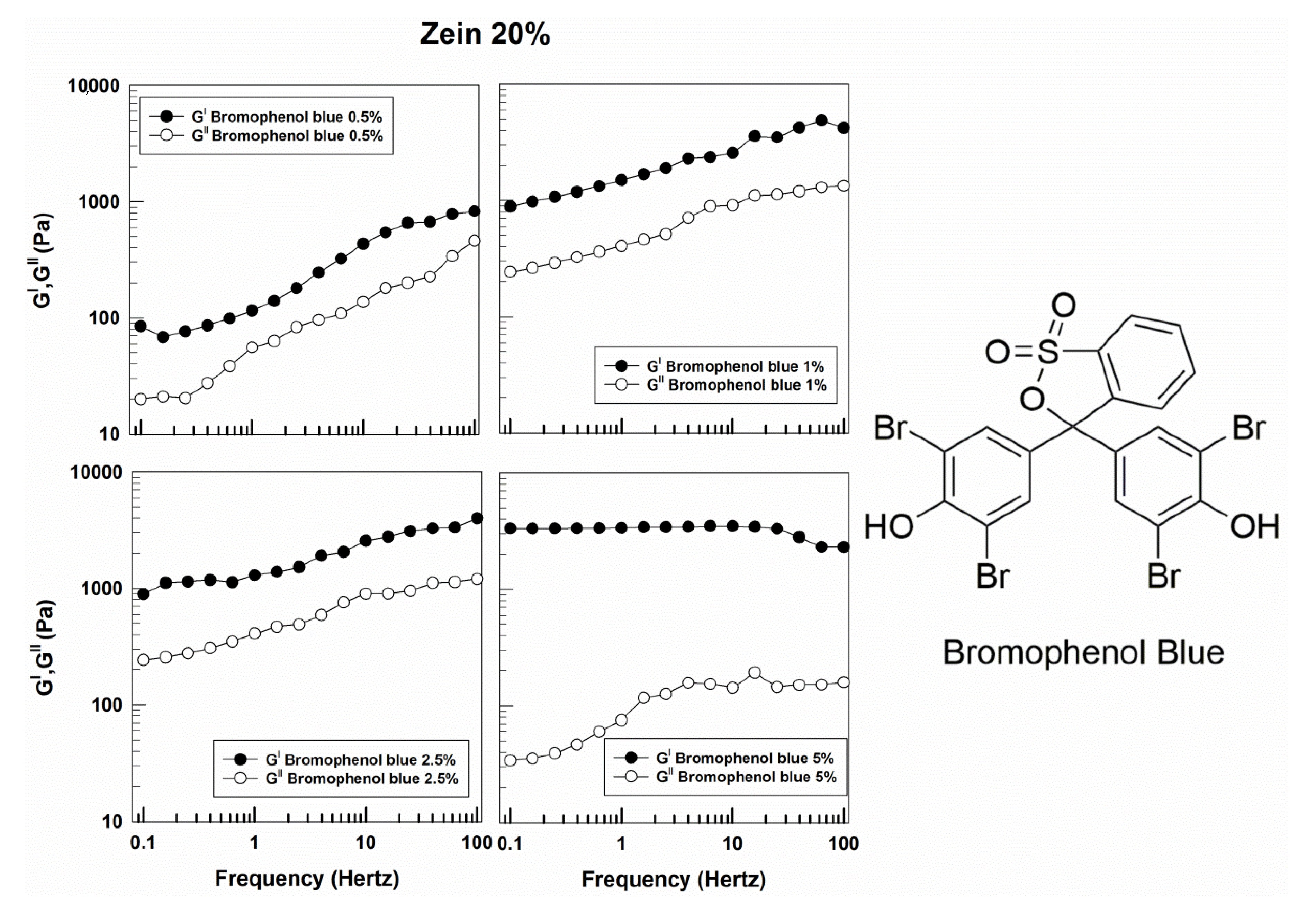
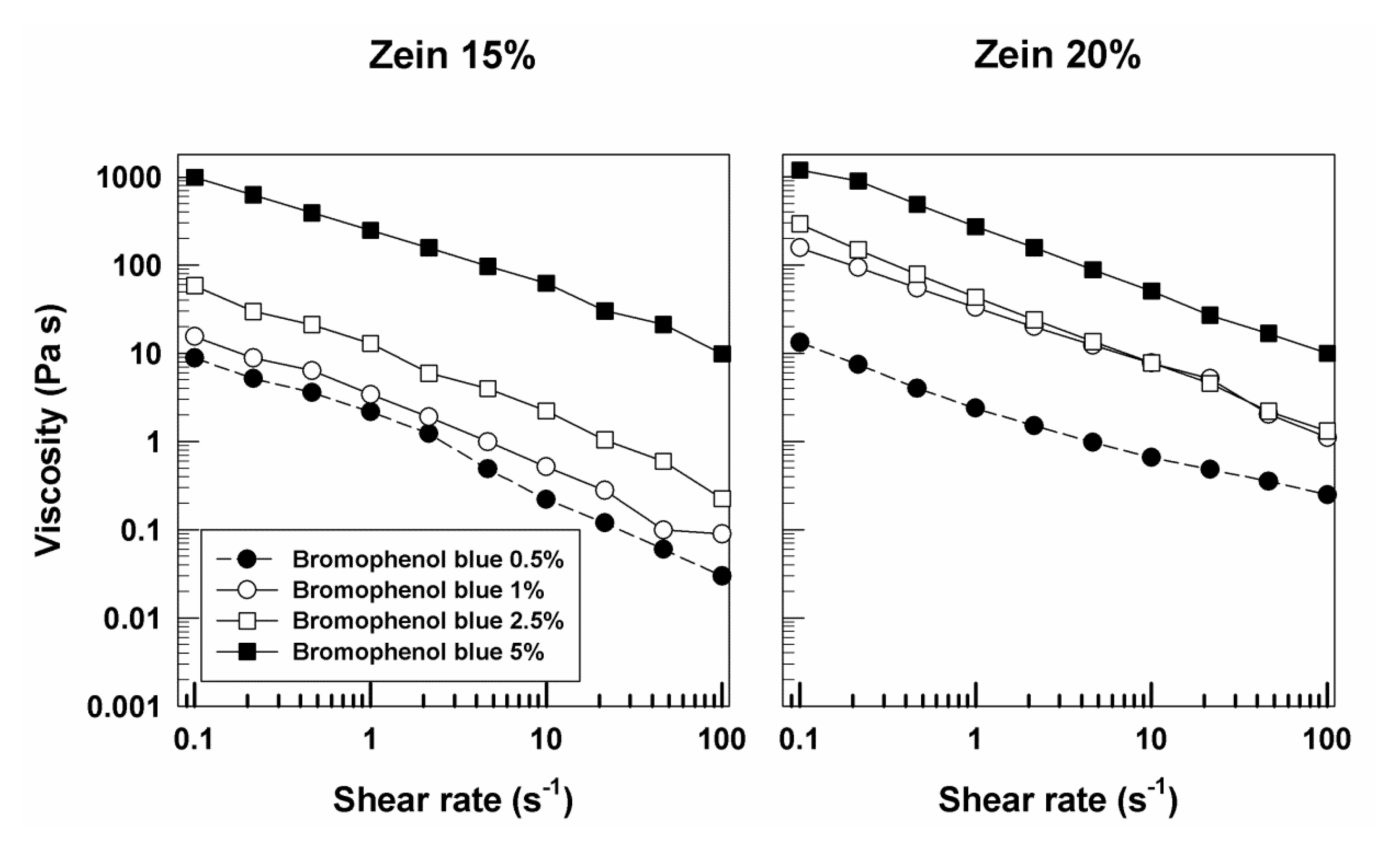
| Sample | Molecular Weight (g/mol) | Solubility in Water (g/L) | Log Pow | Source |
|---|---|---|---|---|
| Disodium fluorescein | 376.27 | 500 | −0.67 | Merck |
| Rhodamine B | 479.02 | 15 | 1.95 | Merck |
| Methylene blue | 319.86 | 25 | 5.85 | LabChem |
| Bromophenol blue | 669.96 | Slightly soluble | 9.2 | Merck |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules 2020, 25, 3174. https://doi.org/10.3390/molecules25143174
Gagliardi A, Voci S, Paolino D, Fresta M, Cosco D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules. 2020; 25(14):3174. https://doi.org/10.3390/molecules25143174
Chicago/Turabian StyleGagliardi, Agnese, Silvia Voci, Donatella Paolino, Massimo Fresta, and Donato Cosco. 2020. "Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels" Molecules 25, no. 14: 3174. https://doi.org/10.3390/molecules25143174
APA StyleGagliardi, A., Voci, S., Paolino, D., Fresta, M., & Cosco, D. (2020). Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules, 25(14), 3174. https://doi.org/10.3390/molecules25143174