The Influence of Single, Tandem, and Clustered DNA Damage on the Electronic Properties of the Double Helix: A Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analysis of Isolated, Tandem, and Closured DNA Damage
2.2. The Ionization Potential of Isolated, Tandem, and Closured DNA Damage
3. Materials and Methods
3.1. Computation Methodology of QM/MM Studies [42,43]
3.2. Computation Methodology of Density Functional Theory (DFT) Study
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Depamphilis, M.L. Review: Nuclear structure and DNA replication. J. Struct. Biol. 2000, 129, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Sudhir Ambekar, S. DNA: Damage and Repair Mechanisms in Humans. Glob. J. Pharm. Pharm. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Aprioku, J.S. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J. Reprod. Infertil. 2013, 14, 158–172. [Google Scholar] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Terato, H.; Ide, H. Clustered DNA damage induced by heavy ion particles. Biol. Sci. Sp. Uchū Seibutsu Kagaku 2004, 18, 206–215. [Google Scholar] [CrossRef][Green Version]
- Imoto, S.; Bransfield, L.A.; Croteau, D.L.; Van Houten, B.; Greenberg, M.M. DNA tandem lesion repair by strand displacement synthesis and nucleotide excision repair. Biochemistry 2008, 47, 4306–4316. [Google Scholar] [CrossRef][Green Version]
- Mangal, D.; Vudathala, D.; Park, J.H.; Seon, H.L.; Penning, T.M.; Blair, I.A. Analysis of 7,8-dihydro-8-oxo-2′-deoxyguanosine in cellular DNA during oxidative stress. Chem. Res. Toxicol. 2009, 22, 788–797. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Lu, K.; Moeller, B.C.; Gao, L.; Upton, P.B.; Nakamura, J.; Starr, T.B. Endogenous versus exogenous DNA adducts: Their role in carcinogenesis, epidemiology, and risk assessment. Toxicol. Sci. 2011, 120, 130–145. [Google Scholar] [CrossRef]
- Guerrero, C.R.; Wang, J.; Wang, Y. Induction of 8,5′-Cyclo-2′-deoxyadenosine and 8,5′-Cyclo-2′-deoxyguanosine in Isolated DNA by Fenton-Type Reagents. Chem. Res. Toxicol. 2013, 26, 1361–1366. [Google Scholar] [CrossRef][Green Version]
- Chatgilialoglu, C.; Ferreri, C.; Terzidis, M.A. Purine 5′,8-cyclonucleoside lesions: Chemistry and biology. Chem. Soc. Rev. 2011, 40, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Di Mascio, P.; Wagner, J.R. Radiation-induced (5′R)-and (5′S)-purine 5′,8-cyclo-2′-deoxyribonucleosides in human cells: A revisited analysis of HPLC-MS/MS measurements. Free Radic. Res. 2019, 53, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Robins, P.; Masutani, C.; Hanaoka, F.; Gasparutto, D.; Cadet, J.; Wood, R.D.; Lindahl, T. Oxygen free radical damage to DNA: Translesion synthesis by human DNA polymerase η and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J. Biol. Chem. 2001, 276, 49283–49288. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Dai, X.; Yuan, B.; Wang, J.; Wang, J.; Brooks, P.J.; Niedernhofer, L.J.; Wang, Y. A quantitative assay for assessing the effects of DNA lesions on transcription. Nat. Chem. Biol. 2012, 8, 817–822. [Google Scholar] [CrossRef]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef]
- Karwowski, B.T. The Influence of (5′R)- and (5′S)-5′,8-Cyclo-2′-Deoxyadenosine on UDG and hAPE1 Activity. Tandem Lesions are the Base Excision Repair System’s Nightmare. Cells 2019, 8, 1303. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease: Induction, Repair and Significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Kong, Q.; Lin, C.G. Oxidative damage to RNA: Mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef]
- Brooks, P.J. The 8,5′-cyclopurine-2′-deoxynucleosides: Candidate neurodegenerative DNA lesions in xeroderma pigmentosum, and unique probes of transcription and nucleotide excision repair. DNA Repair. (Amst) 2008, 7, 1168–1179. [Google Scholar] [CrossRef]
- de Souza-Pinto, N.C. Repair of Oxidative DNA Damage. Brenner’s Encycl. Genet. Second Ed. 2013, 35, 142–143. [Google Scholar] [CrossRef]
- Fortini, P.; Parlanti, E.; Sidorkina, O.M.; Laval, J.; Dogliotti, E. The type of DNA glycosylase determines the base excision repair pathway in mammalian cells. J. Biol. Chem. 1999, 274, 15230–15236. [Google Scholar] [CrossRef]
- Jacobs, A.L.; Schär, P. DNA glycosylases: In DNA repair and beyond. Chromosoma 2012, 121, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. The role of (5′R) and (5′S) 5′,8-cyclo-2′-deoxyadenosine in ds-DNA structure. A comparative QM/MM theoretical study. Comput. Theor. Chem. 2013, 1010, 38–44. [Google Scholar] [CrossRef]
- Olson, W.K.; Bansal, M.; Burley, S.K.; Dickerson, R.E.; Gerstein, M.; Harvey, S.C.; Heinemann, U.; Lu, X.J.; Neidle, S.; Shakked, Z.; et al. A Standard Reference Frame for the Description of Nucleic Acid Base-pair Geometry. J. Mol. Biol. 2001, 313, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomedicine 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.B.; Landman, U. The Mechanism of Long-Distance Radical Cation Transport in Duplex DNA: Ion-Gated Hopping of Polaron-Like Distortions. Proc. Natl. Acad. Sci. USA 2012, 139–161. [Google Scholar] [CrossRef]
- Genereux, J.C.; Barton, J.K. Mechanisms for DNA charge transport. Chem. Rev. 2010, 110, 1642–1662. [Google Scholar] [CrossRef]
- Sontz, P.A.; Mui, T.P.; Fuss, J.O.; Tainer, J.A.; Barton, J.K. DNA charge transport as a first step in coordinating the detection of lesions by repair proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 1–6. [Google Scholar] [CrossRef]
- Fromme, J.C.; Banerjee, A.; Huang, S.J.; Verdine, G.L. Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase. Nat. Mater. 2004, 427, 652–656. [Google Scholar] [CrossRef]
- Merino, E.J.; Boal, A.K.; Barton, J.K. Biological contexts for DNA charge transport chemistry. Curr. Opin. Chem. Biol. 2008, 12, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, P.; Tavernelli, I.; Rothlisberger, U. Vertical Ionization Energies and Electron Affinities of Native and Damaged DNA Bases, Nucleotides, and Pairs from Density Functional Theory Calculations: Model Assessment and Implications for DNA Damage Recognition and Repair. J. Chem. Theory Comput. 2019, 15, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. Clustered DNA Damage: Electronic Properties and Their Influence on Charge Transfer. 7,8-Dihydro-8-Oxo-2′-Deoxyguaosine Versus 5′,8-Cyclo-2′-Deoxyadenosines: A Theoretical Approach. Cells 2020, 9, 424. [Google Scholar] [CrossRef]
- Mayhall, N.J.; Raghavachari, K. Charge transfer across ONIOM QM:QM boundaries: The impact of model system preparation. J. Chem. Theory Comput. 2010, 6, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Truhlar, D.G. QM/MM: What have we learned, where are we, and where do we go from here? Theor. Chem. Acc. 2007, 117, 1–40. [Google Scholar] [CrossRef]
- Plumley, J.A.; Dannenberg, J.J. A comparison of the behavior of functional/basis set combinations for hydrogen-bonding in the water dimer with emphasis on basis set superposition error. J. Comput. Chem. 2011, 32, 1519–1527. [Google Scholar] [CrossRef]
- Cammi, R.; Corni, S.; Mennucci, B.; Tomasi, J. Electronic excitation energies of molecules in solution: State specific and linear response methods for nonequilibrium continuum solvation models. J. Chem. Phys. 2005, 122. [Google Scholar] [CrossRef] [PubMed]
- Jortner, J.; Bixon, M.; Langenbacher, T.; Michel-Beyerle, M.E. Charge transfer and transport in DNA. Proc. Natl. Acad. Sci. USA 1998, 95, 12759–12765. [Google Scholar] [CrossRef] [PubMed]
- Albiser, G.; Lamiri, A.; Premilat, S. The A-B transition: Temperature and base composition effects on hydration of DNA. Int. J. Biol. Macromol. 2001, 28, 199–203. [Google Scholar] [CrossRef]
- Breslin, D.T.; Schuster, G.B. Anthraquinone photonucleases: Mechanisms for GG-selective and nonselective cleavage of double-stranded DNA. J. Am. Chem. Soc. 1996, 118, 554–558. [Google Scholar] [CrossRef]
- Karwowski, B.T. The AT Interstrand Cross-Link: Structure, Electronic Properties, and Influence on Charge Transfer in dsDNA. Mol. Ther. Nucleic Acids 2018, 13, 665–685. [Google Scholar] [CrossRef]
- Romieu, A.; Gasparutto, D.; Molko, D.; Cadet, J. Site-Specific Introduction of (5′S)-5′,8-Cyclo-2′-deoxyadenosine into Oligodeoxyribonucleotides. J. Org. Chem. 1998, 63, 5245–5249. [Google Scholar] [CrossRef]
- Nasr, T.; Li, Z.; Nakagawa, O.; Taniguchi, Y.; Ono, S.; Sasaki, S. Selective fluorescence quenching of the 8-oxoG-clamp by 8-oxodeoxyguanosine in ODN. Bioorganic Med. Chem. Lett. 2009, 19, 727–730. [Google Scholar] [CrossRef]
- Zaliznyak, T.; Lukin, M.; De Los Santos, C. Structure and stability of duplex DNA containing (5′S)-5′,8-cyclo-2′-deoxyadenosine: An oxidatively generated lesion repaired by NER. Chem. Res. Toxicol. 2012, 25, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Hoppins, J.J.; Gruber, D.R.; Miears, H.L.; Kiryutin, A.S.; Kasymov, R.D.; Petrova, D.V.; Endutkin, D.V.; Popov, A.V.; Yurkovskaya, A.V.; Fedechkin, S.O.; et al. 8-oxoguanine affects DNA backbone conformation in the EcoRI recognition site and inhibits its cleavage by the enzyme. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.D.; Becker, D.; Yan, M.; Summerfield, S.R. Relative abundances of primary ion radicals in γ-irradiated DNA: Cytosine vs thymine anions and adenine vs guanine cations. J. Phys. Chem. 1991, 95, 3409–3415. [Google Scholar] [CrossRef]
- Sugiyama, H.; Saito, I. Theoretical studies of GG-specific photocleavage of DNA via electron transfer: Significant lowering of ionization potential and 5′-localization of HOMO of stacked GG bases in B-form DNA. J. Am. Chem. Soc. 1996, 118, 7063–7068. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Grozema, F.C.; Guerra, C.F.; Bickelhaupt, F.M.; Siebbeles, L.D.A. Mapping the Sites for Selective Oxidation of Guanines in DNA. J. Am. Chem. Soc. 2003, 125, 13658–13659. [Google Scholar] [CrossRef]
- Voityuk, A.A.; Jortner, J.; Bixon, M.; Rösch, N. Energetics of hole transfer in DNA. Chem. Phys. Lett. 2000, 324, 430–434. [Google Scholar] [CrossRef]
- Kanvah, S.; Schuster, G.B. Long-range oxidative damage to DNA: Protection of guanines by a nonspecifically bound disulfide. J. Am. Chem. Soc. 2002, 124, 11286–11287. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef]
- Rust, M.; Lappe, J.; Cave, R.J. Multistate effects in calculations of the electronic coupling element for electron transfer using the generalized Mulliken-Hush method. J. Phys. Chem. A 2002, 106, 3930–3940. [Google Scholar] [CrossRef]
- Dreuw, A.; Head-Gordon, M. Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 2005, 105, 4009–4037. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef]
- Miertus, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kinetics. Electronic supplementary information (ESI) available: Mean errors for pure and hybrid DFT methods. See http://www.rsc.org/suppdata/cp/b3/b316260e/. Phys. Chem. Chem. Phys. 2004, 6, 673. [Google Scholar] [CrossRef]
- Davidson, E.R. Basis Set Selection for Molecular Calculations. Chem. Rev. 1988, 86, 681–696. [Google Scholar] [CrossRef]
- Varsano, D.; Felice, R.D.; Marques, M.A.L.; Rubio, A. A TDDFT Study of the Excited States of DNA Bases and Their Assemblies. J. Phys. Chem. B 2006, 110, 7129–7138. [Google Scholar] [CrossRef]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge model 5: An extension of hirshfeld population analysis for the accurate description of molecular interactions in gaseous and condensed phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef]
- Cave, R.J.; Newton, M.D. Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996, 249, 15–19. [Google Scholar] [CrossRef]
- Karwowski, B.T. The influence of the terminal phosphorothioate diester bond on the DNA oxidation process. An experimental and theoretical approach. Molecules 2015, 20, 12400–12411. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zheng, G.; Lu, X.J.; Olson, W.K. Web 3DNA - A web server for the analysis, reconstruction, and visualization of three-dimensional nucleic-acid structures. Nucleic Acids Res. 2009, 37, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. Formation of 5′,8-cyclo-2′-deoxyadenosine in single strand DNA. Theoretical quantum mechanics study. Org. Biomol. Chem. 2010, 8, 1603–1609. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the author. |
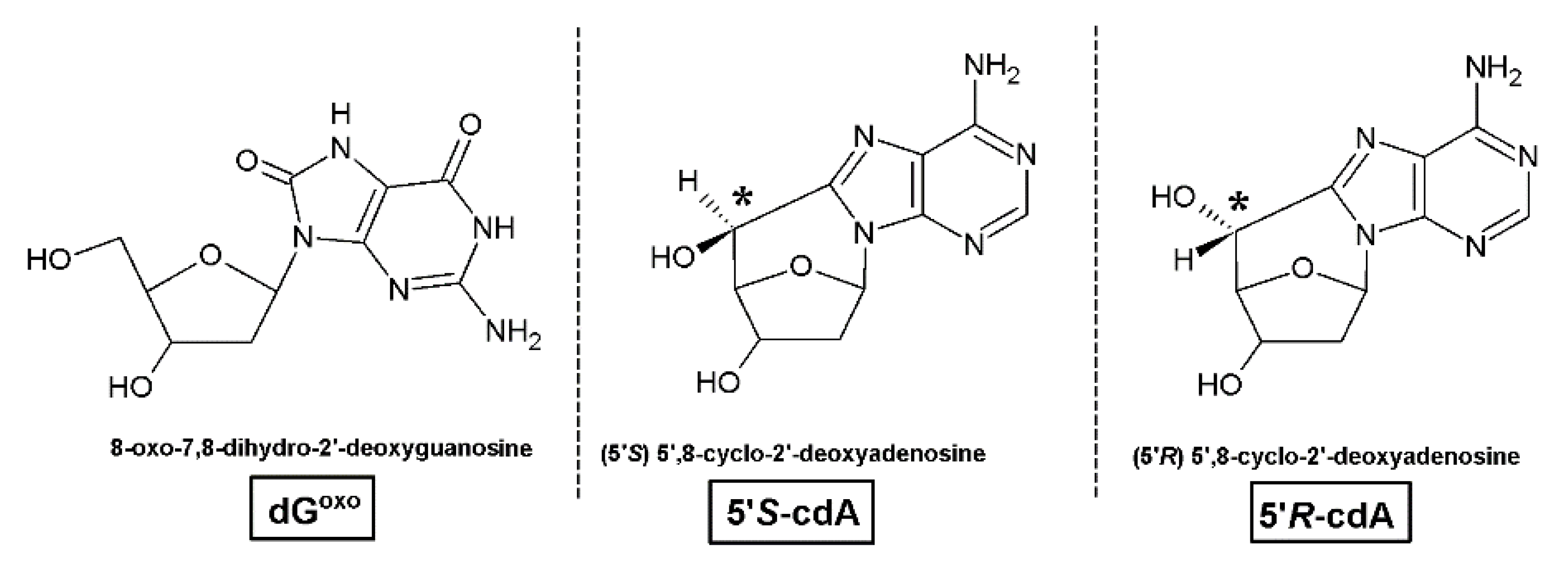
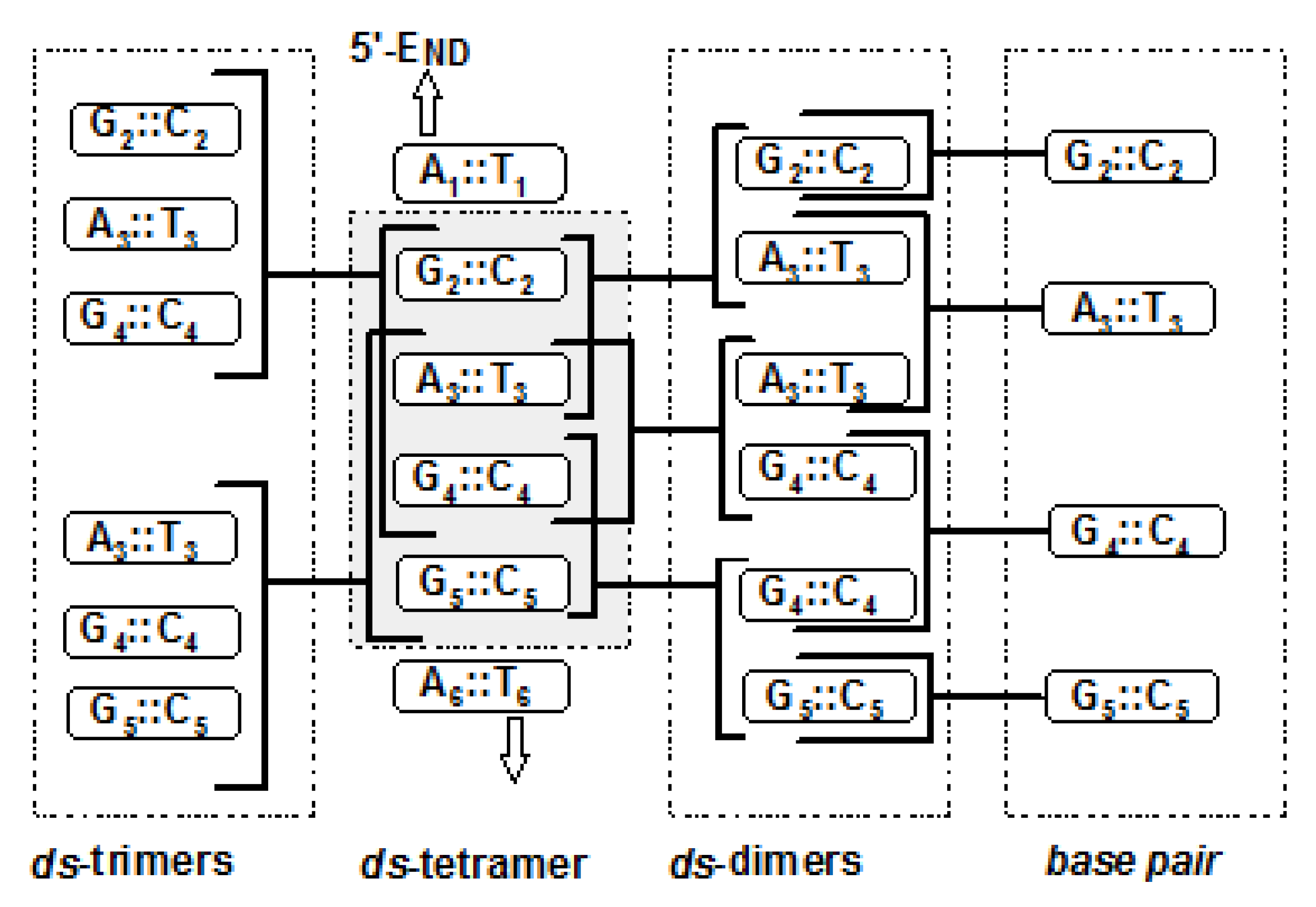
| DNA Damage Type | Oligonucleotide | Oligonucleotide Base Sequence |
|---|---|---|
| Undamaged Native ds-DNA | N-DNA | d[A1G2A3G4G5A6]*d[T6C5C4A3C2T1] |
| Single | 3Goxo-N-DNA | d[A1G2A3oxoG4G5A6]*d[T6C5C4A3C2T1] |
| 5Goxo-N-DNA | d[A1oxoG2A3G4G5A6]*d[T6C5C4A3C2T1] | |
| Tandem | ScA-DNA | d[A1G2(5′S)cA3G4G5A6]*d[T6C5C4A3C2T1] |
| Clustered | 3Goxo-ScA-DNA | d[A1G2(5′S)cA3oxoG4G5A6]*d[T6C5C4A3C2T1] |
| 5Goxo-ScA-DNA | d[A1oxoG2(5′S)cA3G4G5A6]*d[T6C5C4A3C2T1] | |
| Tandem | RcA-DNA | d[A1G2(5′R)cA3G4G5A6]*d[T6C5C4A3C2T1] |
| Clustered | 3Goxo-RcA-DNA | d[A1G2(5′R)cA3oxoG4G5A6]*d[T6C5C4A3C2T1] |
| 5Goxo-RcA-DNA | d[A1oxoG2(5′R)cA3G4G5A6]*d[T6C5C4A3C2T1] |
| ds-DNA Base Dimer h-rise Parameter [Å] | ||||||||
|---|---|---|---|---|---|---|---|---|
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | ||||||
| NEUT. | ARC | NEUT. | ARC | NEUT. | ARC | |||
| G2A3 | 2.96 | 3.01 | G2A3 | 3.06 | 3.02 | oxoG2A3 | 2.89 | 2.86 |
| A3G4 | 3.31 | 2.88 | A3G4oxo | 3.33 | 3.25 | A3G4 | 3.24 | 3.23 |
| G4G5 | 3.34 | 3.14 | oxoG4G5 | 3.28 | 3.07 | G4G5 | 3.34 | 3.38 |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | ||||||
| G2(5′S)cA3 | 3.36 | 3.29 | G2(5′S)cA3 | 3.36 | 3.39 | oxoG2(5′S)cA3 | 3.26 | 3.11 |
| (5′S)cA3G4 | 2.98 | 2.82 | (5′S)cA3G4oxo | 3.05 | 2.87 | (5′S)cA3G4 | 2.94 | 2.97 |
| G4G5 | 3.68 | 3.56 | oxoG4G5 | 3.65 | 3.5 | G4G5 | 3.68 | 3.68 |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | ||||||
| G2(5′R)cA3 | 3.32 | 3.26 | G2(5′R)c A3 | 3.45 | 3.41 | oxoG2(5′R)c A3 | 3.37 | 3.24 |
| (5′R)cA3G4 | 2.8 | 2.7 | (5′R)cA3G4oxo | 3.15 | 3.12 | (5′R)cA3G4 | 2.87 | 3.05 |
| G4G5 | 3.6 | 3.49 | oxoG4G5 | 3.68 | 3.55 | G4G5 | 3.63 | 3.67 |
| ds-DNA Bases Aromatic Rings Overlap [Å2] | ||||||||
|---|---|---|---|---|---|---|---|---|
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | ||||||
| NEUT. | ARC | NEUT. | ARC | NEUT. | ARC | |||
| G2A3 | 2.14 | 1.3 | G2A3 | 2.95 | 2.9 | G2oxoA3 | 1.95 | 1.62 |
| A3G4 | 3.79 | 3.46 | A3G4oxo | 2.56 | 2.93 | A3G4 | 3.31 | 3.66 |
| G4G5 | 1.28 | 1.07 | G4oxoG5 | 0.56 | 0.52 | G4G5 | 0.77 | 0.83 |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | ||||||
| G2A3 | 2.29 | 2.2 | G2A3 | 2.27 | 2.22 | oxoG2A3 | 1.95 | 2.14 |
| (5′S)cA3G4 | 3.10 | 5.59 | (5′S)cA3G4oxo | 6.51 | 6.31 | (5′S)cA3G4 | 5.30 | 5.21 |
| oxoG4G5 | 2.97 | 3.22 | oxoG4G5 | 3.44 | 3.12 | oxoG4G5 | 3.40 | 3.42 |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | ||||||
| G2A3 | 1.99 | 2.11 | G2A3 | 0.99 | 0.89 | oxoG2A3 | 2.05 | 1.86 |
| (5′R)cA3G4 | 2.00 | 5.03 | (5′R)cA3G4oxo | 3.27 | 3.13 | (5′R)cA3G4 | 4.98 | 3.34 |
| oxoG4G5 | 2.81 | 2.99 | oxoG4G5 | 1.86 | 1.99 | oxoG4G5 | 2.89 | 0.94 |
| ds-DNA Stacking Energy (kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | ||||||
| NEUT. | VER.N | NEUT. | VER.N | NEUT. | VER.N | |||
| G2A3 | −14.56 | −14.55 | G2A3 | −14.23 | −14.91 | G2oxoA3 | −14.43 | −13.75 |
| A3G4 | −13.59 | −14.88 | A3G4oxo | −14.69 | −14.63 | A3G4 | −13.38 | −14.55 |
| G4G5 | −12.05 | −13.39 | G4oxoG5 | −12.86 | −13.03 | G4G5 | −12.09 | −12.76 |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | ||||||
| G2A3 | −12.94 | −13.10 | G2A3 | −12.90 | −13.10 | oxo G2A3 | −12.82 | −12.31 |
| (5′S)cA3G4 | −11.25 | −11.20 | (5′S)cA3G4oxo | −11.88 | −11.07 | (5′S)cA3G4 | −11.40 | −11.90 |
| oxoG4G5 | −14.15 | −13.27 | oxoG4G5 | −14.68 | −13.86 | oxoG4G5 | −14.16 | −14.25 |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | ||||||
| G2A3 | −13.23 | −13.39 | G2A3 | −12.92 | −12.86 | oxoG2A3 | −13.05 | −10.58 |
| (5′R)cA3G4 | −13.39 | −12.52 | (5′R)cA3G4oxo | −14.73 | −13.77 | (5′R)cA3G4 | −13.24 | −13.70 |
| oxoG4G5 | −13.50 | −12.92 | oxoG4G5 | −14.84 | −14.28 | oxoG4G5 | −13.48 | −14.54 |
| ds-DNA Hydrogen Bond Energy | ||||||||
|---|---|---|---|---|---|---|---|---|
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | ||||||
| NEUT. | VER.N | NEUT. | VER.N | NEUT. | VER.N | |||
| G2C2 | −17.23 −17.54 (a) −14.36 (b) | −17.22 −18.10 (a) | G2C2 | −17.32 | −17.36 | oxoG2C2 | −17.69 | −18.16 |
| A3T3 | −10.81 −10.95 (a) −8.64 (b) | −10.74 −9.75 (a) | A3T3 | −10.53 | −10.44 | A3T3 | −10.80 | −10.39 |
| G4C4 | −17.20 | −17.00 | oxoG4C4 | −17.74 −18.04 (a) −16.83 (c) | −17.97 −18.49 (a) | G4C4 | −17.26 | −17.14 |
| G5C5 | −17.21 | −17.73 | G5C5 | −17.23 | −17.32 | G5C5 | −17.23 | −17.31 |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | ||||||
| G2C2 | −17.30 | −17.48 | G2C2 | −17.25 | −17.30 | oxoG2C2 | −17.84 | −18.38 |
| (5′S)cA3T3 | −10.62 −10.98 (a) −5.89 (b) | −10.81 −9.77 (a) | (5′S)A3T3 | −10.54 | −10.61 | (5′S)A3T3 | −10.66 | −9.99 |
| G4C4 | −17.10 | −17.80 | oxoG4C4 | −17.58 | −18.07 | G4C4 | −17.12 | −17.04 |
| G5C5 | −17.06 | −17.19 | G5C5 | −17.08 | −17.23 | G5C5 | −17.04 | −17.06 |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | ||||||
| G2C2 | −17.19 | −17.15 | G2C2 | −16.94 | −17.03 | oxoG2C2 | −17.81 | −18.01 |
| (5′R)cA3T3 | −10.60 −10.98 (a) | −10.49 −9.74 (a) | (5′R)A3T3 | −10.65 | −10.55 | (5′R)A3T3 | −10.65 | −9.88 |
| G4C4 | −16.77 | −17.86 | oxoG4C4 | −18.07 | −18.60 | G4C4 | −16.82 | −17.50 |
| G5C5 | −16.95 | −17.17 | G5C5 | −16.81 | −16.85 | G5C5 | −16.96 | −16.80 |
| AIP | VIP | AIP | VIP | AIP | VIP | ||||
|---|---|---|---|---|---|---|---|---|---|
| ds-trimers | |||||||||
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | |||||||
| G2A3G4 | 5.72 | 6.10 | G2A3oxoG4 | 5.51 | 5.85 | oxoG2A3G4 | 5.45 | 5.90 | |
| A3G4G5 | 5.64 | 6.03 | A3oxoG4G5 | 5.37 | 5.79 | A3G4G5 | 6.01 | 6.02 | |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | |||||||
| G2(5′S)cA3G4 | 5.74 | 6.13 | G2(5′S)cA3oxoG4 | 5.51 | 5.91 | oxoG2(5′S)cA3G4 | 5.44 | 5.86 | |
| (5′S)cA3G4G5 | 5.69 | 6.08 | (5′S)cA3oxoG4G5 | 5.45 | 5.88 | (5′S)cA3G4G5 | 6.09 | 6.14 | |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | |||||||
| G2(5′R)cA3G4 | 5.72 | 6.08 | G2(5′R)cA3oxoG4 | 5.50 | 5.94 | oxoG2(5′R)cA3G4 | 5.43 | 5.87 | |
| (5′R)cA3G4G5 | 5.66 | 6.03 | (5′R)cA3oxoG4G5 | 5.47 | 5.91 | (5′R)cA3G4G5 | 6.13 | 6.03 | |
| ds-dimers | |||||||||
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | |||||||
| G2A3 | 6.13 | 6.15 | G2A3 | 6.13 | 6.12 | oxoG2A3 | 5.50 | 5.91 | |
| A3G4 | 5.73 | 6.12 | A3oxoG4 | 5.48 | 5.88 | A3G4 | 6.12 | 6.11 | |
| G4G5 | 5.68 | 6.05 | oxoG4G5 | 5.40 | 5.83 | G4G5 | 6.04 | 6.05 | |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | |||||||
| G2A3 | 6.12 | 6.10 | G2A3 | 6.13 | 6.10 | oxoG2A3 | 5.47 | 5.87 | |
| (5′S)cA3G4 | 5.75 | 6.14 | (5′S)cA3G4 | 5.52 | 5.93 | (5′S)cA3G4 | 6.20 | 6.15 | |
| G4G5 | 5.78 | 6.12 | G4G5 | 5.48 | 5.89 | G4G5 | 6.13 | 6.13 | |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | |||||||
| G2A3 | 6.12 | 6.10 | G2A3 | 6.14 | 6.12 | oxoG2A3 | 5.53 | 5.89 | |
| (5′R)cA3G4 | 5.74 | 6.09 | (5′R)cA3G4 | 5.51 | 5.94 | (5′R)cA3G4 | 6.13 | 6.10 | |
| G4G5 | 5.73 | 6.21 | G4G5 | 5.49 | 5.93 | G4G5 | 6.21 | 6.21 | |
| Single base pairs | |||||||||
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | |||||||
| G2C2 | 6.17 | 6.17 | G2C2 | 6.19 | 6.19 | oxoG2C2 | 5.55 | 5.93 | |
| A3T3 | 6.63 | 6.65 | A3T3 | 6.69 | 6.65 | A3T3 | 6.65 | 6.64 | |
| G4C4 | 5.86 | 6.13 | oxoG4C4 | 5.55 | 5.91 | G4C4 | 6.14 | 6.13 | |
| G5C5 | 6.15 | 6.20 | G5C5 | 6.14 | 6.18 | G5C5 | 6.19 | 6.20 | |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | |||||||
| G2C2 | 6.13 | 6.19 | G2C2 | 6.14 | 6.15 | oxoG2C2 | 5.55 | 5.92 | |
| (5′S)cA3T3 | 6.65 | 6.68 | (5′S)cA3T3 | 6.67 | 6.68 | (5′S)cA3T3 | 6.83 | 6.69 | |
| G4C4 | 5.84 | 6.14 | oxoG4C4 | 5.55 | 5.94 | G4C4 | 6.19 | 6.18 | |
| G5C5 | 6.19 | 6.22 | G5C5 | 6.19 | 6.22 | G5C5 | 6.22 | 6.23 | |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | |||||||
| G2C2 | 6.12 | 6.23 | G2C2 | 6.16 | 6.18 | oxoG2C2 | 5.58 | 5.93 | |
| (5′R)c A3T3 | 6.68 | 6.61 | (5′R)cA3T3 | 6.65 | 6.61 | (5′R)cA3T3 | 6.77 | 6.61 | |
| G4C4 | 5.80 | 6.12 | oxoG4C4 | 5.53 | 5.96 | G4C4 | 6.08 | 6.20 | |
| G5C5 | 6.18 | 6.22 | G5C5 | 6.15 | 6.19 | G5C5 | 6.19 | 6.22 | |
| Ideal Base Pair Model | Isolated from 5ivl.pdb [45] and 21sf.pdb [44] Structure | ||||||||
| AIP | VIP | ||||||||
| G:::C | 5.58 | 6.13 | VIP | ||||||
| Goxo:::C | 5.55 | 5.90 | G:::C** | 6.14 | |||||
| ScA::T | 6.35 | 6.62 | Goxo:::C* | 5.82 | |||||
| RcA::T | 6.35 | 6.62 | ScA::T** | 6.71 | |||||
| A::T | 6.34 | 6.62 | A::T** | 6.66 | |||||
| ds-oligo | Backbone | Bases | All Nucleic Acid |
|---|---|---|---|
| N-DNA | 1.347 | 1.039 | 1.197 |
| 3Goxo-N-DNA | 0.299 | 0.225 | 0.261 |
| 5Goxo-N-DNA | 0.359 | 0.319 | 0.337 |
| ScA-DNA | 0.699 | 0.193 | 0.516 |
| 3Goxo-ScA-DNA | 0.203 | 0.176 | 0.190 |
| 5Goxo-ScA-DNA | 0.247 | 0.214 | 0.231 |
| RcA-DNA | 0.695 | 0.298 | 0.537 |
| 3Goxo-RcA-DNA | 0.119 | 0.082 | 0.103 |
| 5Goxo-RcA-DNA | 1.032 | 0.817 | 0.931 |
| ds-DNA Hirshfeld Charge and Spin Density Population | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | |||||||||||||||
| N | VC | C | N | VC | C | N | VC | C | |||||||||
| Q | Q | S | Q | S | Q | Q | S | Q | S | Q | Q | S | Q | S | |||
| T6 | −0.05 | −0.05 | −0.04 | T6 | −0.05 | −0.05 | −0.04 | T6 | −0.05 | −0.05 | −0.05 | ||||||
| C5 | 0.18 | 0.19 | 0.18 | C5 | 0.17 | 0.17 | 0.17 | C5 | 0.18 | 0.18 | 0.17 | ||||||
| C4 | 0.19 | 0.22 | 0.32 | C4 | 0.21 | 0.24 | 0.29 | C4 | 0.19 | 0.19 | 0.20 | ||||||
| T3 | −0.07 | −0.05 | −0.02 | T3 | −0.07 | −0.05 | −0.05 | T3 | −0.06 | −0.06 | −0.05 | ||||||
| C2 | 0.21 | 0.22 | 0.22 | C2 | 0.20 | 0.21 | 0.21 | C2 | 0.21 | 0.25 | 0.31 | ||||||
| T1 | −0.08 | −0.07 | −0.07 | T1 | −0.08 | −0.07 | −0.07 | T1 | −0.08 | −0.05 | −0.02 | ||||||
| A1 | 0.01 | 0.01 | 0.00 | A1 | 0.01 | 0.01 | 0.01 | A1 | 0.02 | 0.06 | 0.01 | 0.05 | 0.02 | ||||
| G2 | −0.14 | −0.13 | −0.14 | G2 | −0.14 | −0.13 | −0.13 | G2oxo | −0.16 | 0.69 | 0.97 | 0.59 | 0.97 | ||||
| A3 | 0.03 | 0.06 | 0.02 | 0.05 | 0.03 | A3 | 0.03 | 0.07 | 0.02 | 0.07 | 0.02 | A3 | 0.03 | 0.06 | 0.02 | 0.05 | 0.01 |
| G4 | −0.18 | 0.67 | 0.96 | 0.55 | 0.96 | G4oxo | −0.19 | 0.66 | 0.97 | 0.59 | 0.97 | G4 | −0.18 | −0.17 | −0.16 | ||
| G5 | −0.14 | −0.10 | 0.02 | −0.09 | 0.02 | G5 | −0.13 | −0.10 | 0.01 | −0.10 | 0.01 | G5 | −0.14 | −0.14 | −0.13 | ||
| A6 | 0.03 | 0.04 | 0.03 | A6 | 0.04 | 0.04 | 0.05 | A6 | 0.04 | 0.04 | 0.04 | ||||||
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | |||||||||||||||
| T6 | −0.06 | −0.06 | −0.05 | T6 | −0.06 | −0.06 | −0.05 | T6 | −0.06 | −0.06 | −0.06 | ||||||
| C5 | 0.21 | 0.22 | 0.22 | C5 | 0.21 | 0.22 | 0.22 | C5 | 0.21 | 0.21 | 0.22 | ||||||
| C4 | 0.13 | 0.16 | 0.23 | C4 | 0.13 | 0.16 | 0.23 | C4 | 0.12 | 0.12 | 0.13 | ||||||
| T3 | −0.08 | −0.07 | −0.06 | T3 | −0.08 | −0.07 | −0.07 | T3 | −0.08 | −0.07 | −0.07 | ||||||
| C2 | 0.22 | 0.22 | 0.23 | C2 | 0.22 | 0.22 | 0.23 | C2 | 0.22 | 0.25 | 0.31 | ||||||
| T1 | −0.07 | −0.07 | −0.05 | T1 | −0.06 | −0.06 | −0.06 | T1 | −0.05 | −0.03 | −0.01 | ||||||
| A1 | 0.00 | 0.00 | −0.01 | A1 | −0.01 | −0.01 | −0.01 | A1 | 0.06 | 0.11 | 0.02 | 0.10 | |||||
| G2 | −0.16 | −0.16 | −0.15 | G2 | −0.16 | −0.16 | −0.14 | G2oxo | −0.24 | 0.63 | 0.97 | 0.53 | 0.97 | ||||
| ScA3 | 0.04 | 0.08 | 0.03 | 0.07 | 0.03 | ScA3 | 0.03 | 0.07 | 0.03 | 0.07 | 0.02 | ScA3 | 0.03 | 0.06 | 0.01 | 0.06 | 0.03 |
| G4 | −0.15 | 0.72 | 0.96 | 0.62 | 0.96 | G4oxo | −0.16 | 0.71 | 0.96 | 0.61 | 0.97 | G4 | −0.14 | −0.14 | −0.13 | ||
| G5 | −0.11 | −0.08 | 0.01 | −0.09 | 0.01 | G5 | −0.11 | −0.08 | 0.01 | −0.08 | 0.01 | G5 | −0.11 | −0.11 | −0.11 | ||
| A6 | 0.03 | 0.04 | 0.04 | A6 | 0.03 | 0.04 | 0.04 | A6 | 0.03 | 0.03 | 0.03 | ||||||
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | |||||||||||||||
| T6 | 0.05 | −0.05 | −0.05 | T6 | −0.05 | −0.05 | −0.05 | T6 | −0.05 | −0.05 | −0.05 | ||||||
| C5 | −0.21 | 0.21 | 0.22 | C5 | 0.21 | 0.22 | 0.23 | C5 | 0.21 | 0.21 | 0.21 | ||||||
| C4 | 0.13 | 0.16 | 0.24 | C4 | 0.14 | 0.17 | 0.23 | C4 | 0.13 | 0.13 | 0.14 | ||||||
| T3 | −0.06 | −0.04 | −0.04 | T3 | −0.09 | −0.08 | −0.08 | T3 | −0.06 | −0.05 | −0.08 | ||||||
| C2 | 0.22 | 0.23 | 0.23 | C2 | 0.21 | 0.21 | 0.21 | C2 | 0.23 | 0.26 | 0.30 | ||||||
| T1 | −0.03 | −0.03 | −0.03 | T1 | −0.03 | −0.03 | −0.03 | T1 | −0.04 | −0.02 | 0.00 | ||||||
| A1 | −0.02 | −0.02 | −0.02 | A1 | −0.02 | −0.02 | −0.02 | A1 | −0.01 | 0.06 | 0.04 | 0.07 | 0.05 | ||||
| G2 | −0.15 | −0.15 | −0.14 | G2 | −0.15 | −0.14 | −0.13 | G2oxo | −0.17 | 0.66 | 0.95 | 0.55 | 0.92 | ||||
| RcA3 | 0.02 | 0.10 | 0.07 | 0.06 | 0.03 | RcA3 | 0.06 | 0.10 | 0.02 | 0.08 | 0.02 | RcA3 | 0.03 | 0.06 | 0.01 | 0.06 | 0.03 |
| G4 | −0.17 | 0.63 | 0.89 | 0.59 | 0.95 | G4oxo | −0.19 | 0.67 | 0.96 | 0.61 | 0.97 | G4 | −0.16 | −0.16 | −0.13 | ||
| G5 | −0.12 | −0.07 | 0.04 | −0.09 | 0.02 | G5 | −0.12 | −0.08 | 0.02 | −0.09 | 0.01 | G5 | −0.12 | −0.12 | −0.12 | ||
| A6 | 0.03 | 0.04 | 0.04 | A6 | 0.04 | 0.04 | 0.05 | A6 | 0.03 | 0.03 | 0.04 | ||||||
| ds-oligo | Mode | Discussed Trimers | |||||
|---|---|---|---|---|---|---|---|
| G2A3G4 | A3G4 G5 | ||||||
| G2→A3 | A3→G4 | G2→G4 | A3→G4 | G4→G5 | A3→G5 | ||
| N-DNA | Vertical | 0.49 | −0.49 | −0.03 | −0.49 | 0.73 | −0.04 |
| Adiabatic | 0.46 | −0.77 | −0.31 | −0.77 | 0.29 | −0.48 | |
| G2A3 | A3←G4 | G2←G4 | A3←G4 | G4←G5 | A3←G5 | ||
| Vertical | −0.46 | 1.18 | 0.31 | 1.18 | −0.01 | 0.89 | |
| Adiabatic | −0.46 | 0.77 | 0.31 | 0.77 | −0.29 | 0.48 | |
| 3Goxo-N-DNA | G2→A3 | A3→ oxoG4 | G2→ oxoG4 | A3→ oxoG4 | oxoG4→G5 | A3→G5 | |
| Vertical | 0.46 | −0.77 | −0.27 | −0.77 | 0.98 | −0.16 | |
| Adiabatic | 0.50 | −1.14 | −0.64 | −1.14 | 0.59 | −0.54 | |
| G2←A3 | A3←oxoG4 | G2←oxoG4 | A3←oxoG4 | oxoG4←G5 | A3←G5 | ||
| Vertical | −0.49 | 1.44 | 0.64 | 1.44 | −0.23 | 0.85 | |
| Adiabatic | −0.50 | 1.14 | 0.64 | 1.14 | −0.59 | 0.54 | |
| 5Goxo-N-DNA | oxoG2→A3 | A3→G4 | oxoG2→G4 | A3→G4 | G4→G5 | A3→G5 | |
| Vertical | 1.45 | −0.50 | 0.61 | −0.50 | 0.06 | −0.45 | |
| Adiabatic | 1.10 | −0.51 | 0.59 | −0.51 | 0.05 | −0.46 | |
| oxoG2←A3 | A3←G4 | oxoG2←G4 | A3←G4 | G4←G5 | A3←G5 | ||
| Vertical | −0.69 | 0.50 | −0.18 | 0.50 | −0.05 | 0.46 | |
| Adiabatic | −1.10 | 0.51 | −0.59 | 0.51 | −0.05 | 0.46 | |
| ScA-DNA | G2→ScA3 | ScA3→G4 | G2→G4 | ScA3→G4 | G4→G5 | ScA3→G5 | |
| Vertical | 0.55 | −0.51 | 0.02 | −0.51 | 0.71 | −0.11 | |
| Adiabatic | 0.52 | −0.82 | −0.29 | −0.82 | 0.35 | −0.47 | |
| G2←ScA3 | ScA3←G4 | G2←G4 | ScA3←G4 | G4←G5 | ScA3←G5 | ||
| Vertical | −0.46 | 1.16 | 0.35 | 1.16 | −0.05 | 0.82 | |
| Adiabatic | −0.52 | 0.82 | 0.29 | 0.82 | −0.35 | 0.47 | |
| 3Goxo-ScA-DNA | G2→ScA3 | ScA3→oxoG4 | G2→oxoG4 | cSA3→oxoG4 | oxoG4→G5 | ScA3→G5 | |
| Vertical | 0.55 | −0.73 | −0.20 | −0.73 | 1.01 | −0.11 | |
| Adiabatic | 0.53 | −1.12 | −0.59 | −1.12 | 0.64 | −0.48 | |
| G2←ScA3 | ScA3←oxoG4 | G2←oxoG4 | ScA3←oxoG4 | oxoG4←G5 | ScA3←G5 | ||
| Vertical | −0.53 | 1.47 | 0.60 | 1.47 | −0.25 | 0.83 | |
| Adiabatic | −0.53 | 1.12 | 0.59 | 1.12 | −0.64 | 0.48 | |
| 5Goxo-ScA-DNA | oxoG2→ScA3 | ScA3→G4 | oxoG2→G4 | ScA3→G4 | G4→G5 | ScA3→G5 | |
| Vertical | 1.48 | −0.61 | 0.67 | −0.61 | 0.05 | −0.59 | |
| Adiabatic | 1.28 | −0.64 | 0.64 | −0.64 | 0.03 | −0.61 | |
| oxoG2←ScA3 | ScA3←G4 | oxoG2←G4 | ScA3←G4 | G4←G5 | ScA3←G5 | ||
| Vertical | −0.87 | 0.52 | −0.23 | 0.52 | −0.04 | 0.48 | |
| Adiabatic | −1.28 | 0.64 | −0.64 | 0.64 | −0.03 | 0.61 | |
| RcA-DNA | G2→RcA3 | RcA3→G4 | G2→G4 | cA3→G4 | G4→G5 | cA3→G5 | |
| Vertical | 0.49 | −0.52 | 0.02 | −0.52 | 0.69 | −0.18 | |
| Adiabatic | 0.55 | −0.88 | −0.33 | −0.88 | 0.38 | −0.50 | |
| G2←RcA3 | RcA3←G4 | G2←G4 | RcA3←G4 | G4←G5 | RcA3←G5 | ||
| Vertical | −0.42 | 1.08 | 0.46 | 1.08 | −0.06 | 0.70 | |
| Adiabatic | −0.55 | 0.88 | 0.33 | 0.88 | −0.38 | 0.50 | |
| 3Goxo-RcA-DNA | G2→RcA3 | cA3→oxoG4 | G2→G4 | RcA3→oxoG4 | oxoG4→G5 | RcA3→G5 | |
| Vertical | 0.45 | −0.69 | −0.19 | −0.69 | 0.96 | −0.16 | |
| Adiabatic | 0.50 | −1.12 | −0.63 | −1.12 | 0.62 | −0.50 | |
| G2←RcA3 | RcA3←oxoG4 | G2←oxoG4 | RcA3←G4 | oxoG4←G5 | RcA3←G5 | ||
| Vertical | −0.47 | 1.39 | 0.66 | 1.39 | −0.20 | 0.76 | |
| Adiabatic | −0.50 | 1.12 | 0.63 | 1.12 | −0.62 | 0.50 | |
| 5Goxo-RcA-DNA | oxoG2→RcA3 | RcA3→G4 | oxoG2→G4 | RcA3→G4 | G4→G5 | RcA3→G5 | |
| Vertical | 1.36 | −0.53 | 0.67 | −0.53 | 0.08 | −0.61 | |
| Adiabatic | 1.19 | −0.69 | −0.50 | −0.69 | 0.11 | −0.58 | |
| oxoG2←RcA3 | RcA3←G4 | oxoG2←G4 | RcA3←G4 | G4←G5 | RcA3←G5 | ||
| Vertical | −0.80 | 0.47 | −0.11 | 0.47 | 0.01 | 0.36 | |
| Adiabatic | −1.19 | 0.69 | −0.50 | 0.69 | −0.11 | 0.58 | |
| System | ds-DNA Hole Transfer between Stacked Base Pairs | ||||||||||||||||
| λ | ΔG | Ea | V12 | KHT | λ | ΔG | Ea | V12 | KHT | λ | ΔG | Ea | V12 | kHT | |||
| N-DNA | 3Goxo-N-DNA | 5Goxo-N-DNA | |||||||||||||||
| G2←A3 | 0.00 | −0.46 | 18.60 | 0.221 | 0.00 | G2←A3 | 0.01 | −0.50 | 10.09 | 0.220 | 0.00 | oxoG2←A3 | 0.41 | −1.10 | 0.29 | 0.320 | 3.2 × 1010 |
| A3→G4 | 0.28 | −0.77 | 0.22 | 0.246 | 3.8 × 1011 | A3→oxoG4 | 0.37 | −1.14 | 0.41 | 0.363 | 5.2 × 108 | A3→G4 | 0.01 | −0.51 | 4.31 | 0.246 | 0.00 |
| G4←G5 | 0.44 | −0.29 | 0.01 | 0.051 | 4.0 × 1013 | oxoG4←G5 | 0.38 | −0.59 | 0.03 | 0.113 | 1.1 × 1014 | G4←G5 | 0.02 | −0.05 | 0.01 | 0.048 | 1.7 × 1014 |
| ScA-DNA | 3Goxo-ScA-DNA | 5Goxo-ScA-DNA | |||||||||||||||
| G2←ScA3 | 0.06 | −0.52 | 0.86 | 0.263 | 14.58 | G2←cSA3 | 0.02 | −0.53 | 4.30 | 0.271 | 0.00 | oxoG2←ScA3 | 0.41 | −1.28 | 0.45 | 0.367 | 7.3 × 107 |
| ScA3→G4 | 0.31 | −0.82 | 0.21 | 0.264 | 6.4 × 1011 | ScA3→oxoG4 | 0.39 | −1.12 | 0.35 | 0.378 | 5.5 × 109 | ScA3→G4 | 0.04 | −0.64 | 2.62 | 0.271 | 0.00 |
| G4←G5 | 0.36 | −0.35 | 0.00 | 0.035 | 3.4 × 1013 | oxoG4←G5 | 0.37 | −0.64 | 0.05 | 0.157 | 1.0 × 1014 | G4←G5 | 0.01 | −0.03 | 0.01 | 0.038 | 1.5 × 1014 |
| RcA-DNA | 3Goxo-RcA-DNA | 5Goxo-RcA-DNA | |||||||||||||||
| G2←RcA3 | 0.13 | −0.55 | 0.33 | 0.260 | 9.1 × 109 | G2←RcA3 | 0.03 | −0.50 | 1.91 | 0.268 | 0.00 | oxoG2←RcA3 | 0.39 | −1.19 | 0.41 | 0.352 | 3.28 × 108 |
| RcA3→G4 | 0.35 | −0.88 | 0.19 | 0.297 | 1.3 × 1012 | RcA3→oxoG4 | 0.43 | −1.12 | 0.27 | 0.349 | 7.8 × 1010 | RcA3→G4 | 0.16 | −0.69 | 0.43 | 0.291 | 1.75 × 108 |
| G4←G5 | 0.31 | −0.38 | 0.00 | 0.086 | 1.9 × 1014 | oxoG4←G5 | 0.34 | −0.62 | 0.06 | 0.131 | 5.3 × 1013 | G4←G5 | 0.12 | −0.11 | 0.00 | 0.085 | 3.5 × 1014 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. The Influence of Single, Tandem, and Clustered DNA Damage on the Electronic Properties of the Double Helix: A Theoretical Study. Molecules 2020, 25, 3126. https://doi.org/10.3390/molecules25143126
Karwowski BT. The Influence of Single, Tandem, and Clustered DNA Damage on the Electronic Properties of the Double Helix: A Theoretical Study. Molecules. 2020; 25(14):3126. https://doi.org/10.3390/molecules25143126
Chicago/Turabian StyleKarwowski, Bolesław T. 2020. "The Influence of Single, Tandem, and Clustered DNA Damage on the Electronic Properties of the Double Helix: A Theoretical Study" Molecules 25, no. 14: 3126. https://doi.org/10.3390/molecules25143126
APA StyleKarwowski, B. T. (2020). The Influence of Single, Tandem, and Clustered DNA Damage on the Electronic Properties of the Double Helix: A Theoretical Study. Molecules, 25(14), 3126. https://doi.org/10.3390/molecules25143126





