Assembly and Redox-Rich Hydride Chemistry of an Asymmetric Mo2S2 Platform
Abstract
1. Introduction
2. Results
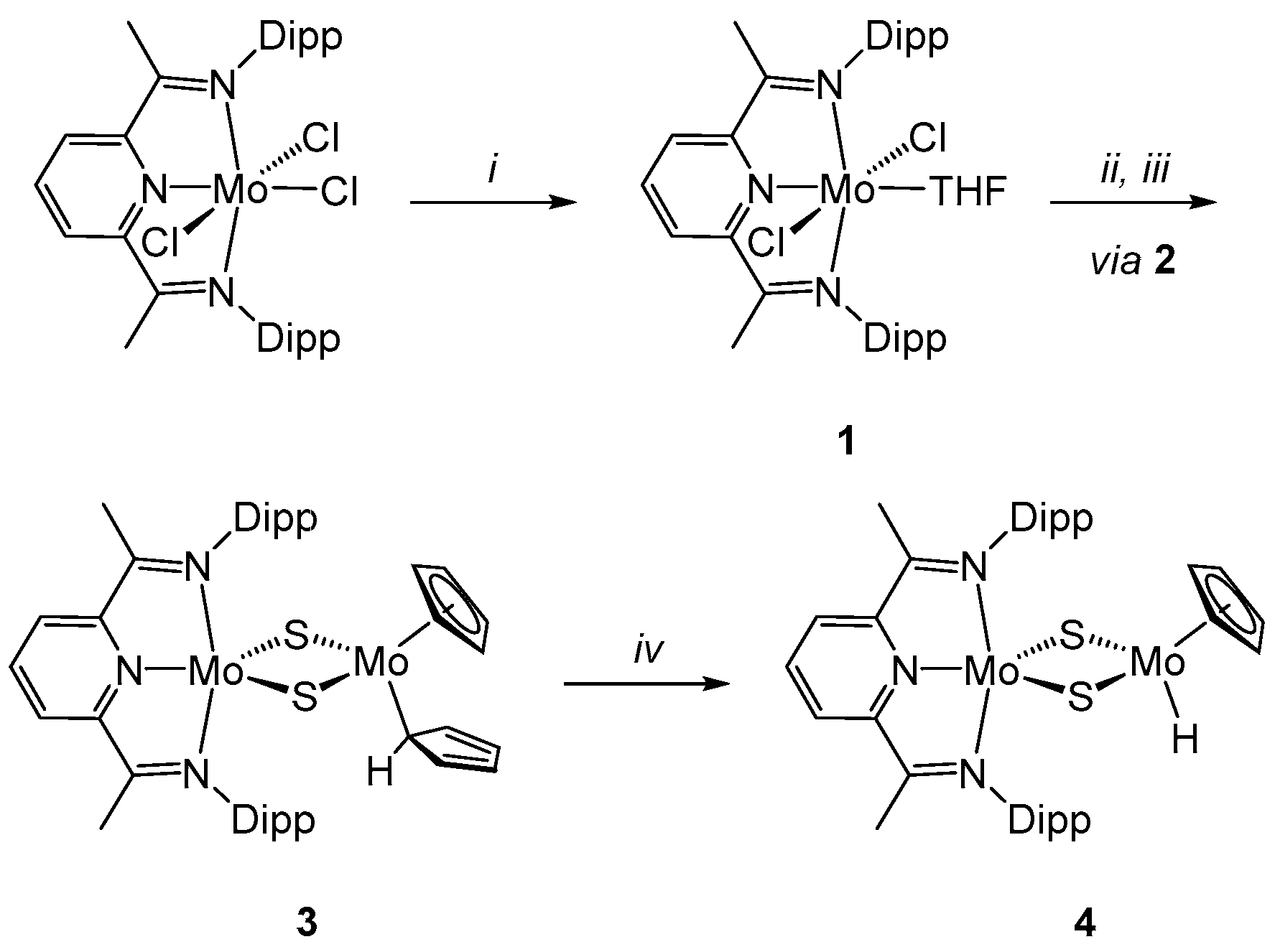
2.1. Synthesis of Complexes
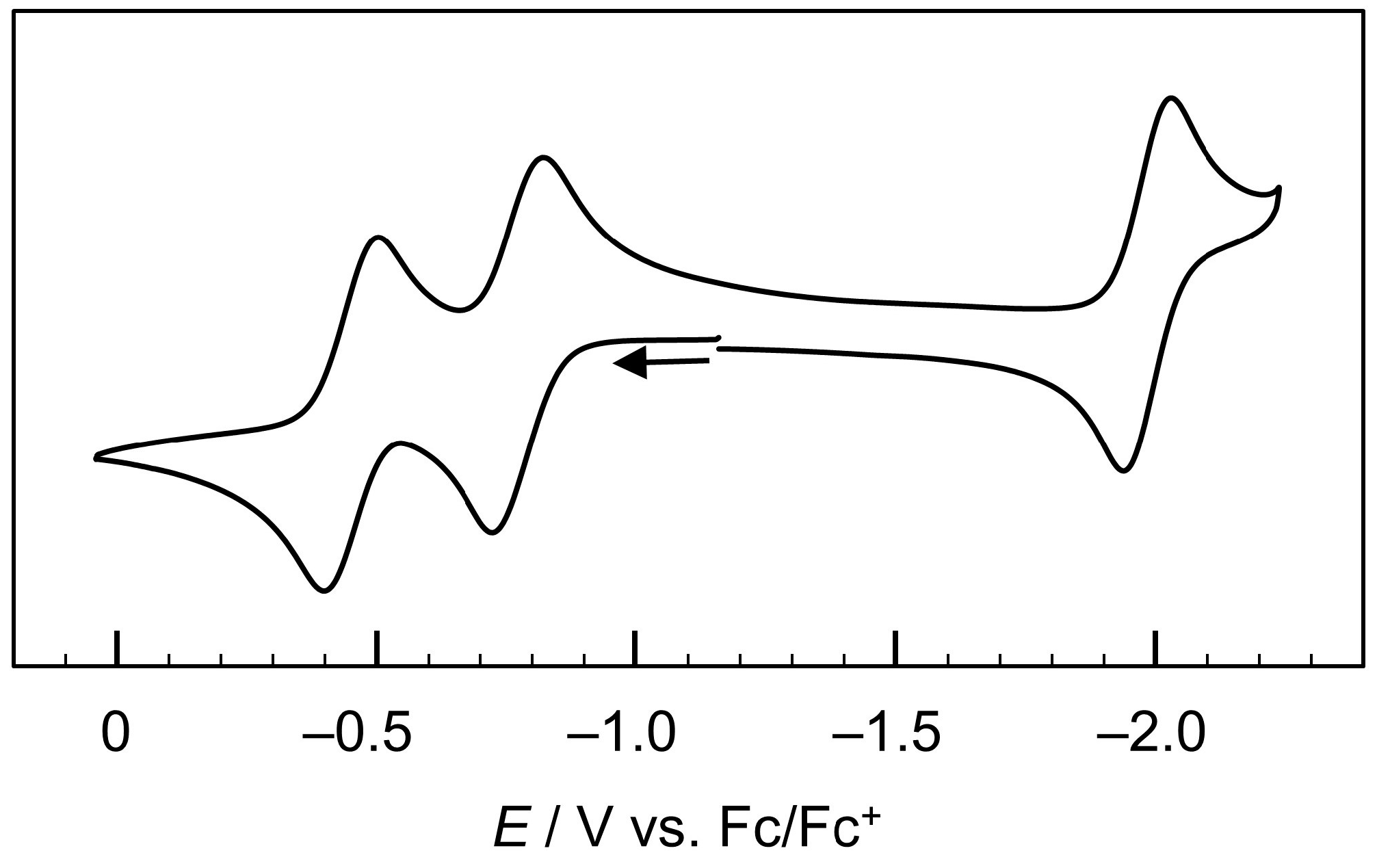
2.2. EPR Spectroscopy
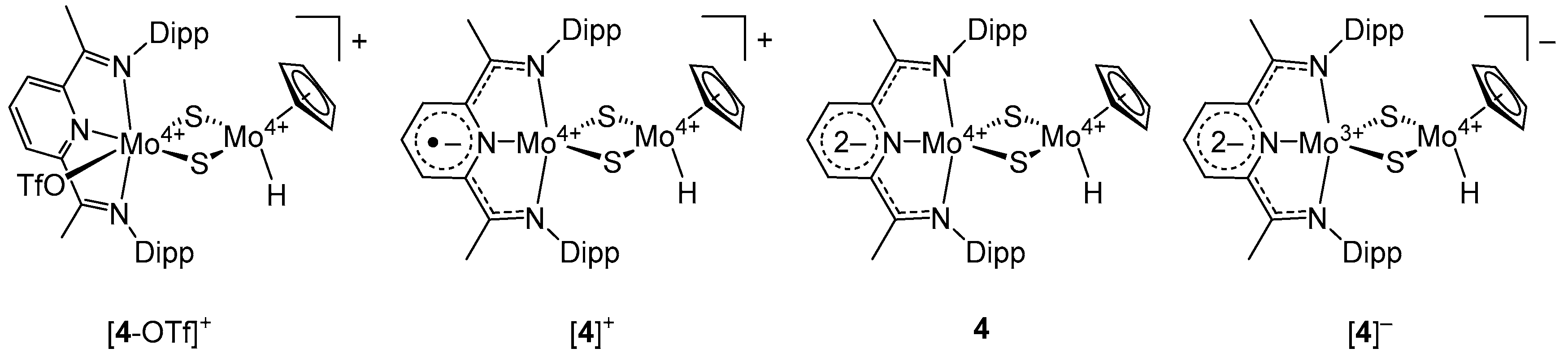
2.3. Crystallography
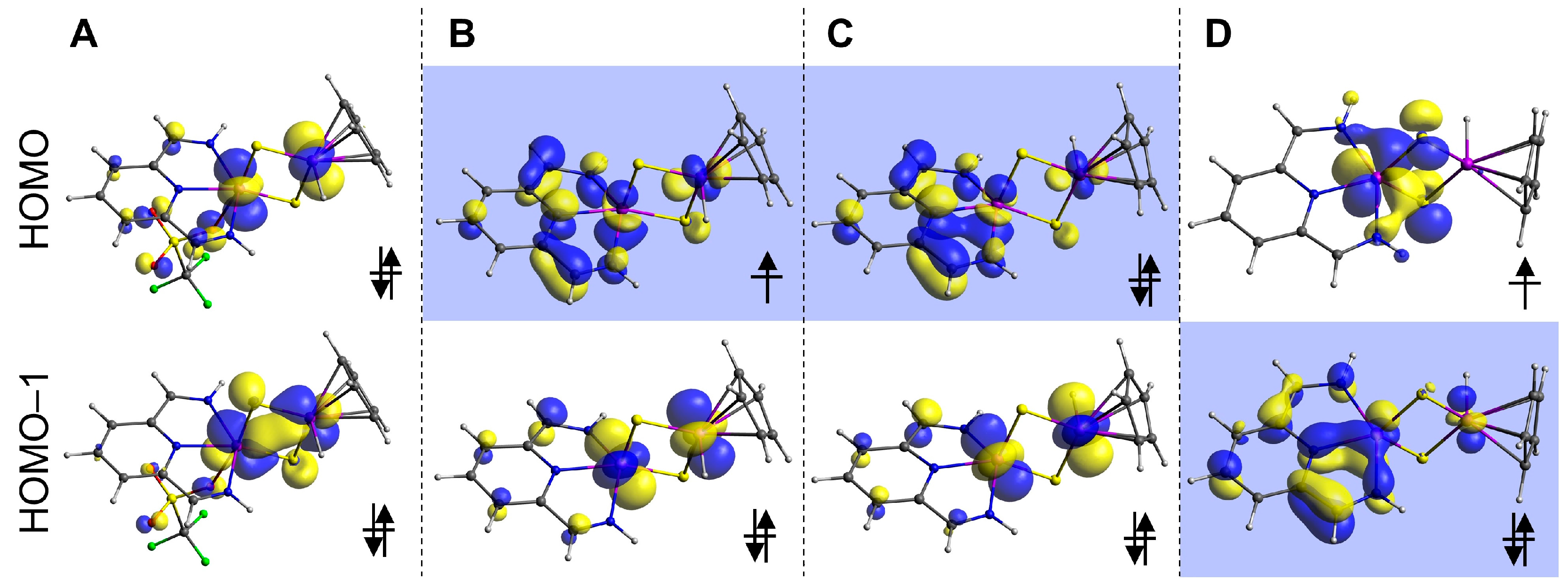
2.4. Density Functional Theory Calculations
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Synthesis
5.1.1. General Considerations
5.1.2. Crystallography
5.1.3. Experimental
trans-(PDI)MoCl2(THF) (1)
trans-(PDI)MoCl2(μ-SH)Mo(SH)Cp2 (2)
(PDI)Mo(μ-S)2Mo(η5-Cp)(η1-Cp)] (3)
(PDI)Mo(μ-S)2Mo(η5-Cp)(H) (4)
[(PDI)Mo(μ-S)2Mo(η5-Cp)(H)][Na(crypt-222)] ([4][Na(crypt-222)])
[(PDI)Mo(μ-S)2Mo(η5-Cp)(H)][BArF4] ([4][BArF4])
[(PDI)Mo(OTf)(μ-S)2Mo(η5-Cp)(H)][OTf] ([4-OTf][OTf])
[(PDI)Mo(MeCN)(μ-S)2Mo(η5-Cp)(MeCN)][BF4]2 (5)
Procedure for the Protonation of [4][Na(crypt-222)] to Give 6:
5.2. DFT Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Esswein, A.J.; Nocera, D.G. Hydrogen Production by Molecular Photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.T.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum Sulfides-Efficient and Viable Materials for Electro-and Photoelectrocatalytic Hydrogen Evolution. Energy. Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Xu, Z.C.; Wang, X. Recent Development of Molybdenum Sulfides as Advanced Electrocatalysts for Hydrogen Evolution Reaction. ACS Catal. 2014, 4, 1693–1705. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Huang, Y.F.; Nielsen, R.J.; Goddard, W.A.; Soriaga, M.P. The Reaction Mechanism with Free Energy Barriers for Electrochemical Dihydrogen Evolution on MoS2. J. Am. Chem. Soc. 2015, 137, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Tran, T.V.; Orio, M.; Torelli, S.; Truong, Q.D.; Nayuki, K.; Sasaki, Y.; Chiam, S.Y.; Yi, R.; Honma, I.; et al. Coordination Polymer Structure and Revisited Hydrogen Evolution Catalytic Mechanism for Amorphous Molybdenum Sulfide. Nat. Mater. 2016, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, D.E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Huang, Y.F.; Nielsen, R.J.; Goddard, W.A. Reaction Mechanism for the Hydrogen Evolution Reaction on the Basal Plane Sulfur Vacancy Site of MoS2 Using Grand Canonical Potential Kinetics. J. Am. Chem. Soc. 2018, 140, 16773–16782. [Google Scholar] [CrossRef]
- Appel, A.M.; DuBois, D.L.; DuBois, M.R. Molybdenum-Sulfur Dimers as Electrocatalysts for the Production of Hydrogen at Low Overpotentials. J. Am. Chem. Soc. 2005, 127, 12717–12726. [Google Scholar] [CrossRef] [PubMed]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.; Majda, M.; Long, J.R.; Chang, C.J. A Molecular MoS2 Edge Site Mimic for Catalytic Hydrogen Generation. Science 2012, 335, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Eckenhoff, W.T.; Brennessel, W.W.; Eisenberg, R. Light-Driven Hydrogen Production from Aqueous Protons Using Molybdenum Catalysts. Inorg. Chem. 2014, 53, 9860–9869. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an Appropriate Active-Site Motif into a Hydrogen-Evolution Catalyst with Thiomolybdate [Mo3S13]2− Clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef]
- Garrett, B.R.; Click, K.A.; Durr, C.B.; Hadad, C.M.; Wu, Y.Y. [MoO(S2)2L](1-) (L = picolinate or pyrimidine-2-carboxylate) Complexes as MoSx-Inspired Electrocatalysts for Hydrogen Production in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 13726–13731. [Google Scholar] [CrossRef]
- Garrett, B.R.; Polen, S.M.; Click, K.A.; He, M.F.; Huang, Z.J.; Hadad, C.M.; Wu, Y. Tunable Molecular MoS2 Edge-Site Mimics for Catalytic Hydrogen Production. Inorg. Chem. 2016, 55, 3960–3966. [Google Scholar] [CrossRef]
- Ji, Z.; Trickett, C.; Pei, X.K.; Yaghi, O.M. Linking Molybdenum-Sulfur Clusters for Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 13618–13622. [Google Scholar] [CrossRef]
- Rajagopal, A.; Venter, F.; Jacob, T.; Petermann, L.; Rau, S.; Tschierlei, S.; Streb, C. Homogeneous visible light-driven hydrogen evolution by the molecular molybdenum sulfide model [Mo2S12]2−. Sustain Energy Fuels 2019, 3, 92–95. [Google Scholar] [CrossRef]
- Sellmann, D.; Zapf, L. Transition-Metal Complexes with Sulfur Ligands XIII*. Syntheses and Reactions of the 1,2-Benzenedithiolato Molybdate Complexes [Mo(C6H4S2)3]0, [Mo(C6S4S2)3]1−, [Mo(C6S4S2)3]2− and the Hydride Complex [Mo(H)(C6H4S2)3]3−. Z. Naturforsch. B 1985, 40, 380–388. [Google Scholar] [CrossRef]
- Lazarowych, N.J.; Morris, R.H. Molybdenum Complexes Containing Hydride and Sulfur Donor Ligands. Synthesis and Properties of Mo(H)2(S2C6H3R)(PMePh2)3, Mo(H)2(S2C6H3H)(PMePh2)3, R = H, Me. J. Chem. Soc. Chem. Commun. 1987, 1865–1866. [Google Scholar] [CrossRef]
- Henderson, R.A.; Hughes, D.L.; Richards, R.L.; Shortman, C. The Preparation, Structure, and Reactions of the Mononuclear Thiolate-Hydride Complexes [MoH(SR)(Dppe)2] (R = Bulky Alkyl or Aryl Group, Dppe = Ph2PCH2CH2PPh2)—X-Ray Structure Determination of Cis-[MoH(SC6H2Pr-2,4,6)(Dppe)2]. J. Chem. Soc. Dalton Trans. 1987, 1115–1121. [Google Scholar] [CrossRef]
- Burrow, T.E.; Lazarowych, N.J.; Morris, R.H.; Lane, J.; Richards, R.L. Hydride Complexes of Molybdenum and Tungsten in a Sulfur Environment. Polyhedron 1989, 8, 1701–1704. [Google Scholar] [CrossRef]
- Lu, S.F.; Huang, J.Q.; Zhuang, H.H.; Li, J.Q.; Wu, D.M.; Huang, Z.X.; Lu, C.Z.; Huang, J.L.; Lu, J.X. Synthesis, Structure and Molecular-Orbital Studies of a Series of Cubane-Type Tetranuclear Molybdenum (Tungsten) Clusters with [S2P(OC2H5)2]− as Ligands. Polyhedron 1991, 10, 2203–2215. [Google Scholar]
- Bernatis, P.; Haltiwanger, R.C.; Dubois, M.R. Sites of Electrophilic Attack in Neutral Dithiolate-Bridged Molybdenum Complexes. Organometallics 1992, 11, 2435–2443. [Google Scholar] [CrossRef]
- Schollhammer, P.; Petillon, F.Y.; Pichon, R.; Poderguillou, S.; Talarmin, J.; Muir, K.W.; Girdwood, S.E. Reactions of Dinuclear and Polynuclear Complexes. 13. Synthesis, Structure and Fluxional Behavior of Hydrido-Bridged Thiolato-Bridged or Selenato-Bridged Complexes [Mo2Cp2(μ-H)(μ-ER)(CO)4] (E = S, R = Me, tBu, Bz, or Ph E = Se, R = Ph). J. Organomet. Chem. 1995, 486, 183–191. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Hughes, D.L.; Maguire, M.J.; Marjani, K.; Richards, R.L. Disproportionation and reduction of hydrazine at a molybdenum-thiolate centre: Crystal structures of [MoH(SC6H2Pr-2,4,6)3(NH3)(PMePh2)] and [MoH(SC6H2Pr-2,4,6)3(NH2NHPh)(PMePh2)]. J. Chem. Soc. Dalton Trans. 1997, 4747–4752. [Google Scholar] [CrossRef]
- Mathur, P.; Ghose, S.; Hossain, M.M.; Satyanarayana, C.V.V.; Drake, J.E. Synthesis and structural characterisation of a mixed Mo/Fe, mixed chalcogen, PhS-bridged cluster. J. Organomet. Chem. 1998, 557, 221–225. [Google Scholar] [CrossRef]
- Takemoto, S.; Otsuka, K.; Otsuka, T.; Seino, H.; Mizobe, Y.; Hidai, M. Synthesis and structures of p-tert-butyltetrathiacalix[4]arene-dihydrides of Mo(V) and W(IV). Chem. Lett. 2002, 31, 6–7. [Google Scholar] [CrossRef]
- Takemoto, S.; Tanaka, S.; Mizobe, Y.; Hidai, M. Ti-Mo Heterobimetallic Thiacalix[4]Arene Complex Containing an Unusual Alpha-Agostic μ-η5:η2-Cyclopentadienyl Ligand. Chem. Commun. 2004, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, H.; Seino, H.; Mizobe, Y. Synthesis of a Sulfido-Capped Trinuclear Cluster [{(η5-C5Me5)Ir}2{Mo(CO)3}(μ3-S)2] and its Reactions at the Molybdenum Site Forming a Series of Ir2MoS2 clusters. Organometallics 2007, 26, 3499–3508. [Google Scholar] [CrossRef]
- Buccella, D.; Parkin, G. Mononuclear and Dinuclear Molybdenum and Tungsten Complexes of p-tert-butyltetrathiacalix[4]arene and p-tert-butyltetrasulfonylcalix[4]arene: Facile cleavage of the calixarene ligand framework by nickel. J. Am. Chem. Soc. 2008, 130, 8617–8619. [Google Scholar] [CrossRef] [PubMed]
- Algarra, A.G.; Basallote, M.G.; Fernandez-Trujillo, M.J.; Feliz, M.; Guillamon, E.; Llusar, R.; Sorribes, I.; Vicent, C. Chiral [Mo3S4H3(diphosphine)3]+ Hydrido Clusters and Study of the Effect of the Metal Atom on the Kinetics of the Acid-Assisted Substitution of the Coordinated Hydride: Mo vs. W. Inorg. Chem. 2010, 49, 5935–5942. [Google Scholar] [CrossRef]
- Sattler, A.; Janak, K.E.; Parkin, G. Modeling Aspects of Hydrodesulfurization by Molybdenum Hydride Compounds: Desulfurization of Thiophene and Benzothiophene and C-S Bond Cleavage of Dibenzothiophene. Inorg. Chim. Acta 2011, 369, 197–202. [Google Scholar] [CrossRef]
- >300 structures on the CCDC as of 2020.
- Margulieux, G.W.; Turner, Z.R.; Chirik, P.J. Synthesis and Ligand Modification Chemistry of a Molybdenum Dinitrogen Complex: Redox and Chemical Activity of a Bis(imino)Pyridine Ligand. Angew. Chem. Int. Ed. 2014, 53, 14211–14215. [Google Scholar] [CrossRef]
- Green, M.L.H.; Lindsell, W.E. Some Bis-π-Cyclopentadienyl-Molybdenum and-Tungsten Complexes with Sulphur or Oxygen Ligands. J. Chem. Soc. A 1967, 1455–1457. [Google Scholar] [CrossRef]
- Kaesz, H.D.; Saillant, R.B. Hydride Complexes of the Transition-Metals. Chem. Rev. 1972, 72, 231–281. [Google Scholar] [CrossRef]
- Silavwe, N.D.; Castellani, M.P.; Tyler, D.R.; Beck, M.A.; Lichtenhan, J.D.; Doherty, N.M. Inorganic Syntheses; John Wiley & Sons, Inc.: Charlottesuille, VA, USA, 1992; Chapter 4; pp. 204–211. [Google Scholar]
- Scarborough, C.C.; Lancaster, K.M.; DeBeer, S.; Weyhermueller, T.; Sproules, S.; Wieghardt, K. Experimental Fingerprints for Redox-Active Terpyridine in [Cr(tpy)2](PF6)(N) (N = 3–0), and the Remarkable Electronic Structure of [Cr(tpy)2]1−. Inorg. Chem. 2012, 51, 3718–3732. [Google Scholar] [CrossRef]
- de Bruin, B.; Bill, E.; Bothe, E.; Weyhermuller, T.; Wieghardt, K. Molecular and electronic structures of bis(pyridine-2,6-diimine)metal complexes [ML2](PF6)n (n=0, 1, 2, 3; M = Mn, Fe, Co, Ni, Cu, Zn). Inorg. Chem. 2000, 39, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Luca, O.R.; Crabtree, R.H. Redox-Active Ligands in Catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. [Google Scholar] [CrossRef]
- Knijnenburg, Q.; Gambarotta, S.; Budzelaar, P.H.M. Ligand-Centred Reactivity in Diiminepyridine Complexes. Dalton Trans. 2006, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Margulieux, G.W.; Bezdek, M.J.; Turner, Z.R.; Chirik, P.J. Ammonia Activation, H2 Evolution and Nitride Formation from a Molybdenum Complex with a Chemically and Redox Noninnocent Ligand. J. Am. Chem. Soc. 2017, 139, 6110–6113. [Google Scholar] [CrossRef] [PubMed]
- Stoffelbach, F.; Saurenz, D.; Poli, R. Improved Preparations of Molybdenum Coordination Compounds from Tetrachlorobis(diethyl Ether)Molybdenum(IV). Eur. J. Inorg. Chem. 2001, 2699–2703. [Google Scholar] [CrossRef]
- Greenhalgh, M.D.; Thomas, S.P. Iron-Catalyzed, Highly Regioselective Synthesis of Alpha-Aryl Carboxylic Acids from Styrene Derivatives and CO2. J. Am. Chem. Soc. 2012, 134, 11900–11903. [Google Scholar] [CrossRef]
- Scott, T.A.; Ooro, B.A.; Collins, D.J.; Shatruk, M.; Yakovenko, A.; Dunbar, K.R.; Zhou, H.C. After 118 Years, the Isolation of Two Common Radical Anion Reductants as Simple, Stable Solids. Chem. Commun. 2009, 65–67. [Google Scholar] [CrossRef]
- Bahr, S.R.; Boudjouk, P. Trityl Tetrakis(3,5-Bis(Trifluoromethyl)Phenyl)Borate—A New Hydride Abstraction Reagent. J. Org. Chem. 1992, 57, 5545–5547. [Google Scholar] [CrossRef]
- Le Bras, J.; Jiao, H.J.; Meyer, W.E.; Hampel, F.; Gladysz, J.A. Synthesis, crystal structure, and reactions of the 17-valence-electron rhenium methyl complex [(η5-C5Me5)Re(NO)(P(4-C6H4CH3)3)(CH3)]+ B(3,5-C6H3(CF3)2)4−: Experimental and computational bonding comparisons with 18-electron methyl and methylidene complexes. J. Organomet. Chem. 2000, 616, 54–66. [Google Scholar] [CrossRef]
- Li, Z.W.; Yeh, A.; Taube, H. Mixed-Valence Molecules Based on Monohydridobis(Ethylenediamine) Osmium(IV) and Metal Cyano Complexes. Inorg. Chem. 1994, 33, 2874–2881. [Google Scholar] [CrossRef]
- Schubert, E.M. Utilizing the Evans Method with a Superconducting NMR Spectrometer in the Undergraduate Laboratory. J. Chem. Educ. 1992, 69, 62. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- SADABS; Version 2014/5; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. Shelxt—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Neese, F. The Orca Program System. Wires Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. 3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Schafer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian-Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian-Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Ab initio Pseudopotentials for the 2nd and 3rd Row Transition-Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 15104–15119. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| (Cp)Mo–S | (PDI)Mo–S | Mo–Mo | NimMo | NpyMo | Nim = Cim | Cim–Cpy | ∠MoSMo | |
|---|---|---|---|---|---|---|---|---|
| [4-OTf]+ | 2.261(2) | 2.2598(5) | 2.770(1) | 2.172(1) | 2.173(1) | 1.297(2) | 1.473(2) | 75.59(4) |
| 2.269(2) | 2.2967(6) | 2.173(1) | 1.298(2) | 1.467(2) | 74.71(4) | |||
| [4]+ | 2.2790(4) | 2.2433(5) | 2.7832(4) | 2.128(2) | 2.126(1) | 1.318(2) | 1.440(3) | 75.97(2) |
| 2.2332(6) | 2.2921(5) | 2.093(2) | 1.345(2) | 1.441(2) | 75.90(2) | |||
| 4 | 2.2419(9) | 2.2912(7) | 2.8237(6) | 2.103(2) | 2.072(2) | 1.352(3) | 1.406(3) | 77.05(2) |
| 2.2964(7) | 2.2525(9) | 2.111(2) | 1.344(2) | 1.412(3) | 76.72(2) | |||
| [4]− | 2.291(1) | 2.299(1) | 2.8255(7) | 2.132(2) | 2.028(2) | 1.364(3) | 1.407(3) | 75.99(4) |
| 2.271(1) | 2.307(1) | 2.162(2) | 1.355(3) | 1.403(3) | 76.22(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McSkimming, A.; Taylor, J.W.; Harman, W.H. Assembly and Redox-Rich Hydride Chemistry of an Asymmetric Mo2S2 Platform. Molecules 2020, 25, 3090. https://doi.org/10.3390/molecules25133090
McSkimming A, Taylor JW, Harman WH. Assembly and Redox-Rich Hydride Chemistry of an Asymmetric Mo2S2 Platform. Molecules. 2020; 25(13):3090. https://doi.org/10.3390/molecules25133090
Chicago/Turabian StyleMcSkimming, Alex, Jordan W. Taylor, and W. Hill Harman. 2020. "Assembly and Redox-Rich Hydride Chemistry of an Asymmetric Mo2S2 Platform" Molecules 25, no. 13: 3090. https://doi.org/10.3390/molecules25133090
APA StyleMcSkimming, A., Taylor, J. W., & Harman, W. H. (2020). Assembly and Redox-Rich Hydride Chemistry of an Asymmetric Mo2S2 Platform. Molecules, 25(13), 3090. https://doi.org/10.3390/molecules25133090






